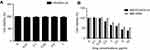Back to Journals » OncoTargets and Therapy » Volume 12
Studies on the treatment of melanoma with folate acid conjugated dextran and lauryl alcohol loaded with IMD0354
Authors Liu C, Chen W, Chen Z , Yan Y , Wang Q, Xie H, Chen X, Wang A , Tang S, Zhou J
Received 5 March 2019
Accepted for publication 27 April 2019
Published 14 June 2019 Volume 2019:12 Pages 4655—4663
DOI https://doi.org/10.2147/OTT.S207685
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Can Liu,1 Wei Chen,2 Zizi Chen,1 Yu Yan,3 Qing Wang,4 Huiqing Xie,5 Xiang Chen,6 Aijun Wang,7 Shijie Tang,8 Jianda Zhou1
1The Third Xiangya Hospital, Central South University, Changsha, Hunan 410013, People’s Republic of China; 2The Xiangya Hospital, Central South University, Changsha, Hunan 410008, People’s Republic of China; 3Xiangya School of Medicine, Central South University, Changsha, Hunan 410013, People’s Republic of China; 4National Institute of Environmental Health, Chinese Center for Disease Control and Prevention, Beijing 102206, People’s Republic of China; 5Department of Rehabilitation, The Third Xiangya Hospital, Central South University, Changsha, Hunan, 410013, People’s Republic of China; 6Department of Dermatology, The Xiangya Hospital, Central South University, Changsha, Hunan, 410008, People’s Republic of China; 7Surgical Bioengeneering Laboratory, School of Medicine, The University of California Davis, Sacramento, CA, 95817, USA; 8Cleft Lip and Palate Treatment Center, The Second Affliated Hospital, Shantou University Medical College, Shantou, Guangdong, 515041, People’s Republic of China
Background: IMD-0354 is a kind of hydrophobic small molecule inhibitor of IKKβ, which can effectively inhibit the NF-κB pathway. Besides, IMD-0354 can inhibit a variety of tumor cells in culture, but its poor water solubility and low utilization have limited its clinical application.
Methods: In this study, IMD-0354 was synthesized through esterifying the folate acid (FA) conjugated dextran (Dex) as well as the lauryl alcohol (LA).
Results:The particle (IMD/FA-Dex-LA) size was 212.13±10.62nm, the encapsulation efficiency was 89.27±6.51%, and the drug loading was 4.25±0.42%. Cell viability studies indicated that the IMD/FA-Dex-LA effectively inhibited survival of B16F10 cells in culture. Meanwhile, Western Blotting results showed that the nuclear transport of NF-κB was reduced after blocking the IKK pathway, which would thereby suppress melanoma cell division and proliferation. Moreover, subcutaneous tumor implantation experiment revealed that, the drug-loading complex had an obvious effect on suppressing melanoma cells. Findings of this study demonstrated that the IMD-0354 loaded FA-Dex-LA was more effective than IMD-0354 alone.
Conclusion: In summary, FA-Dex-LA has been successfully synthesized in this study, which can serve as a carrier for hydrophobic drug. Further, it is believed the FA-Dex-LA can potentially applied in cancer treatment.
Keywords: dextran, lauryl alcohol, folate acid, IKK inhibitor, melanoma
Introduction
It is well established that malignant melanoma (MM) is initiated by mutations in epidermal melanocytes that cause de-regulation of the cell cycle. MM accounts for 65% of skin cancer mortalities and is one of the deadliest of all solid tumors.1–4 Currently, molecular targeted therapy is another option for cancer treatment, but it is distinct from the mechanism of traditional antitumor drug therapy, in that it can block cancer cell division and suppress its replication, thus restraining its killing effect.5,6 Studies have shown that, in melanoma and other cancer cells, activation of transcription factor NF-κΒ can initiate mitotic and anti-apoptotic pathways. Therefore, there have been many attempts to design inhibitors that can block these pathways.7,8 For example, the IKK complex inhibitor IMD-0345 has been shown to suppress cancer cell growth and promote apoptosis in culture. The drug selectivity is increased, but there are still challenges to clinical application. For instance, addition of an organic solvent short half-life.9,10 To this end, polymer nanomaterials are being developed with biocompatibility and release. As drug carriers, dextran microspheres have the advantage of reducing drug side-effects, reducing drug tolerance, but have poor stability.11,12 To solve these problems there is a need to modify the surface of the dextran role in many physiological processes methylation.
In this study, the natural polysaccharide polymer dextran served as the hydrophilic shell, while the lauryl alcohol was used as the hydrophobic nuclear layer, so as to synthesize the amphipathic molecule that could form the micell drug carrier in water solution through self-assembly. Subsequently, FA was introduced onto the dextran surface to improve the targeting ability of the IMD/Dex-LA nanoparticles. Moreover, the hydrophobic molecular inhibitor IMD-0354 was also utilized to bind with the micell material, and the complex was then employed for drug loading by virtue of the hydrophobicity in the micell core layer, thus improving the drug insolubility. Later, drug release was assessed in phosphate buffer solution (PBS) to explore the function of the micell carrier. Finally, the biocompatibility of the micell carrier was evaluated, and the influence of IMD-0354 drug on the melanoma cell survival rate was also assessed.
Materials and methods
Materials
Dex, LA, DMSO, BSA, MTT, and FA were purchased from Sigma (St. Louis, MO, USA). NF-κB and p65 were provided by Lifecore Biomedical (Chaska, MN, USA). Murine B16F10 and L929 cells were obtained from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). Balb/c male mice were purchased from Cyagen Biosciences (Guangzhou) Inc. All animals were performed in conformity to the Animal Welfare Act, and experiments with them were approved by the Institutional Animal Care and Treatment Committee of Central South University.
Preparation of drug microsphere without IMD-0354
Synthesis of FA-Dex
In brief, 0.4 g FA was dissolved into 4 mL mixed solvent, 2 g N,N’-二N,N’-Dicyclohexylcarbodiimide (DCC) was dissolved into 1 mL mixed solvent, 2 g Dex was dissolved into 3 mL mixed solvent, and 2 g dimethyl amine pyridine was dissolved into 10 mL mixed solvent; afterwards, the mixed solvents containing different drugs were mixed to react for 20 hours at room temperature in the dark. Then, the reaction product was filtered, the filtrate was collected in acetone, and the bright yellow precipitate was formed, filtered, and precipitated; then, the dry FA-raw Dex was collected and purified using the dextran gel column, and the first part of the eluent was collected, followed by freeze drying and testing of FA-Dex.
Synthesis of FA-Dex-LA
Briefly, 0.25 g FA-Dex was dissolved into 5 mL anhydrous DMSO. Then, the reaction catalysts DCC and DMAP were added at the molar ratio of LA:DCC:DMAP=1:3:0.3, under nitrogen protection at the constant temperature of 60°C, and the mixture was continuously stirred for 48 hours using a magnetic stirrer. After reaction, the product was transferred into the dialysis bag (MWCO=1 kDa) to remove DMSO at 24 hours following dialysis with deionized water. After dialysis for 24 hours, the product was placed into the separation funnel, and an equivalent volume of ethyl acetate was added for extraction and purification. After purification, the ethyl acetate was completely removed through 24 hours of dialysis with deionized water (MWCO=1 kDa); finally, the powder obtained by freeze-drying was FA-Dex-LA.
Preparation of microspheres loaded with IMD-0354 drug
FA-Dex-LA and IMD-0354 were dissolved into 1 mL DMSO at a mass ratio of 20:1 under room temperature, followed by 30 minutes of stirring using a magnetic stirrer. Afterwards, 1 mL deionized water was added to stir for 3 hours, so as to crosslink the drug in hydrophobic nuclei to form the drug-loaded microsphere solution. Following reaction, deionized water was added for 24 hours of dialysis to completely remove the residual drug and DMSO out of the hydrophobic nuclei. After 24 hours of dialysis, the large-size matters and microsphere clusters in the drug-loaded microsphere solution were filtered with the 0.45 μm sieve, and then the solution was preserved in a refrigerator at −80°C. The drug-loaded microsphere was obtained by freeze-drying, named IMD/FA-Dex-LA, and preserved in a refrigerator at −20°C.
Characterization of the nano drug carrier
The prepared FA-Dex-LA and IMD/FA-Dex-LA were analyzed by infrared (IR) spectroscopy, detection of microsphere diameters, as well as other characterization methods.
Biocompatibility
Lyophilized microspheres (0.01–1 mg/mL) were dissolved in the culture medium to co-culture for 24 hours. Each concentration was repeated for six times. After 24 hours of culture, the microsphere-containing culture medium was extracted and washed with 100 μL PBS twice. Afterwards, 20 μL MTT and 80 μL fresh culture medium were added into the carbon dioxide incubator to react for 4 hours in the dark; the resulting purple crystals were then dissolved and sufficiently mixed with 200 μL DMSO, and the wavelength was measured at 570 nm using ELISA.
Evaluation of the effects of IMD/FA-Dex-LA microspheres and pure IMD-0354 on inhibiting cell survival
Lyophilized IMD/FA-Dex-LA microspheres and pure IMD-0354 were dissolved into the culture medium (at the drug concentration of 0.1–10 μg/mL) for co-culture. Then, the drug-containing culture medium was extracted for MTT assay at 48 hours, respectively, and cell viability was calculated.
Detection of pinocytosis
The microspheres were labeled with fluorescence substance FITC (Ex/Em=492 nm/518 nm), so as to observe the pinocytosis of FA-Dex-LA by microspheres.
Western blotting
Cells were cultured with pure FA-Dex-LA microspheres as well as the culture medium containing IMD-0354 and IMD/FA-Dex-LA (at the drug concentration of 0.5 μg/mL) for 24 hours. Subsequently, the total cell protein was collected, and the expression of IKK, IκB, NF-κB, pIKK, and pIκB was detected.
Animal studies
In this experiment, the 4–5-week-old Balb/c male mice weighing 20–22 g were purchased from Cyagen Biosciences Inc. (Guangzhou, China). Before seeding in animals, B16F10 was washed with PBS and diluted to 105 cells/μL, then 10 μL of the resulting solution was aspirated using a 30 G insulin syringe and seeded in the back of each mouse. After tumor cell seeding, the tumor volume was measured, photographed, and recorded once every 3 days. For all tumor volumes and sizes in the experiment, the long axis (a), short axis (b), and thickness (c) were measured using an electronic light-value scale, and were calculated according to the following formula: tumor volume (mm3)=0.5×a (mm)×b (mm)×c (mm).
- Control Group: No treatment was received, and the volumes were measured, photographed and recorded once every 3 days.
- FA-Dex-LA Group: Treatment was initiated when the tumor grew to ~50 mm3 on Day 10;40 mg/kg FA-Dex-LA was injected via the caudal vein using a 29G syringe once every 7 days for three cycles.
- IMD0354 Group: Treatment was initiated when the tumor grew to ~50 mm3 on Day 10;40 mg/kg IMD0354 was injected via the caudal vein using a 29G syringe once every 7 days for three cycles.
- IMD0354/FA-Dex-LA Group: Treatment was initiated when the tumor grew to ~50 mm3 on Day 10;40 mg/kg Curcumin Micelles was injected via the caudal vein using a 29G syringe once every 7 days for three cycles.
All animal experiments were conducted under the appropriate approval of the Institutional Animal Care and Treatment Committee of Central South University.
Statistical analysis
All data were expressed as mean±standard deviation (SD). The means was compared between two samples using student’s t-test. A difference of p<0.05 was deemed statistically significant.
Results
Structural identification of the FA-Dex-LA microspheres
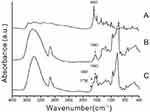
Structural characteristic peak signals of FA-Dextran are shown in Figure 1A, wherein the signals are detected at ν=1,697−1 cm. Figure 1B displays the characteristic peak signals of Lauryl alcohol. As pointed out by the references, the absorption characteristic peak was observed at ν=1,643 cm−1. It can be seen from Figure 1C that the C=O stretching vibration absorption in ester group of FA-Dex-LA was weak, at 1,736 cm−1 in the experiment, which could be ascribed to the low FA graft degree. Moreover, the FTIR spectra further confirmed the formation of the FA-Dex-LA conjugate.
 | Figure 1 FTIR diagram of FA-Dextran (A), Lauryl alcohol (B), and FA-Dex-LA (C). Abbreviations: Dex, dextran; FA, folate acid; FTIR, Fourier-transform infrared spectroscopy; LA, lauryl alcohol. |
Microsphere particle size analysis
The particle size detection results of the pure FA-Dex-LA microsphere and the FA-Dex-LA loaded with drugs are presented in Table 1. As could be observed, the average particle size of FA-Dex-LA was 154.35±8.76 nm, while that of the IMD/FA-Dex-LA was 212.13±10.62 nm.
 | Table 1 Average particle size of FA-Dex-LA and IMD/FA-Dex-LA |
Drug encapsulation rate and loading rate of the microsphere loaded with IMD-0354
Table 2 displays the encapsulation rate and drug loading rate of the drug loading IMD/FA-Dex-LA microsphere. The materials-to-medicines ratio was 20:1 (w:w), the encapsulation rate of FA-Dex-LA was 89.27±6.51%, and the drug loading rate was 4.25±0.42%.
 | Table 2 Encapsulation rate and drug loading rate of FA-Dex-LA microsphere |
Drug release of the IMD-0354 loaded microsphere
Figure 2 shows the drug release curve of the IMD0354/FA-Dex-LA microsphere. As could be observed, the accumulated drug release rate reached 52.7±9.4% within the first 12 hours, 71.8±10.3% within the first 48 hours, and 83.9±7.2% within the first 72 hours, respectively.
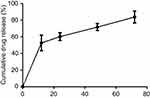 | Figure 2 Drug release curve of IMD/FA-Dex-LA microsphere. Abbreviations: Dex, dextran; FA, folate acid; LA, lauryl alcohol. |
Biocompatibility assessment of the FA-Dex-LA microsphere
Biocompatibility of the material was assessed according to the cytotoxicity test method for ISO10993-5 material. The test results are shown in Figure 3. As could be seen, the survival rates were 92.4, 95.5, 93.3, 95.2 and 83.3%, respectively, at the material concentrations from low to high (Figure 3A).
Evaluation of the effects of IMD0354/FA-Dex-LA microsphere and IMD-0354 drug alone on cancer cell survival rate
As shown in Figure 3B, no significant difference was observed in cell inhibition rate between the two groups at low concentrations. However, the difference in the cell inhibition rate was markedly increased between the two groups as the drug concentration increased (p<0.05). Moreover, the inhibition rate of the carrier group was remarkably higher than that of the single drug alone group.
Cellular pinocytosis of the FITC-FA-Dex-LA microsphere
Figure 4 shows the results of the FITC-FA-Dex-LA microsphere in cancer cells by means of cellular pinocytosis. It could be seen that the microsphere prepared in this study could be taken up by cells and accumulated in the cytoplasm.
Western blotting
In this experiment, Western Blotting was performed to examine the expression of transcription factors IKK, IκB, NF-κB, pIKK, and pIκB in B16F10 cancer cells. There was no significant difference in the expression of IKK, IκB, and NF-κB between the Control group and FA-Dex-LA group after cancer cells were co-cultured with drug for 24 hours, while the pIKK and pIκB activities in IMD-0345 and IMD/FA-Dex-LA groups were evidently dropped (Figure 5).
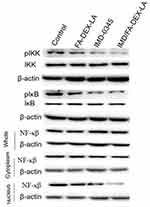 | Figure 5 Western blotting detected the expression of IKK,IκB,NF-κβ,pIKK and pIκB in B16F10 cancer cells.Abbreviations: Dex, Dextran; FA, Folate Acid; LA, Lauryl Alcohol. |
Anti-tumor effect
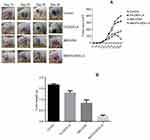
As shown in Figure 6A, tumors in the Control group without any treatment kept growing, and the tumor volume was about 390.6±43.1 mm3 on the 28th day. FA-Dex-LA was injected at a dose of 40 mg/kg once every 3 days a total of five-times. The tumors in the FA-Dex-LA group continued to grow, and the tumor volume was about 300.0±45.3 mm3. Meanwhile, tumors in the IMD0354 group were partially inhibited, with tumor volumes of about 174.2±15.8 mm3. Compared with other groups, tumors in the IMD0354/FA-Dex-LA group were obviously suppressed, with tumor volumes of about 53.2±10.3 mm3.
Moreover, the tumor tissue was removed and weighed on the 28th day, as presented in Figure 6B. It could be observed from the overall trend that the tumor volume size was consistent with that on the 28th day in Figure 6A.
Tumor tissue H&E staining after treatment
At the end of treatment in the Control group, relatively dense nuclei, relatively ordered cytoplasm, and good proliferation capacity could still be observed (Figure 7A). Figure 7B shows the results of FA-Dex-LA treatment. It could be found that tumor cells had relatively complete cytoplasm, although the peritoneal nuclei were slightly loose compared with those in the Control group. Figure 7C displays the results of IMD-0354 treatment. As could be found, the nuclear density was markedly decreased, and many cytoplasmic defects began to appear. Figure 7D reveals the results of IMD/FA-Dex-LA treatment. It could be discovered that the nuclear density was apparently decreased, along with the most serious cytoplasmic defect and numerous residual voids, and only the residual cytoskeleton and part of the cytoplasm wasleft.
Discussion
The dextran molecules have contained abundant active groups (-OH); therefore, chemical reactions, such as amide, can be employed to introduce other groups for modification. This paper was carried out based on this principle, and the active group on the dextran skeleton grafted hydrophobic group cholesterol was utilized to obtain the hydrophobic dextran derivatives. In addition, the final reaction product, dextran derivative, was tested using the KBr tablet and infrared spectrometer. The infrared spectra are displayed in Figure 1. It could be seen from the figure that the characteristic absorption peak of the functional group could be detected at 1,697 cm−1 (C=O). Clearly, the final product of dextran derivatives displayed the characteristic absorption peak of LA at 1,643 cm−1. In the meantime, the O-H absorption peak at 3,365 cm−1 was the C-O-C Dextran characteristic absorption peak; meanwhile, the lauryl alcohol-CH characteristic absorption peak in the hydrophobic chain section of the structure could be detected at ν=2,904 cm−1. All these features could also be detected in FA-Dex-LA, indicating that the FA-Dex-LA had been successfully prepared. According to the detection results of the microsphere particle size, the average particle size was increased after drug encapsulating, which might be because the stereoscopic obstacles would be formed in the microspheres when the hydrophobic drug molecules were embedded in the microsphere core layer.13 Hence, the average particle size would be increased. When the drug release test was conducted in vitro, the water molecules could firstenter the microsphere, so the drugs could be released due to the internal and external concentration difference. Subsequently, the microsphere structure was loosened and broken, as a result of the hydrolysis-induced breaking of the ester linkage bonding on the FA-Dex-LA molecule chain section. Hence, the drugs were released through the core layer of microsphere. Du et al had assessed the release rate of the dextran grafted stearate loaded drugs, and discovered that nearly 50% of the drug amount could be accumulated after 10 h, which was consistent with our results, but the drug release mechanism in their study was hydrolytic degradation.14 In addition, it is also suggested in research that, when the amphoteric molecules in aqueous solution (microcell) can be formed by the self-assembly of water texture features of materials, they will be able to spread into the water molecule micell environment, and connect onto the glucan ester chain key and biodegradable materials through hydrolysis.15,16
In biocompatibility experiments, no statistically significant difference was observed compared with the control group; so the result showed that FA-Dex-LA displayed no cytotoxicity to fibroblast L929, which was a good biocompatibility carrier that could be utilized for subsequent experiments to confirm the feasibility. It could be found in cell proliferation experiments that the inhibition rates in the two groups ofcells showed no statistically significant difference at a drug concentration of <0.25 µg/mL. However, at a drug concentration of >0.25 µg/mL, the inhibition rate in the carrier group on cancer cells was evidently higher than that in the single drug group, which might be due to the different mechanisms of drugs entering into cells. According to a literature report, cell membranes are selectively permeable, which can diffuse material or drug with the molecular weight of <1 kDa into cells.17 Therefore, the simple IMD-0354 drug could enter cells by means of diffusion. Conversely, the IMD/FA-Dex-LA microsphere would enter cells through phagocytosis, which would cause drug accumulation in cells, thus achieving the drug inhibiting effect. To sum up, it is confirmed in this experiment that drug effect was not affected by the loading of microspheres, and an identical drug effect could be achieved at a lower dose. Noteworthily, the FA-Dex-LA microsphere surface prepared in this study had possessed an electrically neutral hydrophilic shell Dextran, so it could relatively reduce the barrier of cell pinocytosis resulting from the material surface charge; besides, it had excellent solubility in aqueous solution. Results of cellular pinocytosis also demonstrated the feasibility of cancer cell phagocytic material. Moreover, Western blotting suggested that both IMD-0345 and IMD/FA-Dex-LA could evidently inhibit the IKK pathway, while the expression of NF-κB and cytoplasmic NF-κB in cells was not changed, and NF-κB expression in nuclei was markedly down-regulated, indicating that the NF-κB nuclei were reduced after blocking the IKK pathway, leading to the inhibition of melanoma cell division and proliferation.
Further, we could more clearly verify whether the vector could inhibit tumor growth after being loaded with IMD0354 through subcutaneous tumor formation in nude mice. On the 28th day after tumor implantation, the tumor volume in nude mice treated with IMD/FA-Dex-LA was the lowest. Apparently, the tumor growth rate in this group was lower than those in other groups. Also, the tumor weight in this group was lower than those in other groups. In addition, it could be observed in HE staining experiment that more severe tumor tissue damage could be detected after IMD/FA-Dex-LA treatment. Hence, it can be concluded that IMD/FA-Dex-LA can powerfully inhibit the growth of the subcutaneously implanted tumors from melanoma B16F10 cells.
Conclusions
Results in this study show that the IMD/FA-Dex-LA nanoparticles can reduce the toxicity of IMD0354 in the meantime of obviously improving the antitumor effect. Therefore, FA-Dex-LA can serve as a safe and effective carrier with great potential for application.
Acknowledgments
The present study was supported by the Special Foundation of Basic Science and Technology Resources Survey of Ministry of Science and Technology of China (no.2017FY101204), the National Natural Science Foundation of China (no.81572689,81872219), the Natural Science Foundation of Hunan Province (no.2014JJ7020), the Project of Science and Technology of Hunan Province (no.2016SK2087), the Project of Health and Family Planning Commission of Hunan Province (no.2016-126,2015-40), and the Project of Science and Technology of Guizhou Province (no.2016-7189).
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ocanha-Xavier JP, X JCC, M MEA. Melanoma: clinical, evolutive and histopathological characteristics of a series of 136 cases. An Bras Dermatol. 2018;93:373–376. doi:10.1590/abd1806-4841.20186690
2. Gadducci A, Carinelli S, Guerrieri ME, Aletti GD. Melanoma of the lower genital tract: prognostic factors and treatment modalities. Gynecol Oncol. 2018;150:180–189. doi:10.1016/j.ygyno.2018.04.562
3. Liu C, Xie HQ, Yu JG, et al. A targeted therapy for melanoma by graphene oxide composite with microRNA carrier. Drug Des Devel Ther. 2018;12:3095–3106. doi:10.2147/DDDT.S160088
4. Nacchiero E, Vestita M, Robusto F, Maruccia M, Annoscia P, Giudice G. Surgical management of tumor-positive interval node in melanoma patients: an observational study. Medicine (Baltimore). 2018;97:e0584. doi:10.1097/MD.0000000000010584
5. Hepner A, Salgues A, Cad A, et al. Treatment of advanced melanoma-A changing landscape. Rev Associacao Med Bras. 2017;63:814–823. doi:10.1590/1806-9282.63.09.814
6. Atkinson V. Recent advances in malignant melanoma. Intern Med J. 2017;47:1114–1121. doi:10.1111/imj.13574
7. Xie B, Cao K, Li JJ, et al. Hmgb1 inhibits Klotho expression and malignant phenotype in melanoma cells by activating NF-κB. Oncotarget. 2016;49:80765–80782.
8. Tiwari V, Kamran MZ, Ranjan A, Nimesh H, Singh M, Tandon V. Akt1/NFκB signaling pathway activation by a small molecule DMA confers radioprotection to intestinal epithelium in xenograft model. Free Radic Biol Med. 2017;108:564–574. doi:10.1016/j.freeradbiomed.2017.04.029
9. Chen YM, Chiang WC, Yang Y, Lai CF, Wu KD, Lin SL. Pentoxifylline attenuates proteinuria in anti-Thy1 glomerulonephritis via downregulation of nuclear factor-κB and Smad2/3 signaling. Mol Med. 2015;21:276–284. doi:10.2119/molmed.2015.00023
10. Liu C, Fonken LK, Wang A, et al. Central IKKβ inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part Fibre Toxicol. 2014;11:53. doi:10.1186/s12989-014-0053-5
11. Pradhan M, Alexander A, Singh MR, Singh D, Saraf S, Saraf S. Ajazuddin, understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed Pharmacother. 2018;107:447–463. doi:10.1016/j.biopha.2018.07.156
12. Ghassami E, Varshosaz J, Taymouri S. Redox sensitive polysaccharide based nanoparticles for improved cancer treatment: a comprehensive review. Curr Pharm Des. 2018. doi:10.2174/1381612824666180813114841
13. Pal S, Saha C. Solvent effect in the synthesis of hydrophobic drug-loaded polymer nanoparticles. IET Nanobiotechnol. 2017;11:443–447. doi:10.1049/iet-nbt.2016.0116
14. Du YZ, Weng Q, Yuan H, Hu FQ. Synthesis and antitumor activity of stearate-g-dextran micelles for intracellular doxorubicin delivery. ACS Nano. 2010;4:6894–6902. doi:10.1021/nn100927t
15. Prasad M, Lambe UP, Brar B, et al. Nanotherapeutics: an insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother. 2018;97:1521–1537. doi:10.1016/j.biopha.2017.11.026
16. Jeong YI, Kim DH, Chung CW, et al. Doxorubicin-incorporated polymeric micelles composed of dextran-b-poly(DL-lactide-co-glycolide) copolymer. Int J Nanomedicine. 2011;6:1415–1427. doi:10.2147/IJN.S19491
17. Tian C, Asghar S, Xu Y, et al. Tween80-modified hyaluronic acid-ss-curcumin micelles for targeting glioma: synthesis, characterization and their in vitro evaluation. Int J Biol Macromol. 2018. doi:10.1016/j.ijbiomac.2018.09.034
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.