Back to Journals » International Journal of Women's Health » Volume 14
Struma Ovarii Associated with Ascites and Elevated CA125: Two Case Reports and Review of the Literature
Authors Wang S , He X, Yang H , Chen L
Received 27 June 2022
Accepted for publication 25 August 2022
Published 7 September 2022 Volume 2022:14 Pages 1291—1296
DOI https://doi.org/10.2147/IJWH.S379128
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Shaoyu Wang, Xinqin He, Huijuan Yang, Lihong Chen
Department of Obstetrics and Gynecology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, 350005, People’s Republic of China
Correspondence: Lihong Chen, Department of Obstetrics and Gynecology, The First Affiliated Hospital of Fujian Medical University, 20 Chazhong Road, Fuzhou, 350005, People’s Republic of China, Tel +86 591-87982062, Email [email protected]
Abstract: Struma ovarii is a rare variety of specialized monodermal mature ovarian teratoma, it is composed predominantly of thyroid tissue. Ascites is present in one third of patients. The combination of struma ovarii, marked ascites and elevated CA125 is a rare condition, which may mimic ovarian cancer. We described two cases presenting with pelvic mass, ascites and elevated serum CA125 levels, frozen section and final pathology turned out to be struma ovarii. Ascites disappeared and the level of CA125 returned to normal level after operation. One of the cases was associated with pleural effusion, leading to a condition called pseudo-Meigs’ syndrome. Then we reviewed the related literatures to explore the possible mechanism of ascites and pleural effusion, the reason of CA125 elevation and imaging manifestations of struma ovarii. In conclusion, struma ovarii should be considered in the differential diagnosis preoperatively, when presented with pelvic mass, ascites and an elevated CA125 level.
Keywords: struma ovarii, ascites, CA125, case report, pseudo-Meigs’ syndrome
Introduction
Struma ovarii is a rare variety of specialized monodermal mature ovarian teratoma, accounting for nearly 2.7% of teratomas and 1% of all ovarian tumors.1 It is predominantly or entirely composed of thyroid tissue, which means more than half of the components are follicles, varying in size and filled with eosinophilic colloid and cuboidal epithelial cells.2 It is usually benign in nature, but approximately 5% of cases undergo malignant transformation into thyroid-type carcinoma.3,4 The combination of struma ovarii, marked ascites and elevated CA125 is a rare condition, since the patient’s clinical manifestations and imaging examinations are unrepresentative, it is relatively difficult to diagnose before surgery, even misdiagnosed as ovarian cancer. Meigs’ syndrome refers to a benign and solid ovarian tumor (eg. fibroma, thecoma, or granulosa cell tumor) present with ascites and pleural effusion, symptoms disappear after the resection of tumor.5 When similar clinical manifestations presented in other benign pelvic tumors are termed as pseudo-Meigs’ syndrome.6 Here, we reported two cases of struma ovarii with gross ascites and elevated CA125 level, and reviewed the related literature as well.
Case Presentation
Case 1 A 45-year-old woman was admitted to our institution in March 2016, complaining of abdominal distention for six months. An abdominal magnetic resonance imaging (MRI) revealed a 4.1×5.0×6.1cm left adnexal mass with gross ascites. A chest computed tomography (CT) scan showed a small amount of right hydrothorax. The serum CA125 level was 711.5U/mL. Thyroid function tests preoperatively were within normal limits.
An exploratory laparotomy was performed. 2 liters of straw-colored ascites was evacuated upon entrance to the peritoneal cavity. The uterus and right adnexa were normal. The left-sided adnexal cystic-solid mass measured 6×5×5cm. There was no evidence of intraperitoneal metastasis or retroperitoneal adenopathy. The left adnexa was removed and frozen section suggested a struma ovarii. Then, hysterectomy and right salpingo-oophorectomy were performed due to the strongly insistence of the family members. The final pathology confirmed the diagnosis of struma ovarii with benign thyroid tissue confined in the left ovary (Figure 1). IHC: CK7(+), CK19(+), CK20(-), TTF1(+), Ki67(1%+), P53(-), TG(+), Syn(-), CgA(-), ER(-), vimentin(+). The uterus, right ovary and fallopian tubes were histologically unremarkable. The cytology of ascitic fluid was negative for malignant cells.
 |
Figure 1 Microscopic appearance of the left ovary showing thyroid follicles (H&E). |
One month after surgery, the patient had no evidence of ascites and pleural effusion, also the serum levels of CA125 had fallen to normal.
Case 2 A 66-year-old woman was admitted to our institution in August 2021 with complaints of abdominal distention for the preceding three months. The patient had a history of right ovarian surgery 20 years ago. Physical examination revealed a markedly distended abdomen and shifting dullness. An abdominal CT revealed a 10.3×12.7×13.8cm pelvis mass with large amount of ascites (Figure 2). There was no indication of hydrothorax on chest CT. The serum CA125 level was 463.78U/mL, whereas the remaining tumor markers were within normal limits. Thyroid function tests were unremarkable.
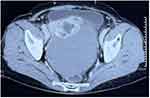 |
Figure 2 Computed tomography scan of the abdomen revealed a 10.3×12.7×13.8cm complex cystic and solid pelvic mass. |
An exploratory laparotomy was performed. 3 liters of straw-colored ascites was evacuated. The left-sided adnexal mass measured 20×13×10cm, with a smooth external surface. The uterus was atrophic and the right adnexa was absent. Neither abdominal metastasis nor retroperitoneal enlarged lymph node was found. A left salpingo-oophorectomy was performed. Frozen section revealed a struma ovarii. The final pathology confirmed the diagnosis of struma ovarii with benign thyroid tissue (Figure 3). The ascitic fluid contained no malignant cells.
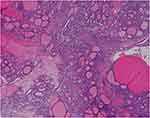 |
Figure 3 Microscopic appearance of the left ovary showing various thyroid follicles (H&E). |
Three months after surgery, she had no evidence of ascites and the serum levels of CA125 had fallen to normal.
Discussion
When facing a patient presenting with a pelvic mass, large amount of ascites and elevated serum CA125, the first thing comes to a surgeon’s mind may be malignant tumor, especially epithelial ovarian cancer. However, our cases which were suspected to be malignant tumor preoperatively turned out to be struma ovarii. As a rare ovarian germ cell tumor, struma ovarii mainly occurs in the fifth decade or older, but it has also been reported to occur during the reproductive stage.7 Despite the predominance of thyroid tissue, hyperthyroidism is present only in 5% of struma ovarii.2 The mechanism leading to hyperthyroidism might be the antibodies produced by the struma ovarii for the TSH receptor.8 Thyroid function tests are usually within normal limits postoperatively.
One-third of patients with struma ovarii have coexistent ascites.2 As described in case1, the condition that struma ovarii associated with ascites and pleural effusion simultaneously is called pseudo-Meigs’ syndrome, which was first described by Meigs in 1954.6 We made a literature search in PubMed on struma ovarii associated with ascites and elevated serum CA125. The details of those cases were showed in Table 1.1,4,7–22
 |
Table 1 Overview of Reported Cases of Struma Ovarii Associated with Ascites and Elevated CA125 |
The ascites and pleural effusion are usually serous. Unlike malignant process, hypoproteinemia is exceedingly rare. Compared with histologic type, tumor size is a more important factor in the formation of ascites.21 The etiology of ascites and pleural effusion is still uncertain, several theories have been put forward. Most believed that ascites formation resulted from peritoneal irritation and pelvic lymphatics obstruction caused by the solid pelvic tumor.6 Meanwhile, the increasing peritoneal pressure from the ascites lead to a process of peritoneal inflammation, the release of toxins and inflammatory factors can aggravate the situation.23,24 Some scholars suggested that the discrepancy between the blood vessels that supplying tumor and lymphatic drainage leads to stromal edema and transudation.25
Struma ovarii is associated with pleural effusion and ascites in only 5% of cases.16 The pleural effusion often appears in the right thoracic cavity, but can also occur in the left side or both sides.5 There is a hypothesis that hydrothorax originated from the transdiaphragmatic transport of the ascitic fluid. Once attained adequate volume and pressure, the ascites find its way through the diaphragm through intercellular gaps.23 There was no pleural effusion in case 2. The condition that struma ovarii associated with ascites but without pleural effusion is probably due to the early diagnosis and timely treatment.
In the reviewed literatures, the range for CA125 is enormous, from 120 to 5218U/mL. The exact reason for the elevated CA125 in struma ovarii also remains unclear. Strong immunoreactivity for CA125 was observed in the omentum, whereas the tumor was negative.26 It shows that the expression of CA125 originates from mesothelial cells rather than the tumor.27 The mechanical irritation and inflammatory response from the tumor and ascites increase the expression of CA125 in adjacent mesothelial cells. The serum level of CA125 and the amount of ascites influenced each other, but there was no parallel relationship.
Although struma ovarii with ascites and elevated CA125 may actually mimic an ovarian cancer, there are still some different characteristics on the imaging findings. Struma ovarii usually appears as a unilateral adnexal multilocular lobulated cystic-solid lesion, with thickened septa in the solid components. On ultrasound, because thyroid tissue is richer in vascularization, struma ovarii displays the characteris of hypervascularity than other mature ovarian teratoma.17 Sometimes one or more struma pearls can be observed on Doppler examination, which refers to well circumscribed, roundish solid components with smooth contours.28 Another sonographic appearance of struma ovarii include acoustic shadowing and signs of dermoid cysts.29 On MRI, the solid component shows low intensity on T2-weighted images and punctuate foci shows high intensity on T1-weighted images. On CT, calcifications along the cyst walls or septa may be the key feature.30
Struma ovarii is usually a benign tumor, classical pattern of variable-sized follicles filled with colloid are easily visible microscopically. Malignant transformation has already been reported, but first we should exclude the primary thyroid carcinoma metastasis by thyroid imaging examination. Malignant struma ovarii usually presents as a well-differentiated thyroid cancer, with “ground glass” nuclei, increased mitotic activity and/or vascular invasion.4 Papillary thyroid carcinoma (PTC) is the predominant malignant type. Oncogene mutations including KRAS, NRAS, BRAF and JAK3 have been reported.31,32 It would be beneficial to investigate the mutations of ovarian germ cell tumors in guiding postoperative treatment.
Conclusion
As demonstrated by our case reports, pseudo-Meigs’ syndrome should be considered preoperatively in the differential diagnosis of patients presenting with a pelvic mass, ascites, pleural effusion and elevated CA125. Struma ovarii is usually a benign neoplasm, patients have a preferable prognosis with the removal of tumor.
Ethics Approval
The study was approved by the Ethics Committee of First Affiliated Hospital of Fujian Medical University. Written informed consent were obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Acknowledgments
We thank Dr. Xuan Tao at pathology department of our hospital for her kindly analysis of the patients’ tissue samples.
Funding
No funding was received.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Jiang W, Lu X, Zhu ZL, Liu XS, Xu CJ. Struma ovarii associated with pseudo-Meigs’ syndrome and elevated serum CA 125: a case report and review of the literature. J Ovarian Res. 2010;3:18. doi:10.1186/1757-2215-3-18
2. Roth LM, Talerman A. The enigma of struma ovarii. Pathology. 2007;39(1):139–146. doi:10.1080/00313020601123979
3. Lara C, Cuenca D, Salame L, Padilla-Longoria R, Mercado M. A hormonally active malignant struma ovarii. Case Rep Oncol Med. 2016;2016:2643470. doi:10.1155/2016/2643470
4. Zannoni GF, Gallotta V, Legge F, Tarquini E, Scambia G, Ferrandina G. Pseudo-Meigs’ syndrome associated with malignant struma ovarii: a case report. Gynecol Oncol. 2004;94(1):226–228. doi:10.1016/j.ygyno.2004.03.045
5. Meigs JV. Fibroma of the ovary with ascites and hydrothorax—Meigs’ syndrome. Am J Obstet Gynecol. 1954;67(5):962–987. doi:10.1016/0002-9378(54)90258-6
6. Meigs JV. Pelvic tumors other than fibromas of the ovary with ascites and hydrothorax. Obstet Gynecol. 1954;3(5):471–486.
7. Mancuso A, Triolo O, Leonardi I, De Vivo A. Struma ovarii: a rare benign pathology which may erroneously suggest malignancy. Acta Obstet Gynecol Scand. 2001;80(11):1075–1076. doi:10.1034/j.1600-0412.2001.801121.x
8. Anastasilakis AD, Ruggeri RM, Polyzos SA, et al. Coexistence of Graves’ disease, papillary thyroid carcinoma and unilateral benign struma ovarii: case report and review of the literature. Metabolism. 2013;62(10):1350–1356. doi:10.1016/j.metabol.2013.05.013
9. Bethune M, Quinn M, Rome R. Struma ovarii presenting as acute pseudo-Meigs syndrome with an elevated CA 125 level. Aust N Z J Obstet Gynaecol. 1996;36(3):372–373. doi:10.1111/j.1479-828X.1996.tb02734.x
10. Huh JJ, Montz FJ, Bristow RE. Struma ovarii associated with pseudo-Meigs’ syndrome and elevated serum CA 125. Gynecol Oncol. 2002;86(2):231–234. doi:10.1006/gyno.2002.6741
11. Loizzi V, Cormio G, Resta L, Fattizzi N, Vicino M, Selvaggi L. Pseudo-Meigs syndrome and elevated CA125 associated with struma ovarii. Gynecol Oncol. 2005;97(1):282–284. doi:10.1016/j.ygyno.2004.12.040
12. Obeidat BR, Amarin ZO. Struma ovarii with pseudo-Meigs’ syndrome and elevated CA125 levels. J Obstet Gynaecol. 2007;27(1):97–98. doi:10.1080/01443610601076267
13. Mitrou S, Manek S, Kehoe S. Cystic struma ovarii presenting as pseudo-Meigs’ syndrome with elevated CA125 levels. A case report and review of the literature. Int J Gynecol Cancer. 2008;18(2):372–375. doi:10.1111/j.1525-1438.2007.00998.x
14. Paladini D, Vassallo M, Sglavo G, Nappi C. Struma ovarii associated with hyperthyroidism, elevated CA 125 and pseudo-Meigs syndrome may mimic advanced ovarian cancer. Ultrasound Obstet Gynecol. 2008;32(2):237–238. doi:10.1002/uog.5399
15. Mui MP, Tam KF, Tam FK, Ngan HY. Coexistence of struma ovarii with marked ascites and elevated CA-125 levels: case report and literature review. Arch Gynecol Obstet. 2009;279(5):753–757. doi:10.1007/s00404-008-0794-1
16. Rana V, Srinivas V, Bandyopadhyay S, Ghosh SK, Singh Y. Bilateral benign non functional struma ovarii with Pseudo-Meigs’ syndrome. Indian J Pathol Microbiol. 2009;52(1):94–96. doi:10.4103/0377-4929.44978
17. Peyron N, Coulon A. Struma ovarii, pseudo-Meigs’ syndrome and raised CA125, a rare association. Answer to May e-quid. Diagn Interv Imaging. 2012;93(7–8):643–647. doi:10.1016/j.diii.2012.03.012
18. Sivrioglu AK, Saglam M, Sonmez G, Deveer M. Pseudo-Meigs’ syndrome associated with struma ovarii. BMJ Case Rep. 2013;2013(apr23 1):bcr2013009189–bcr2013009189. doi:10.1136/bcr-2013-009189
19. Jin C, Dong R, Bu H, Yuan M, Zhang Y, Kong B. Coexistence of benign struma ovarii, pseudo-Meigs’ syndrome and elevated serum CA 125: case report and review of the literature. Oncol Lett. 2015;9(4):1739–1742. doi:10.3892/ol.2015.2927
20. Yadav S, Tomar R, Verma N, Khurana N, Triathi R. Struma ovarii with Pseudo-Meigs’ syndrome and raised cancer antigen-125 levels masquerading as an ovarian carcinoma case report and literature review. Sultan Qaboos Univ Med J. 2017;17(2):e229–e233. doi:10.18295/squmj.2016.17.02.017
21. Mostaghel N, Enzevaei A, Zare K, Fallahian M. Struma ovarii associated with Pseudo-Meig’s syndrome and high serum level of CA 125; a case report. J Ovarian Res. 2012;5:10. doi:10.1186/1757-2215-5-10
22. Guida M, Mandato VD, Di Spiezio Sardo A, Di Carlo C, Giordano E, Nappi C. Coexistence of Graves’ disease and benign struma ovarii in a patient with marked ascites and elevated CA-125 levels. J Endocrinol Invest. 2005;28(9):827–830. doi:10.1007/BF03347575
23. Amant F, Gabriel C, Timmerman D, Vergote I. Pseudo-Meigs’ syndrome caused by a hydropic degenerating uterine leiomyoma with elevated CA 125. Gynecol Oncol. 2001;83(1):153–157. doi:10.1006/gyno.2001.6251
24. Jimerson SD. Pseudo-Meigs’s syndrome. An unusual case with analysis of the effusions. Obstet Gynecol. 1973;42(4):535–537. doi:10.1097/00006250-197310000-00008
25. Samanth KK, Black WC. Benign ovarian stromal tumors associated with free peritoneal fluid. Am J Obstet Gynecol. 1970;107(4):538–545. doi:10.1016/s0002-9378(16)33939-4
26. Buttin B, Cohn D, Herzog T. Meigs’ syndrome with an elevated CA 125 from benign Brenner tumors. Obstet Gynecol. 2001;98:980–982. doi:10.1016/s0029-7844(01)01562-9
27. Lin JY, Angel C, Sickel JZ. Meigs syndrome with elevated serum CA 125. Obstet Gynecol. 1992;80(3 Pt 2):563–566.
28. Savelli L, Testa AC, Timmerman D, Paladini D, Ljungberg O, Valentin L. Imaging of gynecological disease (4): clinical and ultrasound characteristics of struma ovarii. Ultrasound Obstet Gynecol. 2008;32(2):210–219. doi:10.1002/uog.5396
29. Weinberger V, Kadlecova J, Minář L, et al. Struma ovarii - ultrasound features of a rare tumor mimicking ovarian cancer. Med Ultrason. 2018;20(3):355–361. doi:10.11152/mu-1526
30. Ikeuchi T, Koyama T, Tamai K, et al. CT and MR features of struma ovarii. Abdom Imaging. 2012;37(5):904–910. doi:10.1007/s00261-011-9817-7
31. Poli R, Scatolini M, Grosso E, et al. Malignant struma ovarii: next-generation sequencing of six cases revealed Nras, Braf, and Jak3 mutations. Endocrine. 2021;71(1):216–224. doi:10.1007/s12020-020-02438-7
32. Stanojevic B, Dzodic R, Saenko V, et al. Unilateral follicular variant of papillary thyroid carcinoma with unique KRAS mutation in struma ovarii in bilateral ovarian teratoma: a rare case report. BMC Cancer. 2012;12:224. doi:10.1186/1471-2407-12-224
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
