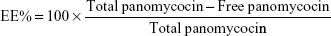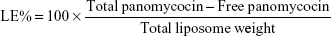Back to Journals » International Journal of Nanomedicine » Volume 12
Stratum corneum lipid liposome-encapsulated panomycocin: preparation, characterization, and the determination of antimycotic efficacy against Candida spp. isolated from patients with vulvovaginitis in an in vitro human vaginal epithelium tissue model
Authors İzgü F, Bayram G, Tosun K, İzgü D
Received 16 May 2017
Accepted for publication 24 June 2017
Published 3 August 2017 Volume 2017:12 Pages 5601—5611
DOI https://doi.org/10.2147/IJN.S141949
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Fatih İzgü,1 Günce Bayram,2 Kübra Tosun,2 Demet İzgü3
1Department of Molecular Biology and Genetics, Middle East Technical University, Ankara, Turkey; 2Department of Biotechnology, Graduate School of Natural and Applied Sciences, Middle East Technical University, Ankara, Turkey; 3Biology Department, TED Ankara College, Ankara, Turkey
Abstract: In this study, a liposomal lyophilized powder formulation of panomycocin was developed for therapeutic purposes against vulvovaginal candidiasis which affects 80% of women worldwide. Panomycocin is a potent antimycotic protein secreted by the yeast Wickerhamomyces anomalus NCYC 434. This study involved the preparation of panomycocin-loaded stratum corneum lipid liposomes (SCLLs), characterization of the SCLLs, and determination of antimycotic efficacy of the formulation against Candida albicans and Candida glabrata clinical vaginal isolates in a human vaginal epithelium tissue model. The encapsulation and loading efficiencies of SCLLs were 73% and 76.8%, respectively. In transmission electron microscopy images, the SCLLs appeared in the submicron size range. Dynamic light scattering analyses showed that the SCLLs had uniform size distribution. Zeta potential measurements revealed stable and positively charged SCLLs. In Fourier transform infrared spectroscopy analyses, no irreversible interactions between the encapsulated panomycocin and the SCLLs were detected. The SCLLs retained >98% of encapsulated panomycocin in aqueous solution up to 12 hours. The formulation was fungicidal at the same minimum fungicidal concentration values for non-formulated pure panomycocin when tested on an in vitro model of vaginal candidiasis. This is the first study in which SCLLs and a protein as an active ingredient have been utilized together in a formulation. The results obtained in this study led us to conduct further preclinical trials of this formulation for the development of an effective topical anti-candidal drug with improved safety.
Keywords: antifungal protein, panomycocin, exo-β-1,3-glucanase, stratum corneum lipid liposome, vulvovaginitis, Candida spp.
Introduction
Vulvovaginal candidiasis is a yeast infection which affects 80% of women of childbearing ages worldwide at least once in their lifetime. Candida albicans strains are the major pathogens isolated from the vagina of patients with vulvovaginal candidiasis.1 Almost all of the remaining strains (10% of the total) belong to Candida glabrata species while Candida tropicalis, Candida parapsilosis, and Candida krusei strains are also associated with this infection very rarely.2 Candida spp. multiply by adhering to the stratum corneum layer of the epithelium in the infected vaginal tissue.2,3 In 40%–50% of the cases, recurrence is seen with 8%–10% risk of becoming chronic which may then develop into life-threatening invasive Candidiasis infection.4 In the current treatment of vulvovaginal candidiasis, antimycotic agents such as azoles, polyenes, allylamines, echinocandins, and antimetabolites are commonly used.5 Resistance of pathogens to antimycotic drugs, mild to serious adverse effects such as gastrointestinal disturbances and hepatotoxicity caused by these drugs, and drug interactions in immunosuppressed patients are the known major complications associated with the current therapies.5 Also, some active ingredients that can be used in treatments (such as amphotericin B and Ciclopirox olamine)6,7 are optimally effective at neutral pH values in laboratory conditions.8 Healthy women have an acidic vaginal pH in the range of 3.7–5.7 worldwide9 which remains unchanged in vulvovaginal candidiasis.10,11 Thus, to achieve high efficacy in acidic environments, large doses of previously mentioned active ingredients are required in clinical conditions. For the previously mentioned reasons, there is a continuing need for new classes of antimycotic agents that have little or no toxicity toward mammalian cells and a low tendency to elicit resistance.5 Among the different approaches, naturally produced antifungal/antimycotic proteins are attracting increasing attention. In various studies, panomycocin, which is produced and secreted into the environment by the killer (K+) yeast strain Wickerhamomyces anomalus (formerly known as Pichia anomala) NCYC 434, has been shown as a promising potential antifungal agent in biomedicine.12–14 Panomycocin is a 49 kDa monomeric glycoprotein with an exo-β-1,3-glucanase activity. It hydrolyzes the exo-β-1,3-glucans which are vital polymers for the integrity of the fungal cell wall, leading to the disruption of the cell wall and death of the target cells.13 The mammalian cells lack the β-1,3-glucans in their structure, and this highlights the use of panomycocin as a selective antifungal/antimycotic agent in therapy. In several studies, the potent in vitro antifungal activity of panomycocin against human dermatophytes and Candida spp. was shown.15,16 In solution, panomycocin is stable and active at the pH range between 3.0 and 5.5 up to 37°C.12,13 In lyophilized form, it retains its stability and activity up to 38.5°C (unpublished data) and in the vaginal pH range.
The most common formulation type for the topical delivery of proteins for therapeutic purposes is their encapsulation in carrier systems which will allow the retention of protein integrity and activity. Being biocompatible, biodegradable, and nontoxic, liposomes are among the most preferable protein carrier systems in therapeutic applications.17,18
Liposomes with lipid composition similar to that of lipid matrix of the stratum corneum layer of the epithelium are referred to as stratum corneum lipid liposomes (SCLLs) and are virtually devoid of phospholipids commonly used in conventional liposomes.19 SCLLs adhere to the surface of the stratum corneum layer of the epithelium and integrate into the stratum corneum structure, releasing the encapsulated active ingredient.20,21 In literature, there are studies with SCLLs carrying non-proteinaceous substances.21,22 To the best of our knowledge, this is the first report describing the encapsulation of a protein in SCLLs in the biologically active form.
Efficacy evaluations of the topical formulations by alternative (non-animal) methods such as the use of 3-D tissue constructs which exhibit in vivo-like morphological and ultrastructural characteristics have significantly contributed to the minimization of animal utilization for ethical23 and cost reduction purposes. In vitro reconstructed human vaginal epithelium (HVE) tissue models have been recently utilized and shown to be valuable tools for assessing the efficacy of topically applied gynecological compounds and products.24
In the present study, a topical liposomal lyophilized powder formulation of panomycocin was developed for therapeutic purposes against vulvovaginal candidiasis. This study involved the preparation of SCLLs and the encapsulation of panomycocin, characterizations of the formulation, and the biological activity tests on HVE tissue model infected with C. albicans and C. glabrata clinical vaginal isolates.
Materials and methods
Strains and growth media
W. anomalus from the National Collection of Yeast Cultures, UK (NCYC 434, K+) was used as the source of panomycocin and maintained on YEPD agar (yeast extract [Sigma-Aldrich Co., St Louis, MO, USA] 1% [w/v], peptone [Sigma-Aldrich Co.] 2% [w/v], dextrose [Sigma-Aldrich Co.] 2% [w/v], and agar [EMD Millipore, Billerica, MA, USA] 2% [w/v], pH 5.5). Candida strains (four in total; three C. albicans and one C. glabrata vaginal isolates) were from German Hospital, İstanbul, Turkey and maintained on Sabouraud dextrose agar (SDA) (peptone [Sigma-Aldrich Co.] 2% [w/v], dextrose [Sigma-Aldrich Co.] 2% [w/v], and agar [EMD Millipore] 2% [w/v], pH 5.6). The identity of all isolates was confirmed by MALDI-TOF mass spectroscopic species identification technique (MALDI Biotyper, Bruker Corporation, Billerica, MA, USA) according to the manufacturer’s instructions.
Production and purification of panomycocin
Panomycocin-producing strain W. anomalus NCYC 434 was cultured in 5 L of YEPD at 20°C until the stationary phase in a 7 L bioreactor (BioBundle; Applikon, Delft, the Netherlands) at an impeller speed of 450 rpm. The pH of the culture medium was maintained at 4.5 by automatic addition of 2 M KOH (ADI 1030 Bio-Controller; Applikon). The culture chamber was fed with sterilized air by a mass-flow control unit with a flow rate of 2 L/min to ensure dissolved oxygen concentration above 30% measured by Applikon oxygen probe. The culture medium was clarified by centrifugation (5,000 rpm for 10 min at 4°C) and filtered through 0.45 and 0.2 μm cellulose acetate membranes (Sartorius AG, Göttingen, Germany). Panomycocin in the cell-free medium was concentrated by ultrafiltration using 30 and 5 kDa cut-off ultrafilters (Vivaflow200; Sartorius AG).
The crude panomycocin was purified and analyzed as described previously.12 The biological activity of the pure panomycocin was tested on vaginal C. albicans and C. glabrata isolates using the microdilution broth method according to the Clinical and Laboratory Standards Institute reference document M27-A325 with modifications (assay medium pH was adjusted to 4.5 and the assay was performed at 30°C).
Preparation of SCLLs and encapsulation of panomycocin in the vesicles
A lipid mixture similar to the composition of the stratum corneum was prepared as described by Wertz et al.26 Ceramide:cholesterol:palmitic acid:cholesteryl sulfate were combined in 4:2.5:2.5:1 ratio (w/w). For the construction of positively charged vesicles, stearylamine (18% by mole) was added to the mixture,27 and as an antioxidant, it was supplemented with alpha tocopherol (3% by mole)28 (chicken egg ceramide was from Avanti Lipids, Alabaster, AL, USA and all other lipids and chemicals were from Sigma-Aldrich Co.). From this mixture, empty liposomes were prepared by thin film hydration method of Bangham et al29 with modifications. The lipid mixture was dissolved in chloroform-methanol (2:1 v/v) to yield a lipid concentration of 10 mg/mL. Then, the solvent was removed in a rotary evaporator (Steroglass, Perugia, Italy) at 25°C and 40 rpm under vacuum (525 mmHg) until a homogeneous thin film was formed (~10 min). It was further incubated at the same conditions in the absence of vacuum for 2 h, and any remaining solvent was removed with nitrogen flush. Then the resulting dry film was rehydrated in 1 M Na2HPO4-citric acid buffer, pH 4.0 containing 0.1 M Na2SO4 in a water bath (95°C) under vigorous agitation (120 rpm). Sterile glass beads (426–600 microns; Sigma-Aldrich Co.) were also included to aid the removal of the lipid film from the inner surface of the flask in the course of agitation. The resulting milky liposome dispersion was bath-ultrasonicated (Isolab, Wertheim, Germany) at 95°C, 40 Hz, 180 W for 15 min.
Panomycocin was encapsulated in SCLL vesicles by the freeze-thaw technique described by Zhao and Lu30 with minor modifications. Briefly, liposome dispersion (0.95 mg/mL) and purified panomycocin solution (2 mg/mL) were mixed in 2:1 ratio (v/v). This mixture was rapidly frozen in liquid nitrogen (−196°C) for 5 min and slowly thawed at 4°C for 40 min in a vacuum desiccator. The freeze-thaw cycle was repeated three times. An adequate volume of the resulting dispersion was aliquoted for further analyses of encapsulation and loading efficiencies. The free panomycocin was separated from the remaining dispersion by a combined ultrafiltration/centrifugation technique at 2,500 rpm for 15 min (Centrisart I, 100 kDa cut-off, polyethersulfone membrane; Sartorius AG) at 4°C. An adequate volume of the resulting panomycocin-loaded liposomal dispersion was stored at 4°C for further zeta potential and dynamic light scattering (DLS) analyses which require liquid samples. The rest of the loaded liposomal dispersion was lyophilized at −115°C, 80 Torr (Maxi-Dry LYO; Thermo Fisher Scientific, Waltham, MA, USA) and stored at 4°C.
Determination of encapsulation efficiency (EE) and loading efficiency (LE)
EE and LE were calculated after the determination of the concentration of non-encapsulated panomycocin in the liposomal dispersion. The free panomycocin was separated from the loaded SCLL dispersion (1.5 mL) by a combined ultrafiltration/centrifugation technique as described previously, and the concentration of free panomycocin in the supernatant was determined according to Bradford31 in a nanodrop spectrophotometer (Biodrop, Cambridge, UK). The assay was done in triplicate. EE and LE calculations were done according to the following equations.32
|
|
Total panomycocin is the amount of panomycocin (by weight) added to the liposome dispersion during encapsulation.
Total liposome is the amount of lipids (by weight) present in the liposome dispersion during encapsulation. From the loaded SCLL dispersion, 1.5 mL aliquots were used for both EE and LE calculations.
Characterization of the empty and the panomycocin-loaded SCLLs
The morphology and size of the empty and the panomycocin-loaded SCLLs were examined with high contrast transmission electron microscope (TEM) (FEI Tecnai G2 Spirit BIOTWIN; Thermo Fisher Scientific) at an acceleration voltage of 80 kV.
The stability and surface charge of empty and loaded liposomal colloidal systems were determined with zeta potential analysis in 1 M Na2HPO4-citric acid buffer, pH 4.0 containing 0.1 M Na2SO4 at 25°C with Malvern Nano ZS90 (Malvern Instruments, Malvern, UK).
Investigation of irreversible intermolecular interactions between encapsulated panomycocin and SCLLs was done by Fourier transform infrared spectroscopy. Lyophilized empty and panomycocin-loaded SCLLs were scanned over a wave number range of 4,000–400 cm−1 by a Bruker Corporation IFS 66/S spectrometer.
Hydrodynamic size and polydispersity index of empty and panomycocin-loaded SCLLs were determined by DLS technique in 1 M Na2HPO4-citric acid buffer, pH 4.0 containing 0.1 M Na2SO4 at 20°C with a 90° scattering angle using a Malvern CGS-3 multi-angle goniometer (Malvern Instruments).
In vitro release studies of panomycocin-loaded SCLLs were done based on the Franz diffusion cell technique.33 Donor and receiver chambers of the cell were separated by a 100 kDa cut-off membrane (EMD Millipore). Lyophilized liposomal powder containing 1.5 mg panomycocin was rehydrated in 2 mL of sterile distilled water and loaded into the donor chamber. The receiver chamber (5 mL) was completely filled with 1 M Na2HPO4-citric acid buffer, pH 4.0 containing 0.1 M Na2SO4. The system was incubated at 37°C for 12 h with slow agitation and 1 mL of the medium from the receiver chamber was removed and replaced with fresh buffer solution at 1 h intervals. The assay was done in triplicate. The percentage of released protein in each sample was determined and plotted against time to generate in vitro release profile of SCLLs.
Determination of antimycotic efficacy of panomycocin-loaded lyophilized SCLLs
The antimycotic efficacy of panomycocin was tested on a commercially available reconstructed HVE (Episkin, Lyon, France) based on the vulvar epidermoid carcinoma cell line A431 which forms a 3-D epithelial tissue similar to the human in vivo vaginal mucosa when cultivated in vitro on a polycarbonate filter in a chemically defined medium. Tissues were inoculated separately with the C. albicans and C. glabrata vaginal isolates (1×103–5×103 CFU/mL)25 and incubated at 37°C for 2 h in a humidified CO2 incubator to enable the pathogens to adhere to the surface of the tissues. Then, three different amounts of liposomal lyophilized powder containing panomycocin which correspond to 1×, 1.5×, and 2× minimum fungicidal concentration (MFC) values of non-formulated pure panomycocin for each Candida spp. were applied onto the surface of infected tissues (Table 1).
After further incubation for 48 h at the previously mentioned conditions, the growth of each Candida strain on the tissues was determined by a magnifying glass in comparison with the control groups (growth control, sterility control, and negative control). Swab samples were taken from the tissue inserts with no visible growth and sub-cultured onto SDA plates for 7 days at 37°C to ascertain the killing effect of SCLL-encapsulated panomycocin.
Results
The pure panomycocin was encapsulated in SCLLs by freeze-thawing of the liposome–protein mixture. Encapsulation and LE studies were performed in 1.5 mL of liposome dispersion. The non-encapsulated panomycocin was separated from the liposomal dispersion as described in “Determination of encapsulation efficiency (EE) and loading efficiency (LE)” section, and the amount of free panomycocin was found to be 270.6 μg as determined by Bradford assay. The percentage of panomycocin incorporated in SCLLs relative to the initial total amount of panomycocin in the solution [EE% =100× (1,000 μg − 270.6 μg)/1,000 μg] was 73.0%, and the percentage of panomycocin incorporated in SCLLs relative to the content of the total lipid in 1.5 mL of liposome dispersion [LE% =100× (1,000 μg − 270.6 μg)/950 μg] was 76.8%.
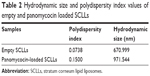
Empty and panomycocin-loaded SCLLs appeared as spherical particles in submicron size range in TEM images (Figure 1A and B). DLS experiments showed monodisperse size distribution for each SCLL sample in the dispersion as indicated with their polydispersity index values approaching zero. These experiments also showed that the hydrodynamic sizes were larger than the static sizes observed with TEM (Table 2; Figure 2). Empty and panomycocin-loaded SCLLs were stable and had positively charged surfaces as determined by a zeta potential value of +22.0 and +21.4 mV, respectively (Figure 3A and B). Also, no obvious irreversible interactions were observed between the liposome particles and panomycocin when the infrared spectra of both empty and loaded SCLL samples were compared (Figure 4A and B). In vitro release studies showed that the liposomal preparation retained 98.29% of the initially encapsulated panomycocin in 12 h in buffer solution with a plateau time of 4 h (Figure 5).
  | Figure 1 Transmission electron microscopy images. |
  | Table 2 Hydrodynamic size and polydispersity index values of empty and panomycocin loaded SCLLs |
The in vitro antimycotic efficacy of the liposomal panomycocin formulation was tested on HVE tissues. The tissues that were infected separately with the vaginal isolates of C. albicans and C. glabrata were observed visually with the aid of a magnifying glass after 48 h of incubation and compared to the cream-colored and shiny candidal growth in the growth control wells. Candidal growth was not observed on the tissues in any of the inserts which contained liposomal lyophilized powder with predetermined panomycocin quantities which correspond to 1×, 1.5×, and 2× MFC values of non-formulated pure panomycocin for each Candida spp. (Figure 6A and B). There was also no cell growth at the end of 7 days of incubation for any of the strains, after the swab samples taken from the tissue inserts with no visible candidal growth were sub-cultured on SDA plates.
Discussion
In the present study, we have developed a liposomal lyophilized powder formulation of panomycocin for the treatment of human vulvovaginal candidiasis. We have isolated panomycocin from the culture supernatant of W. anomalus NCYC 434 cells in crude form and purified it by using an fast protein liquid chromatography (FPLC) system as previously described by Izgu and Altinbay.12 Candida spp. majorly adhere to the stratum corneum layer of the vaginal epithelium during infections.2,3 The liposomes that we have constructed as panomycocin carriers were composed of lipids which are found in the composition of the stratum corneum layer of the vagina which is similar to that of the stratum corneum layer of the skin34 so that they would be adsorbed and mixed into the stratum corneum layer26 and release their contents where the Candida spp. localize. We have used the freeze-thaw technique to encapsulate panomycocin in the SCLLs for its high efficiency in encapsulation. In this method, the thawing temperature is determined according to the lipid Tm value which is the highest among the lipids in the liposome structure.30 In the composition of SCLLs, ceramide is the lipid with the highest Tm value which is between 80°C and 90°C.35 Since panomycocin is irreversibly inactive in this temperature range, we have used a modified freeze-thaw method in which the thawing was done at 4°C under vacuum30 in order to preserve protein stability. The zeta potential values for empty and loaded liposomes were +22.0 and +21.4 mV, respectively, indicating a very low protein binding on the surface of the liposomes. This also indicated highly efficient separation of the free proteins from the loaded liposomes. The EE that we have obtained with the modified freeze-thaw technique was reasonably high (73%), as reported previously.30 The buffer solution of panomycocin where it was stable and bioactive was at pH 4. At this pH value, panomycocin is negatively charged.12 To enhance the EE, we have included stearylamine into the lipid composition of SCLLs to give liposomes a final positive charge.27 The zeta potential values of the empty and loaded SCLLs were in the positive range, which is the indication of overall positive charge on the surface of the liposomes. We believe that, the opposite charges on SCLLs and panomycocin contributed to the achievement of high encapsulation and loading efficiencies. TEM images of panomycocin loaded SCLLs, when compared to that of their empty counterparts, appeared as quasi-spherical particles with smaller sizes. This most probably is the result of the repeated freeze-thaw steps where the liposomes break open and re-assemble into vesicles during loading process. In previous studies where liposomes were loaded by a freeze-thaw method in a buffer solution, similar morphological observations were also reported.36,37 Both types of liposomes had hydrodynamic sizes larger than their static sizes observed in TEM images. This is because in DLS analyses liposomes are dynamic, freely active in solution, and interacting with solvents.38 This state is contrary to that in transmission electron microscopy analyses in which drying of the samples is required which leads to liposome shrinkage.39 In DLS analyses we have obtained polydispersity index values close to 0 for both empty and panomycocin loaded SCLLs. This showed that the liposomes were monodisperse as a polydispersity index value closer to 0 in the range of 0–1 is the indication of monodispersity which is a significant factor in the efficacy of drug delivery and the stability studies.21,40–42 Also, an absolute value of zeta potential >20 mV for the liposomes shows their stability.43 Thus, we have concluded that empty and panomycocin-loaded SCLLs with zeta potential values of +22.0 mV and +21.4 mV, respectively, were stable in the colloidal system. An essential criterion for the active ingredient to be released at the target site in high amounts and in an active form is the lack of covalent bonds with its encapsulating vesicle. To investigate the presence of any irreversible interactions between panomycocin and SCLLs, we have inspected the infrared spectra of both loaded and empty liposomes and observed no significant differences between the spectra which signaled the presence of any irreversible interactions after encapsulation.44 The retention of panomycocin in the SCLLs in buffer solution at 37°C up to 12 h was very high (>98%), which is the indication of high stability of SCLLs characterized by low leakage of active ingredient in aqueous medium.32 The high stability is most probably due to the long chain lipids with high phase transition temperatures found in the composition of SCLL structure, and this is also supported by the studies reporting a highly ordered structure and increased phase transition temperature for the lipid bilayers containing ceramides.35,45 We have performed a biological efficacy test of the liposomal panomycocin formulation on an in vitro model of vaginal candidiasis. Liposomal lyophilized powder with predetermined panomycocin quantities for each Candida spp., which correspond to 1× MFC value of non-formulated pure panomycocin, was found to be inhibitory for all the tested strains. We have cultured the swabs from the tissues with no visible microbial growth onto SDA plates. The lack of any microbial growth on the plates at the end of 7 days of incubation showed that the inhibitory effect of the formulation at quantities corresponding to 1× MFC value of non-formulated pure panomycocin on both Candida spp. was fungicidal, which was also an indication of total release of the encapsulated panomycocin onto the tissues. The full release of the active ingredient from the SCLLs was most likely due to the effective electrostatic interaction of the tissues with the positively charged SCLLs which enabled the liposomes to be adsorbed onto the tissue layer effectively,46,47 and the acidic pH of the formulation (pH 4), which enabled the SCLLs to be fused with the lamellar structure of the tissue surface where they released their contents effectively.20 The efficacy of the formulation was tested after storage of the lyophilized liposomal panomycocin at 4°C for 30 days under the same experimental conditions as stated in the “Materials and methods” section, and no significant change in efficacy was observed.
It is well known that cryoprotectants are incorporated into the liposomal formulations to preserve their stability during lyophilization. The buffer solution in our liposomal formulation is composed of sodium phosphate and sodium sulfate salts and citric acid which are known for their cryoprotectant activities.48,49 Thus, we have not used any additional cryoprotectants in our formulation.
In the literature, although there are studies describing the use of stearylamine containing delivery systems for therapeutics and vaccines,27,47,50 positively charged liposomes may present toxicity to normal cells.51,52 The target of the formulation described in this study is the stratum corneum layer of the epithelium which is the protective layer composed of dead cells. Although it is known that the SCLLs will integrate into the stratum corneum structure and will not pass to the lower layers of the epithelium,20 further studies to evaluate any cytotoxicity related to this formulation are underway in our laboratory.
As a conclusion, we have described the development of a liposomal formulation of an antimycotic agent. This is the first study in which SCLLs and a naturally produced protein were both used to construct a formulation intended for topical therapeutic applications. The liposomes that we have constructed from stratum corneum lipids encapsulated panomycocin in high amounts and were stable in aqueous medium. The formulation carried the active ingredient to its target efficiently where it released its content completely and in a biologically active form. The results we have obtained here paved the way for further preclinical trials of liposomal formulation of panomycocin in the development of an effective topical anti-candidal drug with improved safety and high activity up to 38.5°C at vaginal pHs. Long-term stability studies are currently underway in our laboratory.
Acknowledgments
The authors thank METU Central Laboratory for providing facilities. This work was supported by a grant from the Scientific and Technological Research Council of Turkey (TUBITAK) (Project no: 115Z376). The sponsor had no role in study design, data collection, data interpretation, and preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Sobel JD. Genital candidiasis. Medicine. 2014;42(7):364–368. | ||
Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369(9577):1961–1971. | ||
Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9(7):327–335. | ||
Lim CS, Rosli R, Seow HF, Chong PP. Candida and invasive candidiasis: back to basics. Eur J Clin Microbiol Infect Dis. 2012;31(1):21–31. | ||
Kathiravan MK, Salake AB, Chothe AS, et al. The biology and chemistry of antifungal agents: a review. Bioorg Med Chem. 2012;20(19):5678–5698. | ||
Mendling W, Brasch J; German Society for Gynecology and Obstetrics; Working Group for Infections and Infectimmunology in Gynecology and Obstetrics; German Society of Dermatology, the Board of German Dermatologists; German Speaking Mycological Society. Guideline vulvovaginal candidosis (2010) of the German Society for Gynecology and Obstetrics, the Working Group for Infections and Infectimmunology in Gynecology and Obstetrics, the German Society of Dermatology, the Board of German Dermatologists and the German Speaking Mycological Society. Mycoses. 2012;55(Suppl 3):1–13. | ||
Niewerth M, Kunze D, Seibold M, Schaller M, Korting HC, Hube B. Ciclopirox olamine treatment affects the expression pattern of candida albicans genes encoding virulence factors, iron metabolism proteins, and drug resistance factors. Antimicrob Agents Chemother. 2003;47(6):1805–1817. | ||
Danby CS, Boikov D, Rautemaa-Richardson R, Sobel JD. Effect of pH on in vitro susceptibility of Candida glabrata and Candida albicans to 11 antifungal agents and implications for clinical use. Antimicrob Agents Chemother. 2012;56(3):1403–1406. | ||
Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–4687. | ||
Sobel JD, Faro S, Force RW, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178(2):203–211. | ||
Zhou X, Westman R, Hickey R, et al. Vaginal microbiota of women with frequent vulvovaginal candidiasis. Infect Immun. 2009;77(9):4130–4135. | ||
Izgu F, Altinbay D. Isolation and characterization of the K5-type yeast killer protein and its homology with an exo-beta-1,3-glucanase. Biosci Biotechnol Biochem. 2004;68(3):685–693. | ||
Izgu F, Altinbay D, Sertkaya A. Enzymic activity of the K5-type yeast killer toxin and its characterization. Biosci Biotechnol Biochem. 2005;69(11):2200–2206. | ||
Walker GM. Pichia anomala: cell physiology and biotechnology relative to other yeasts. Antonie Van Leeuwenhoek. 2011;99(1):25–34. | ||
Izgu F, Altinbay D, Türeli AE. In vitro activity of panomycocin, a novel exo-β-1,3-glucanase isolated from Pichia anomala NCYC 434, against dermatophytes. Mycoses. 2006;50(1):31–34. | ||
Izgu F, Altinbay D, Tureli A. In vitro susceptibilities of Candida spp. to Panomycocin, a novel exo-β-1,3-glucanase isolated from Pichia anomala NCYC 434. Microbiol Immunol. 2007;51(9):797–803. | ||
Swaminathan J, Ehrhardt C. Liposomal delivery of proteins and peptides. Expert Opin Drug Deliv. 2012;9(12):1489–1503. | ||
Onyuksel H, Sejourne F, Suzuki H, Rubinstein I. Human VIP-alpha: a long-acting, biocompatible and biodegradable peptide nanomedicine for essential hypertension. Peptides. 2006;27(9):2271–2275. | ||
Pierre MB, Dos Santos Miranda Costa I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res. 2011;303(9):607–621. | ||
Abraham W, Downing DT. Interaction between corneocytes and stratum corneum lipid liposomes in vitro. Biochim Biophys Acta. 1990;1021(2):119–125. | ||
Pierre MB, Tedesco AC, Marchetti JM, Bentley MV. Stratum corneum lipids liposomes for the topical delivery of 5-aminolevulinic acid in photodynamic therapy of skin cancer: preparation and in vitro permeation study. BMC Dermatol. 2001;1:5. | ||
Suhonen M, Li SK, Higuchi WI, Herron JN. A liposome permeability model for stratum corneum lipid bilayers based on commercial lipids. J Pharm Sci. 2007;97(10):4278–4292. | ||
European Commission. Communication from the Commission to the European Parliament and the Council. On the Animal Testing and Marketing Ban and on the State of Play in Relation to Alternative Methods in the Field of Cosmetics. Brussels: European Commision; 2013. Available from: http://ec.europa.eu/consumers/sectors/cosmetics/files/pdf/animal_testing/com_at_2013_en.pdf. Accessed July 3, 2017. | ||
Costin GE, Raabe HA, Priston R, Evans E, Curren RD. Vaginal irritation models: the current status of available alternative and in vitro tests. Altern Lab Anim. 2011;39(4):317–337. | ||
CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard M27-A. Wayne, PA; 2007. | ||
Wertz PW, Abraham W, Landmann L, Downing DT. Preparation of liposomes from stratum corneum lipids. J Invest Dermatol. 1986;87(5):582–584. | ||
Villasmil-Sánchez S, Drhimeur W, Ospino SC, Rabasco Alvarez AM, González-Rodríguez ML. Positively and negatively charged liposomes as carriers for transdermal delivery of sumatriptan: in vitro characterization. Drug Dev Ind Pharm. 2010;36(6):666–675. | ||
Tabandeh H, Mortazavi SA. An investigation into some effective factors on encapsulation efficiency of alpha-tocopherol in MLVs and the release profile from the corresponding liposomal gel. Iran J Pharm Res. 2013;12(Suppl):21–30. | ||
Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–252. | ||
Zhao YZ, Lu CT. Increasing the entrapment of protein-loaded liposomes with a modified freeze-thaw technique: a preliminary experimental study. Drug Dev Ind Pharm. 2009;35(2):165–171. | ||
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. | ||
Mugabe C, Azghani AO, Omri A. Liposome-mediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother. 2005;55(2):269–271. | ||
Franz TJ. Percutaneous absorption on the relevance of in vitro data. J Invest Dermatol. 1975;64(3):190–195. | ||
Anderson DJ, Marathe J, Pudney J. The structure of the human vaginal stratum corneum and its role in immune defense. Am J Reprod Immunol. 2014;71(6):618–623. | ||
Morrow MR, Helle A, Perry J, Vattulainen I, Wiedmer SK, Holopainen JM. Ceramide-1-phosphate, in contrast to ceramide, is not segregated into lateral lipid domains in phosphatidylcholine bilayers. Biophys J. 2009;96(6):2216–2226. | ||
Mayer LD, Hope MJ, Cullis PR, Janoff AS. Solute distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles. Biochim Biophys Acta. 1985;817(1):193–196. | ||
Traïkia M, Warschawski DE, Recouvreur M, Cartaud J, Devaux PF. Formation of unilamellar vesicles by repetitive freeze-thaw cycles: Characterization by electron microscopy and 31P-nuclear magnetic resonance. Eur Biophys J. 2000;29(3):184–195. | ||
Domingos RF, Baalousha MA, Ju-nam Y, et al. Characterizing manufactured nanoparticles in the environment: multimethod determination of particle sizes. Environ Sci Technol. 2009;43(19):7277–7284. | ||
Chattopadhyay S, Modesto-Lopez LB, Venkataraman C, Biswas P. Size distribution and morphology of liposome aerosols generated by two methodologies. Aerosol Sci Tech. 2010;44(11):972–982. | ||
Zhu TF, Szostak JW. Preparation of large monodisperse vesicles. PLoS One. 2009;4(4):e5009. | ||
Zelphati O, Nguyen C, Ferrari M, Felgner J, Tsai Y, Felgner PL. Stable and monodisperse lipoplex formulations for gene delivery. Gene Ther. 1998;5(9):1272–1282. | ||
van Swaay D, deMello A. Microfluidic methods for forming liposomes. Lab Chip. 2013;13(5):752–767. | ||
Chibowski E, Szcześ A. Zeta potential and surface charge of DPPC and DOPC liposomes in the presence of PLC enzyme. Adsorption. 2016;22(4–6):755–765. | ||
Campardelli R, Espirito Santo I, Albuquerque EC, De Melo SV, Della Porta G, Reverchon E. Efficient encapsulation of proteins in submicro liposomes using a supercritical fluid assisted continuous process. J Supercrit Fluids. 2016;107:163–169. | ||
Ibarguren M, López DJ, Escribá PV. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim Biophys Acta. 2014;1838(6):1518–1528. | ||
Pagano RE, Weinstein JN. Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng. 1978;7:435–468. | ||
Kusonwiriyawong C, Atuah K, Alpar OH, Merkle HP, Walter E. Cationic stearylamine-containing biodegradable microparticles for DNA delivery. J Microencapsul. 2004;21(1):25–36. | ||
Bujacz G, Wrzesniewska B, Bujacz A. Cryoprotection properties of salts of organic acids: a case study for a tetragonal crystal of HEW lysozyme. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 7):789–796. | ||
Fransen GJ, Salemink PJ, Crommelin DJ. Critical parameters in freezing of liposomes. Int J Pharm. 1986;33:27–35. | ||
Watarai S, Sasaki Y. Evaluation of stearylamine-modified liposomes for the oral vaccine adjuvant. J Infect Dis Ther. 2014;2(3):2–7. | ||
Sharma A, Madhunapantula SV, Robertson GP. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin Drug Metab Toxicol. 2012;8(1):47–69. | ||
Knudsen KB, Northeved H, Kumar PE, et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine. 2015;11(2):467–477. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.