Back to Journals » Cancer Management and Research » Volume 12
Strategies for Increasing the Effectiveness of Aromatase Inhibitors in Locally Advanced Breast Cancer: An Evidence-Based Review on Current Options
Authors Grizzi G , Ghidini M, Botticelli A, Tomasello G, Ghidini A , Grossi F, Fusco N , Cabiddu M, Savio T, Petrelli F
Received 23 November 2019
Accepted for publication 20 January 2020
Published 30 January 2020 Volume 2020:12 Pages 675—686
DOI https://doi.org/10.2147/CMAR.S202965
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Giulia Grizzi,1 Michele Ghidini,2 Andrea Botticelli,3,4 Gianluca Tomasello,5 Antonio Ghidini,6 Francesco Grossi,2 Nicola Fusco,7,8 Mary Cabiddu,9 Tommaso Savio,10 Fausto Petrelli9
1Oncology Unit, Oncology Department, ASST of Cremona, Cremona, Italy; 2Oncology Unit, Internal Medicine Department, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; 3Medical Oncology Department, Sant’Andrea Hospital, Rome, Italy; 4Department of Clinical and Molecular Medicine, “Sapienza” University of Rome, Rome, Italy; 5Oncology Unit, Niguarda Cancer Center, Grande Ospedale Metropolitano Niguarda, Milan, Italy; 6Medical Oncology Unit, Casa Di Cura Igea, Milan, Italy; 7Division of Pathology, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy; 8Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy; 9Oncology Unit, Medical Sciences Department, ASST of Bergamo Ovest, Treviglio, Italy; 10Breast Unit, ASST of Bergamo Ovest, Treviglio, Italy
Correspondence: Fausto Petrelli
Oncology Unit, Medical Sciences Department, ASST of Bergamo Ovest, Piazzale Ospedale 1, Bergamo 24047, Treviglio, Italy
Tel +39 03 6342 4420
Fax +39 03 6342 4380
Email [email protected]
Abstract: Neoadjuvant hormonal therapy (NEO-HT) is a possible treatment option for breast cancer (BC) patient with estrogen receptor positive (ER+) and HER2 negative (HER2-) disease. The absence of solid data on the type of drugs to be used and duration of treatment as well as lack of clear evidence of effectiveness of NEO-HT compared to chemotherapy (CT) reserve its use for patients with old age or frail conditions. However, the low pathologic complete response rate (pCR) obtained with tamoxifen or aromatase inhibitors (AIs) alone does not make NEO-HT as a suitable option for the neoadjuvant treatment of HR+ HER2-. The use of the cyclin-dependent kinase 4 and 6 (CDK 4/6) inhibitors palbociclib, ribociclib and abemaciclib of the mammalian target of rapamycin (mTOR) inhibitor everolimus and of the phosphoinositide 3 kinase (PI3K) inhibitor taselisib together with endocrine therapy (ET) has become a standard in advanced breast cancer, showing clinical effectiveness and significantly prolonging median progression-free survival compared to ET only. In the early phase disease, the use of ET together with CDK 4/6, mTOR and PI3K inhibitors is still investigational. Data from recent studies are promising even though less impressive than in metastatic setting. In this context, the use of genomic-transcriptomic tools (such as ONCOTYPE, PAM50) and the identification of novel biomarkers (ESR1, PI3Kca, PDGF-R) on tissue or with liquid biopsy could help to select patient prone to respond to endocrine-combined therapy and able to achieve pCR. With our review, we aimed at evaluating the current state of the art in the treatment of locally advanced breast cancer with NEO-HT.
Keywords: neoadjuvant endocrine therapy, breast cancer, CDK 4/6 inhibitors, mTOR inhibitors, PI3K inhibitors, aromatase inhibitors
Introduction
Endocrine therapy is the standard of care for estrogen receptor-positive and human epidermal receptor-2 negative (ER+/HER2-) breast cancer (BC). Luminal A ER+ represents the most common subtype of BC, accounting for approximately 75% of cases.1 Moreover, ER+ BC has a good prognosis and lower risk of mortality than ER- and/or progesterone receptor negative (PgR-) disease.2 Endocrine therapy acts wither by targeting the ER itself or by inhibiting the production of estrogens so that no ligand is available to activate the receptor. The first mechanism of action is typical of tamoxifen, a selective ER modulator, and fulvestrant, a selective ER degrader. On the other hand, aromatase inhibitors (AIs) block the aromatase enzyme and reduce estrogens level in post-menopause while and luteinizing hormone-releasing hormone agonists block the ovarian production of estrogens in pre-menopause.1
The role of preoperative (neoadjuvant) treatment for BC is a suitable treatment option for tumors of 2 cm of diameter or more, and for the locally advanced disease, not amenable for primary surgery. Neoadjuvant treatment may downstage tumor size, allowing a breast conservative surgery instead of radical mastectomy.3
Although the role of neoadjuvant chemotherapy (CT) is well established, the use of endocrine therapy (ET), alone or in combination in preoperative setting is still investigational and less diffuse. Since 2001, many studies have explored the efficacy of NEO-HT in ER+ BC, showing significant response rates of good tolerability. However, these series included small number of patients, had low statistical power and did not lead to robust conclusions in this setting of disease.1
The lack of solid data on the type of drugs to be used, on the duration of treatment and the absence of clear evidence of effectiveness of neoadiuvant hormonotherapy (NEO-HT) compared to CT do not allow a wide use of ET alone in the neoadjuvant setting, reserving its administration to patients who cannot tolerate CT because of its poor tolerability or for comorbidities. The association between ET and new molecules such as the cyclin-dependent kinase 4 and 6 (CDK 4/6) inhibitors, the mammalian target of rapamycin (mTOR) and phosphoinositide 3 kinase (PI3K) inhibitors aims at increasing the effectiveness of NEO-HT.
We conducted a comprehensive review in order to evaluate new combination treatments in the neoadjuvant setting of ER+ BC, focusing mainly on (CDK 4/6), mTOR and PI3K inhibitors associated with ET.
The Role of ET Alone
Early NEO-HT studies assessed the role of tamoxifen as a treatment of choice for older women, showing clinical response rates of more than 30% and overall survival similar to that achieved with the surgery-tamoxifen sequence but with worse loco-regional disease control.4 Recent trials evaluated the effectiveness of AIs against tamoxifen. The IMPACT study compared the effectiveness of anastrozole vs (vs) tamoxifen vs the combination of anastrozole and tamoxifen in the neoadjuvant ER+ BC. The conversion to conservative surgery for mastectomy-candidate patients was greater in the arm with anastrozole compared to tamoxifen alone and to the combination (46%, 22% and 26%, respectively).5 Similarly, in the PROACT study, which evaluated 3 months of preoperative treatment with anastrozole versus (vs) tamoxifen, conversion to conservative surgery for patients not immediately resectable was greater in the arm with anastrozole (44% vs 31%).6 Similar results were obtained with letrozole vs tamoxifen in study P024.7
Only 2 Phase II studies directly compared ET alone to CT in a neoadjuvant setting. The first trial enrolled 239 postmenopausal patients with stage IIa-IIIb ER+ BC to receive AIs for 3 months or anthracyclines-based CT plus paclitaxel. The primary endpoint was pCR. No significant differences were found between the 2 treatment arms.8 Similar results emerged from the GEICAM/2006-03 study that randomized 96 pre- and postmenopausal patients to receive exemestane for 24 weeks or CT based on anthracyclines and taxanes. The primary endpoint of the study was the clinical response but no statistical differences in the two treatment arms were observed (48% of responses in the exemestane arm, 66% in the chemotherapy arm, p=0.075).9
Few years ago, a large meta-analysis including 20 prospective, randomized, NEO-HT clinical trials and 3490 patients was conducted. Compared with the combination CT, NEO-HT as monotherapy with AIs had similar clinical response rate (odds ratio [OR] 1.08), radiological response rate (OR 1.38) and breast conservative surgery (OR 0.65) but with lower toxicity. AIs were associated with a significantly better clinical, radiological and breast conservative surgery rates compared to tamoxifen. The incidence of pCR was low (<10%).1
CDK 4/6, PI3K and mTOR Inhibitors in ER+ BC
Cyclin D-CDK 4/6 Retinoblastoma Pathway
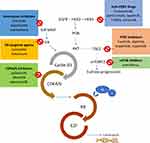
In normal breast tissue, cyclin D1 and CDK4 are important for the proliferation of the luminal epithelium (Figure 1). 10–13
During the G1 phase of the cell cycle, cyclin D binds to the cyclin-dependent kinase 4 (CDK4) creating an activated complex cyclin D-CDK4.14 The activated complex phosphorylates the retinoblastoma protein (RB). In its hypo-phosphorylates state, RB creates an inactive and stable complex with the E2F transcription factor, blocking cell replication. On the contrary, RB phosphorylated removes the inhibition of E2F transcriptional activity and promotes cell replication. Active E2F induces transcription of cyclin E, promoting the transition to the S phase of the cell cycle.15
CDK4/6 inhibitors act by blocking RB phosphorylation and thus causing the G1 cell cycle arrest resulting in a cellular senescence process.16 In vitro, cancer cells with a non-functioning RB protein are resistant to CDK4/6 inhibitors probably because the canonical target of these agents is missing.17,18
The enzymatic activity of CDK4/6 is regulated by different mechanisms.19 First, several signaling pathways positively regulate the activity of CDK4/6 by increasing the expression of CCND1 (encoding cyclin D) and/or increasing the stability of the cycline D. These signaling pathways usually start from tyrosin-kinase receptors (such as EGFR and HER2), the PI3K-AKT-mTOR axis and the ER (Figure 1).20,21
In particular, cyclin D1 is a transcriptional target of ER and estrogens promote the transit from G1 phase to S phase in ER-positive breast cancer cells.22 On the contrary, anti-estrogen therapies such as tamoxifen, AIs and fulvestrant reduce cyclin D expression leading to cell cycle arrest in G1 phase.23 Finally, about 15% of breast cancers have amplification of CCND1 itself and these tumors show higher levels of cyclin D1 proteins.24
PI3K-AKT-mTOR Pathway
The mammalian target of rapamycin (mTOR), a kinase in the phosphoinositide-3-kinase (PI3K)/AKT signaling pathway, integrates growth factor stimulation with energy and nutrient signaling to control cell growth and proliferation (Figure 1).25 In BC, the PI3K/AKT/mTOR pathway modulates responses to signals communicated through the ER and the human epidermal growth factor receptor (HER) family of receptors (HER-2 in particular),26,27 and this pathway is important in the clinical sensitivity of breast cancer cells to antiendocrine therapy. Preclinical studies have shown that breast cancer cells with upregulated AKT signaling are resistant to hormonal therapy, but sensitivity may be restored by treatment with everolimus or other mTOR inhibitors.28
The efficacy of mTOR inhibitors plus hormonal therapies in HR+ advanced BC has been known for several years and the combination is routinely used in clinical practice. Recently, about 40% of patients with ER+ HER2- BC have been identified as carriers of activating mutations in the PI3K gene and the effectiveness of PI3K inhibitors emerged both in advanced BC and in the neoadjuvant setting and will be discussed later.
ER+ Advanced BC
Clinical Data of CDK 4/6 Inhibitors in Advanced ER+ BC
The first generation of CDK 4/6 inhibitors revealed futility due to the toxicity and selectivity of drugs.29 Next-generation CDK inhibitors were developed with greater selectivity towards isoforms 4 and 6, resulting in more effective and less toxic compounds.23 To date, there are three CDK4/6 inhibitors available in clinical practice, palbociclib, ribociclib and abemaciclib, which are slightly different in pharmacokinetics profiles and toxicities. All these three agents demonstrated effectiveness in first and subsequent metastatic lines, with an approximate doubling of PFS compared to ET alone. Following the results of PALOMA-1,30 PALOMA-231 and PALOMA-3,32 palbociclib was approved by FDA in the United States and EMA in Europe for the treatment of patients with metastatic BC ER-positive HER2-negative in combination with AIs or fulvestrant. Ribociclib has been approved by the FDA and EMA for the same indications as palbociclib, based on the results of MONALEESA-2,33 MONALEESA-334 and MONALEESA-7.35 The Phase 3 MONARCH study demonstrated the clinical efficacy and safety of abemaciclib, achieving similar results in combination with AIs and fulvestrant.36
Clinical Data of PI3K and mTOR Inhibitors in Advanced ER+ BC
Targeting the PI3K-AKT-mTOR axis in ER-positive breast cancer demonstrated significant results in both advanced and early BC. The study published by Baselga et al in 2009, conducted in a neoadjuvant setting, accurately predicted outcomes of using everolimus plus exemestane in advanced ER+ HER2- BC.37 In particular, in the phase 3 BOLERO-2 study, the addition of everolimus to exemestane showed a higher PFS in patients with HR-positive tumors but not in those with PI3KCA mutations, suggesting that a direct inhibition of PI3K could be the preferred option in these patients.38,39
Two recent phase 3 studies evaluated the efficacy and safety of different PI3K inhibitors in patients with pretreated ER+ HER2- advanced BC.
The multicenter, placebo-controlled, BELLE-3 trial evaluated the efficacy and safety of buparlisib (pan-PI3K inhibitor) plus fulvestrant in 432 patients with ABC pretreated with endocrine therapy and mTOR inhibitors. The study was positive in its primary endpoint demonstrating an increase in median PFS in favor of buparlisib plus fulvestrant vs placebo plus fulvestrant (3.9 months vs. 1.8 months; HR 0.67, 95% CI 0.53–0.84, p=0.00030). Despite these positive data, the safety profile of buparlisib plus fulvestrant (G3-4 adverse events in 61% of patients) did not support the development of the combination in this setting while providing the rationale for the use of PI3K inhibitors plus endocrine therapy in patients with PIK3CA-mutation.40
The SOLAR-1 study evaluated the efficacy of alpelisib (α-specific PI3K inhibitor) vs placebo both plus fulvestrant in patients with ER+ HER2- ABC who had a disease that had relapsed or progressed during or after the receipt of an endocrine therapy. Patients were enrolled in two different cohorts on the basis of tumor-tissue PIK3CA mutation status. Primary endpoint of the study was PFS in the cohort with PIK3CA-mutated cancer, but PFS was analysed also in the cohort of patients without the mutation. 572 patients were enrolled, 341 with confirmed PIK3CA mutations. After a median follow-up of 20 months, PFS was 11.0 months in the alpelisib plus fulvestrant arm vs 5.7 months (HR 0.65; 95% CI, 0.50–0.85, P<0.001). No difference in PFS in the cohort of patients without the PIK3CA mutation. With a good safety profile of the combination, a higher incidence of hyperglycemia, rash and diarrhea occurred in the alpelisib arm as expected and in line with data available from previous studies.41
ER+ Locally Advanced BC
Clinical Data of CDK 4/6 Inhibitors in NEO-HT
So far, there are different clinical trials with available results that evaluate the effectiveness of the combination of CDK4/6 inhibitors plus ET in the neoadjuvant setting and are summarized in Table 1. The Phase 2 NeoPalAna is a single-arm study that enrolled 50 patients with stage II-III HR+/HER2- BC to receive monotherapy with anastrozole for 28 days, followed by the addition of palbociclib for 4 cycles (of 28 days).42 In some patients, palbociclib was stopped, maintaining monotherapy with anastrozole for 28 days before surgery while a subgroup of patients received a sixth course of palbociclib and anastrozole until surgery. The primary endpoint was the complete stop of the cell cycle (CCCA) defined as Ki67 < 2.7% and observed after 15 days of combination therapy (C1D15). The CCCA rate at C1D15 was significantly higher than C1D1 (87% vs 26%, p<0.001), regardless of PIK3CA mutational state and luminal subtype. The benefit of palbociclib was observed in grade 3 or negative progesterone-receptor tumors. The most common adverse events were neutropenia (48% any grade, 22% grade 3, 4% grade 4), leucopenia (22% any degree, 22% grade 2) and fatigue (14% any degree, 14% degree 2). The main limitation of this study is the lack of evaluation of pCR as primary endpoint.
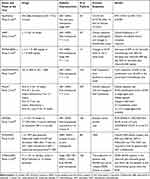 |
Table 1 Clinical Trials About CDK4/6 Inhibitors in Neoadjuvant Setting with Available Results |
N007 is a phase 2 single arm study that enrolled 20 postmenopausal patients with HR+ HER2- tumors (greater than 2 cm in diameter) to receive palbociclib and letrozole for 16 weeks. The co-primary endpoints were the objective response rate (with radiological assessment) and changes in EndoPredict scores after combined treatment. Clinical response of 50% or more was achieved in 17 patients, including 8 clinical complete response and 1 pathologic complete response. Every lesion showed a reduction in size and one patient obtained pCR. Median Ki-67 values were significantly reduced as a result of treatment (p=0.044). In addition, EndoPredict’s values were significantly lower than baseline values (p<0.0001). The safety profile was in line with previous data. Non-febrile neutropenia occurred in all patients during the first cycle. More than half of patients developed a G3/G4 neutropenia that required a reduction of dose or discontinuation of the therapy.43
The results of the PALLET study were recently published. A total of 307 postmenopausal women with HR+/HER2- (>2 cm in size) tumors were randomized to receive letrozole alone for 14 weeks (arm A), letrozole single-agent for 2 weeks followed by the addition of palbociclib for 12 weeks (arm B), palbociclib single-agent for 2 weeks followed by the addition of letrozole for 12 weeks (arm C) or letrozole plus palbociclib for 14 weeks (arm D). During the interval between the 14th week of therapy and surgery, all patients received letrozole. Primary endpoints were overall response rate (ORR) and reduction of ki-67 values observed in the four arms. The CCCA (defined as Ki-67 lower or equal to 2.7%), pCR rate, safety profile and analysis of molecular/genetic profiles of tumor samples were secondary endpoints. The clinical response was not significantly different between the single-agent arm and the combination groups. Complete or partial responses were observed in 49.5% with letrozole compared to 54.3% in the combination groups (p=0.2). However, the median change of Ki67 expression was most evident in the combination groups compared to letrozole alone (−4.1 vs −2.2, p<0.001). In addition, CCCA was observed in 38 (58.5%) of 65 patients in the letrozole group compared to 113 (90.4%) of 125 patients in the combination groups (P<0.001). This suggests that the addition of palbociclib to letrozole confirmed the suppression of proliferation but without any evidence of increasing clinical response. More patients experienced a grade 3/4 associated-toxicity with the combination drugs than letrozole monotherapy (49.8% vs 17%, p<0.001). The principal adverse event in the palbociclib plus letrozole arms was asymptomatic neutropenia.44
The role of ribociclib as neoadjuvant treatment was explored in the MONALEESA-1 study. The study compared ribociclib (at two doses: 400 or 600 mg/d) plus letrozole and single-agent letrozole in 14 postmenopausal women with G2/G3 HR+ HER2- BC (at least one breast lesion of 1 cm). The primary endpoint was the comparative level of Ki67 between the three study arms. The results showed that Ki67 levels decreased in the two combination arms compared to letrozole alone (a decrease of 69% in the letrozole arm, 96% for letrozole plus ribociclib 400 mg/die, 92% for letrozole plus ribociclib 600 mg/die). Combined therapy was well tolerated with no grade 3/4 adverse events reported. Unfortunately, the low number of patients represents a strong limitation of this study and makes it difficult to transfer results in clinical practice.37 NeoMONARCH is a phase 2 study that enrolled 223 postmenopausal women with primary breast cancer HR+ HER2- (≥ 1 centimeter) to receive abemaciclib in monotherapy, monotherapy with anastrozole or both in combination for 2 weeks, followed by 14 weeks of combined treatment. The primary endpoint was defined as the change in Ki67 expression from baseline to 2 weeks after starting. Secondary endpoints were clinical, radiological and pathological evaluation, safety profile and pharmacokinetics. The study showed a reduction in Ki67 of 92.62% in the combination arm and 63.24% in the anastrozole arm. Results of the monotherapic abemaciclib arm have not yet been published. Overall radiological responses were 46.4% with a 53.6% reduction in tumor size in all patients. However, only 3.7% of patients reached a pCR, but the authors do not provide an explanation that justifies this unusually low rate of pCR. Grade 3 diarrhea occurred in 4% of patients, despite a prophylactic therapy protocol with loperamide. Other common adverse events were constipation (any degree 43.5%; G3 1.8%) nausea (any degree 41.7%; G3 2.2%).45
To date, it remains unclear whether the combination of CDK4/6 inhibitors plus ET is more effective than chemotherapy in a neoadjuvant setting. Currently, there are no definitive answers and many clinical trials are still ongoing. However, the results of the NEOPAL French study have recently been published. This is a phase II trial comparing chemotherapy to standard neoadjuvant ET plus palbociclib in 106 patients with HR+, HER2- stage II-III BC. The primary endpoint was the residual cancer burden (RCB) frequency. The rate of patients with RCB 0/I (no or minimal residual disease) was higher in the chemotherapy arm than in the ET-palbociclib arm (15.5% vs. 7.7%), but the response rate and conservative breast surgery rate were comparable. Ki-67 median expression was significantly lower in palbociclib arm (3% vs 8%, p-0.017) and there were 2 adverse events in the ET-palbociclib arm vs 17 in the CT arm, demonstrating a better safety profile of ET-palbociclib.46
In the phase-2 PETREMAC trial (NCT02624973), patients with large tumor (>4cm) or locally advanced breast cancers and luminal A characteristics (ER> 50%, HER2-) received neoadjuvant ET and CDK4/6 inhibition in concert. NEO-HT consisted of letrozole in postmenopausal patients or tamoxifen plus goserelin in premenopausal. Palbociclib was added if Ki-67 had decreased <50% after 14 days on NEO-HT. Neoadjuvant chemotherapy (docetaxel) was introduced if NEO-HT + Palbociclib decreased Ki-67 < 50% or if NEO-HT ± Palbociclib did not cause an objective response. Eighty-eight patients were enrolled (2 patients lacked Ki-67 data for analysis, 1 was a screening failure). The results showed that 55% (47/85) had a drop > 50% on NEO-HT alone. Among the remaining 38 patients, 31 had Palbociclib added in concert. NEO-HT + Palbociclib showed a Ki-67 drop > 50% in 71% (22/31) of patients. Neoadjuvant CT was required in 33% (28/84) of patients after NEO-HT ± Palbociclib.
The overall ORR before surgery was 85%, and ORR for NEO-HT ± Palbociclib was 77%. pCR was observed in 4/75 patients at surgery, where 3/4 with pCR received neoadjuvant CT after NEO-HT ± Palbociclib. Pre-treatment tumor biopsies underwent targeted DNA sequencing of 360 cancer-related genes were performed: CDH1 mutations were associated with a higher probability of Ki67 reduction >50% on NEO-HT alone (CDH1 mutated: 15/20 vs CDH1 WT: 32/66; p=0.042).47
In conclusion, NEO-HT ± Palbociclib was effective at reducing cell proliferation and showed an ORR of 77% in these ER+ HER2-negative breast cancers. NAC was required only in 33% of the patients. CDH1 mutations seem predictive of response to NEO-HT in this setting.
In the recently published CORALLEEN phase 2 trial, 106 postmenopausal women with stage I-IIIA, HR+ HER2-negative, luminal B by PAM50 tumours were randomized to receive either six 28-days cycles of ribociclib (oral 600 mg once daily for 3 weeks on, 1 week off) plus daily letrozole or four cycles of doxorubicin and cyclophosphamide every 21 days followed by weekly paclitaxel for 12 weeks. The primary endpoint of the study was to evaluate the proportion of patients with PAM50 low-risk-of-relapse (ROR) disease at surgery in the modified intention-to-treat population. The PAM50 ROR risk class integrated gene expression data, tumour size, and nodal status to define prognosis. At baseline, 92 patients had high ROR disease (44 of 52 in the ribociclib and letrozole group and 48 of 54 in the chemotherapy group). At surgery, 23 of 49 (46.9%) patients in the ribociclib plus letrozole group and 24 of 52 (46.1%) patients in the chemotherapy group were low-ROR. These results suggest that some patients with high-risk, early stage, HR+ HER2- breast cancer could achieve molecular downstaging of their disease with CDK4/6 inhibitor and endocrine therapy.48
Clinical Data of mTOR and PI3K Inhibitors in NEO-HT
In Table 2, the conducted studies in this setting are listed. A phase 2 placebo-controlled study published in 2009 enrolled 270 postmenopausal women with operable ER+ tumors to receive 4 months of letrozole plus everolimus or placebo as neoadjuvant treatment. The primary endpoint was clinical response by palpation. Everolimus plus letrozole as neoadjuvant treatment resulted in higher objective responses compared with placebo plus letrozole, although this difference was not significant (68% vs 595, p=0.062).49
 |
Table 2 Clinical Trials About mTOR- and PI3K-Inhibitors in Neoadjuvant Setting with Available Results |
The phase-2 LORELEI trial evaluated the efficacy of letrozole plus taselisib or placebo in patients with operable ER+ HER2-negative BC, stage I-III. 334 patients were randomized to receive letrozole continuously plus taselisib 4 mg or placebo (on a 5 days-on, 2 days-off schedule) for 16 weeks, followed by surgery. Co-primary endpoints were the proportion of patients who achieved an objective response by centrally assessed breast magnetic resonance (MRI) and a locally assessed pathological complete response in the breast and axilla (ypT0/Tis, ypN0) at surgery in all randomly assigned patients and in patients with PIK3CA mutant tumors. The addition of taselisib to letrozole was associated with a higher proportion of patients achieving an objective response in all randomly assigned patients (39% patients in the placebo group vs 50% in the taselisib group; OR 1.55, 95% CI 1.00–2.38; p=0.049) and in the PIK3CA-mutant subset (38% vs 56%; OR 2.03, 95% CI 1.06–3.88; p=0.033). No significant differences were observed in pCR between the two groups, either in the overall population (2% in the taselisib group vs 1% in the placebo group; OR 3.07 [95% CI 0.32–29·85], p=0.37) or in the PIK3CA-mutant cohort (1% vs 0%; OR not estimable, p=0.48). The most common grade 3–4 adverse events in the taselisib group were gastrointestinal (8%), infections (5%), and skin-subcutaneous tissue disorders (5%). There was no grade 4 hyperglycemia and grade 3 cases were asymptomatic.
The rate of patients who achieved a greater objective response receiving taselisib plus letrozole in a neoadjuvant setting is in line with the clinical benefit observed in studies of advanced breast cancer disease. Achieving a pCR correlates with long-term outcomes such as event-free survival after neoadjuvant chemotherapy but after NEO-HT the evidence of pCR achievement is anecdotal.50
Ongoing Trials
In the neoadjuvant treatment landscape of HR+ HER2- early BC there are many ongoing studies aiming at evaluating the association of new target molecules with standard hormonal therapies.
PREDIX LumA (NCT02592083) is a phase-2 study enrolling pre- and post-menopausal patients with early BC (tumors < 2 cm), node negatives and luminal A phenotype (defined as expression of ER and PgR > 50% in IHC, KI-67 < 20% and no HER2 amplification) to receive neoadjuvant ET (tamoxifen or AIs ± goserelin) for 4 weeks. After this period, patients who experience a reduction in Ki-67 ≥ 20% respect of baseline level are randomized to continue ET-alone or add palbociclib for the next 12 weeks. Patients who do not experience a significant reduction in Ki-67 automatically receive ET plus palbociclib combination for 12 weeks. Primary endpoints are the clinical and radiological response. The trial started in October 2015 and recruitment is currently active.
A similar and ongoing study is the PREDIX LumB (NCT02603679) trial which assesses the efficacy and toxicity of palbociclib plus ET (tamoxifen or AIs ± goserelin) vs CT (weekly paclitaxel 80 mg/mq) for 12 weeks in patients with stage II-III luminal B tumors (defined as ER and Ki-67 expression ≥ 20%, HER2-). Primary endpoint of the study is the radiological response evaluated by breast ultrasound, mammography or MRI. After the first 12 weeks, patients with no signs of progression are switched to the other treatment arm for additional 12 weeks. In case of progression, surgery is offered as first-choice treatment.
The FELINE trial (NCT02712723) aim is to evaluate whether the combination of ribociclib plus letrozole for 24 weeks is able to obtain a higher rate of PEPI (pre-operative endocrine prognostic index) score equal to 0, at time of surgery, compared to letrozole in monotherapy. PEPI is a score used to predict the risk of recurrence in patients treated with neoadjuvant hormonal therapy and is calculated by evaluating pathological T and N, Ki-67 level and ER status at the time of surgery. This score was already validated in the IMPACT trial, which showed no 5-year recurrences in patients who reached a PEPI score of 0.51 The FELINE study evaluates two different ribociclib schedules in combination with letrozole: ribociclib 400 mg/day continuously or ribociclib 600 mg/day with a schedule “3 weeks on, 1 week off.” The study closed the enrollment.
The NEOLBC (NCT03283384) trial that evaluates the efficacy of ribociclib plus letrozole vs standard CT (AC for 4 cycles followed by paclitaxel for 12 weeks) in HR+ HER2- stage II-III breast tumors that do not obtain CCCA (primary endpoint of the study and defined as an expression of Ki-67 > 1%) after 2 weeks of treatment with letrozole monotherapy (considered as surrogate marker of resistance to ET). Recruiting is ongoing.
Finally, the triple blockade obtained from the combination of letrozole, palbociclib and copanlisib (a PI3K inhibitor) in patients with ER+ HER2- stage I-IV breast tumors is evaluated in the Phase 1–2 study (NCT03128619) which aim is to verify the safety profile and maximum tolerated dose of the triplet in the neoadjuvant and metastatic setting. The study is currently suspended (dose level-1 cohort suspended due to DLT) (NCT03128619).
Bona Fide Biomarkers to Overcome Resistance and Improve Patients’ Outcome
The research of biomarkers able to predict the response or resistance to treatment is an urgent need also in the scenario of endocrine therapy. Indeed, despite the success of endocrine-combined therapy in metastatic setting, the treatment response is observed in only 40% of first-line metastatic patients, with the vast majority of initial responders eventually developing resistance and/or recurrence over the time.52 In this setting, novel predictive biomarkers are warranted to further improve the outcome of AI-treated postmenopausal, luminal breast cancer patients. Over the past few years, numerous bona fide mechanisms of AIs resistance have been identified. However, only few studies have aimed to explore the clinical value of specific biomarkers predictive of AIs resistance. Among them, the secondary loss of hormone receptors expression (or development of negative clones) and acquired overexpression of HER2 are recognized as clinically relevant.52,53 It should be noted, however, that while the loss of PgR expression in patients who relapse or progress after AIs treatment can be observed in approximately 50% of cases, loss of ER expression is a rare event, occurring in approximately 7% of cases.52 Activating mutations in the estrogen receptor-1 (ESR1) gene affecting the ligand-binding domain have been identified in the metastatic site of a subset of neoplasms after AI treatment, but not in their corresponding primary tumors, suggesting the secondary acquisition of this alteration.54,55 Importantly, the p.Tyr537Ser and p.Asp538Gly alterations in ESR1 have been shown to favor an agonistic conformation of the ER, potentially leading to hormone receptor independent activation.56 Activation of the platelet-derived growth factor receptor (PDFG-R) family signaling has been documented both in vitro and in vivo in relapse samples of AI-treated breast cancers.57 For this reason, PDFG-R have been recently proposed as putative targets in these patients.57 Other recurrent molecular changes after AIs therapy involve the receptor tyrosine-kinase (RET) and the Ret-ligand glial-derived neurotrophic factor (GDNF).58 Interestingly, after neoadjuvant AIs treatment of postmenopausal ER+ BC patients a higher gene expression immune response signature and mismatch repair alterations have also been observed.59,60 However, the real-life clinical value of these observations remains a matter of controversy. The between-tumor genotypic and phenotypic heterogeneity among AIs-resistant breast cancers, however, is extremely complex. A wide gene array-based analysis has identified 26 genes showing heterogeneous expression between tumors from postmenopausal ER+ BC patients receiving adjuvant AIs who experienced recurrence.54 Among these alterations, those targeting the trefoil factor 3 (TFF3) and ESR1 were dominant. These data provide further credence to the notion that biopsy at recurrence would be beneficial to the identification of relevant targets at an individualized level.
Conclusion
Neoadjuvant endocrine therapy can be a safe and effective option for the treatment of ER+/HER2- early BC in particular for those patients considered too frail to receive primary chemotherapy. The low pCR rate does not allow considering endocrine therapy alone as the first-choice option for the neoadjuvant treatment of HR+ HER2-. However, data from recent studies combining in neoadjuvant setting the CDK4/6- and PI3K-inhibitors drugs to standard endocrine therapy are promising even though less impressive than in metastatic setting.
In the context of multiple choice of strategy and treatment the routine use of genomic-transcriptomic tools (such as ONCOTYPE, PAM50) and the identification of novel biomarkers (ESR1, PI3Kca, PDGF-R, the reduction of ki-67) on tissue or with liquid biopsy could be crucial to select patient prone to respond to endocrine-combined therapy and to achieve pCR.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1477–1486. doi:10.1001/jamaoncol.2016.1897
2. Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9(1):R6. doi:10.1186/bcr1639
3. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. doi:10.1200/JCO.2007.15.0235
4. Chia YH, Ellis MJ, Ma CX. Neoadjuvant endocrine therapy in primary breast cancer: indications and use as a research tool. Br J Cancer. 2010;103(6):759–764. doi:10.1038/sj.bjc.6605845
5. Smith IE, Dowsett M, Ebbs SR, et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J Clin Oncol. 2005;23(22):5108–5116. doi:10.1200/JCO.2005.04.005
6. Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trial. Cancer. 2006;106(10):2095–2103. doi:10.1002/(ISSN)1097-0142
7. Ellis MJ, Ma C. Letrozole in the neoadjuvant setting: the P024 trial. Breast Cancer Res Treat. 2007;105(Suppl 1):33–43. doi:10.1007/s10549-007-9701-x
8. Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110(2):244–254.
9. Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23(12):3069–3074. doi:10.1093/annonc/mds132
10. Jeselsohn R, Brown NE, Arendt L, et al. Cyclin D1 kinase activity is required for the self-renewal of mammary stem and progenitor cells that are targets of MMTV-ErbB2 tumorigenesis. Cancer Cell. 2010;17(1):65–76. doi:10.1016/j.ccr.2009.11.024
11. Sicinski P, Donaher JL, Parker SB, et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82(4):621–630. doi:10.1016/0092-8674(95)90034-9
12. Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411(6841):1017–1021. doi:10.1038/35082500
13. Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9(1):23–32. doi:10.1016/j.ccr.2005.12.012
14. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–1512. doi:10.1101/gad.13.12.1501
15. Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife. 2014;3. doi:10.7554/eLife.02872
16. Choi YJ, Li X, Hydbring P, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22(4):438–451. doi:10.1016/j.ccr.2012.09.015
17. Gong X, Litchfield LM, Webster Y, et al. Genomic aberrations that activate D-type cyclins are associated with enhanced sensitivity to the CDK4 and CDK6 inhibitor abemaciclib. Cancer Cell. 2017;32(6):761–776 e766. doi:10.1016/j.ccell.2017.11.006
18. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. doi:10.1186/bcr2419
19. Knudsen ES, Witkiewicz AK. The strange case of CDK4/6 inhibitors: mechanisms, resistance, and combination strategies. Trends Cancer. 2017;3(1):39–55. doi:10.1016/j.trecan.2016.11.006
20. Goel S, Wang Q, Watt AC, et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell. 2016;29(3):255–269. doi:10.1016/j.ccell.2016.02.006
21. Vora SR, Juric D, Kim N, et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26(1):136–149. doi:10.1016/j.ccr.2014.05.020
22. Watts CK, Sweeney KJ, Warlters A, Musgrove EA, Sutherland RL. Antiestrogen regulation of cell cycle progression and cyclin D1 gene expression in MCF-7 human breast cancer cells. Breast Cancer Res Treat. 1994;31(1):95–105. doi:10.1007/BF00689680
23. Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11(8):558–572. doi:10.1038/nrc3090
24. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi:10.1158/2159-8290.CD-12-0095
25. Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–430. doi:10.1038/nature04869
26. Kurokawa H, Lenferink AE, Simpson JF, et al. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60(20):5887–5894.
27. Perez-Tenorio G, Stal O, Southeast Sweden Breast Cancer G. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86(4):540–545. doi:10.1038/sj.bjc.6600126
28. Behrens D, Lykkesfeldt AE, Fichtner I. The mTOR pathway inhibitor RAD001 (everolimus) is highly efficacious in tamoxifen-sensitive and -resistant breast cancer xenografts. Targ Oncol. 2007;2(3):135–144. doi:10.1007/s11523-007-0054-5
29. Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14(2):130–146. doi:10.1038/nrd4504
30. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. doi:10.1016/S1470-2045(14)71159-3
31. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi:10.1056/NEJMoa1607303
32. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi:10.1016/S1470-2045(15)00613-0
33. O’Shaughnessy J, Petrakova K, Sonke GS, et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2- advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat. 2018;168(1):127–134. doi:10.1007/s10549-017-4518-8
34. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472. doi:10.1200/JCO.2018.78.9909
35. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904–915. doi:10.1016/S1470-2045(18)30292-4
36. Sledge GW
37. Curigliano G, Gomez Pardo P, Meric-Bernstam F, et al. Ribociclib plus letrozole in early breast cancer: a presurgical, window-of-opportunity study. Breast. 2016;28:191–198. doi:10.1016/j.breast.2016.06.008
38. Hortobagyi GN, Chen D, Piccart M, et al. Correlative analysis of genetic alterations and everolimus benefit in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from BOLERO-2. J Clin Oncol. 2016;34(5):419–426. doi:10.1200/JCO.2014.60.1971
39. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi:10.1056/NEJMoa1109653
40. Di Leo A, Johnston S, Lee KS, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(1):87–100. doi:10.1016/S1470-2045(17)30688-5
41. Andre F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi:10.1056/NEJMoa1813904
42. Ma CX, Gao F, Luo J, et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res. 2017;23(15):4055–4065. doi:10.1158/1078-0432.CCR-16-3206
43. Chow LWC, Morita S, Chow CYC, Ng WK, Toi M. Neoadjuvant palbociclib on ER+ breast cancer (N007): clinical response and EndoPredict’s value. Endocr Relat Cancer. 2018;25(2):123–130. doi:10.1530/ERC-17-0396
44. Johnston S, Puhalla S, Wheatley D, et al. Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor-positive early breast cancer: PALLET trial. J Clin Oncol. 2019;37(3):178–189. doi:10.1200/JCO.18.01624
45. Martin M, Hurvitz S, Chan D, et al. Abstract PD5-01: final results of NeoMONARCH: a phase 2 neoadjuvant study of abemaciclib in postmenopausal women with hormone receptor positive (HR+), HER2 negative breast cancer (BC). Cancer Res. 2018;78(4Supplement):
46. Cottu P, D’Hondt V, Dureau S, et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol. 2018;29(12):2334–2340. doi:10.1093/annonc/mdy448
47. Lønning PE, Clausen C, Blix ES, et al. 183PDNeoadjuvant endocrine therapy with palbociclib in patients with high-risk breast cancer. Ann Oncol. 2019;30(Supplement_5):v59–v60. doi:10.1093/annonc/mdz240.009
48. Prat A, Saura C, Pascual T, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):33–43. doi:10.1016/S1470-2045(19)30786-7
49. Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27(16):2630–2637. doi:10.1200/JCO.2008.18.8391
50. Saura C, Hlauschek D, Oliveira M, et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2019;20(9):1226–1238. doi:10.1016/S1470-2045(19)30334-1
51. Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100(19):1380–1388. doi:10.1093/jnci/djn309
52. Arnedos M, Drury S, Afentakis M, et al. Biomarker changes associated with the development of resistance to aromatase inhibitors (AIs) in estrogen receptor-positive breast cancer. Ann Oncol. 2014;25(3):605–610. doi:10.1093/annonc/mdt575
53. Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30(6):587–592. doi:10.1200/JCO.2010.33.5232
54. Thomsen KG, Lyng MB, Elias D, et al. Gene expression alterations associated with outcome in aromatase inhibitor-treated ER+ early-stage breast cancer patients. Breast Cancer Res Treat. 2015;154(3):483–494. doi:10.1007/s10549-015-3644-4
55. Ellis MJ, Ding L, Shen D, et al. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486(7403):353–360. doi:10.1038/nature11143
56. Toy W, Shen Y, Won H, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45(12):1439–1445. doi:10.1038/ng.2822
57. Weigel MT, Banerjee S, Arnedos M, et al. Enhanced expression of the PDGFR/Abl signaling pathway in aromatase inhibitor-resistant breast cancer. Ann Oncol. 2013;24(1):126–133. doi:10.1093/annonc/mds240
58. Morandi A, Martin LA, Gao Q, et al. GDNF-RET signaling in ER-positive breast cancers is a key determinant of response and resistance to aromatase inhibitors. Cancer Res. 2013;73(12):3783–3795. doi:10.1158/0008-5472.CAN-12-4265
59. Dunbier AK, Ghazoui Z, Anderson H, et al. Molecular profiling of aromatase inhibitor-treated postmenopausal breast tumors identifies immune-related correlates of resistance. Clin Cancer Res. 2013;19(10):2775–2786. doi:10.1158/1078-0432.CCR-12-1000
60. Fusco N, Lopez G, Corti C, et al. Mismatch repair protein loss as a prognostic and predictive biomarker in breast cancers regardless of microsatellite instability. JNCI Cancer Spectr. 2018;2(4):pky056. doi:10.1093/jncics/pky056
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.