Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Stimuli may have little impact on the deficit of visual working memory accuracy in first-episode schizophrenia
Authors She S , Zhang B, Mi L, Li H, Kuang Q, Bi T , Zheng Y
Received 25 September 2018
Accepted for publication 17 January 2019
Published 18 February 2019 Volume 2019:15 Pages 481—489
DOI https://doi.org/10.2147/NDT.S188645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Shenglin She,1,* Bei Zhang,1,* Lin Mi,1 Haijing Li,1 Qijie Kuang,1 Taiyong Bi,2 Yingjun Zheng1
1Department of Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), National Clinical Research Center on Mental Disorders (Changsha), Guangzhou 510370, China; 2School of Management, Zunyi Medical University, Guizhou 563000, China
*These authors contributed equally to this work
Purpose: Working memory (WM) deficits have been observed in people with schizophrenia (SZ) and are considered a core cognitive dysfunction in these patients. However, little is known about how stimuli and memory load influence visual WM deficits.
Patients and methods: In the present study, we adopted a match-to-sample task to examine the visual WM in 18 first-episode patients with SZ and 18 healthy controls (HCs). Faces and houses were used as the stimuli, and there were two levels of memory load – one item and two items; the average accuracy (ACC) and reaction time were calculated for each condition. The Positive and Negative Syndrome Scale and the Personal and Social Performance scale were used to assess the psychiatric symptoms and social function, respectively.
Results: The results showed equivalent levels of WM deficit when using face and house stimuli. Moreover, the WM deficits were not related to the duration of illness, medication, or SZ symptoms.
Conclusion: These results demonstrate that stimuli may have little impact on ACC in WM tasks in people with SZ. In addition, the memory load may have little impact on WM ACC when the load is relatively low.
Keywords: visual working memory, first-episode schizophrenia, face perception, match-to-sample, memory load, working memory deficit
Introduction
Schizophrenia (SZ) is considered as a complex illness with multiple cognitive dysfunctions.1 Working memory (WM) is one of the most examined cognitive functions in people with SZ and has been demonstrated to be impaired in a wide range of studies.2 A meta-analysis showed that WM deficits could be observed across all WM subdomains and across a variety of tasks, indicating a robust and reliable deficit in SZ.3 In this study, researchers investigated WM deficits in all three WM subdomains, namely, phonological, visuospatial, and central executive WM functions. The tasks included digit span, verbal learning, facial recognition, spatial span, and others. The meta-analysis results showed that although large deficits were observed in all the domains and tasks, there were no clear differences across the different domains or tasks. Some studies found that WM ability correlated with a series of other cognitive functions in SZ, such as visual retention, visual orientation, motor function, and even intelligence, suggesting the core role of WM deficits in SZ.4,5 Neurophysiological studies have consistently shown that prefrontal cortex (PFC) abnormalities are related to the WM deficits in SZ.6 Using different tasks and stimuli, researchers have found abnormalities in the activation of the dorsolateral prefrontal cortex (DLPFC) or the ventrolateral prefrontal cortex (VLPFC).7–9 These findings suggest a relationship between WM deficits and PFC abnormalities across a variety of tasks.
Visual WM is well investigated in healthy population through the approach of psychophysics.10 Delayed discrimination or delayed match-to-sample (DMTS) tasks are usually adopted to measure the precision or the capacity of the memory.11–13 In this paradigm, a sample display is first presented. Subjects are required to remember the stimuli in the display as accurately as possible. A few seconds after the disappearance of the sample display, a test display is provided, and subjects are asked to make a two alternative force choice or an old/new judgment about the difference between the test and sample stimuli. Unlike other kinds of WM, visual WM emphasizes both the perceptual processing and the information retention.14–17
Although the deficits in WM in SZ patients have been widely investigated,3 whether there are differences in WM tasks involving different stimuli is unknown, eg, face stimuli vs other types of stimuli. It was previously shown that facial perception is severely impaired in SZ.18 For example, using a matching task, Martin et al19 found that patients with SZ performed worse than control patients in terms of face recognition, ie, lower accuracy (ACC) rates. Using a visual search task, She et al20 also found a face-related impairment in visual search performance in patients with SZ. These results indicate that there is a face-specific processing deficit in people with SZ. On the neurophysiological level, studies have found abnormalities related to face processing in both anatomical structures and functional activities. For example, the gray matter volume of the fusiform gyrus was revealed to be smaller in patients with SZ than in healthy controls (HCs) by magnetic resonance imaging (MRI) studies.21,22 His area, which was termed the fusiform face area (FFA), was found to be crucial for face perception.23,24 Importantly, the neural response to face stimuli in the FFA was also found to be smaller in patients with SZ than in HCs.25 In summary, the results of these studies suggest that SZ may lead to physiological changes that affect face processing.
As described earlier, no evidence shows that stimuli could influence the WM deficit in patients with SZ. Therefore, the first motivation of the current study was examining the effect of stimuli on the WM deficit. As the processing of face stimuli may be specifically impaired in SZ, we hypothesized that patients with SZ may have problems in encoding face stimuli before holding the information in WM, thus resulting in a more severe deficit in WM related to faces than in memorizing other stimuli.
Furthermore, there was evidence showing that the memory load may not influence WM deficits in patients with SZ.2 However, previous studies usually manipulated the load by changing the duration of the delay, which may introduce confounding factors such as the time of rehearsal and the patience of the participants. To exclude these factors, we manipulated the memory load by changing the number of items in the current study. If the memory load has no effect on the WM deficit, we would observe equivalent amount of WM deficits in tasks with different memory loads.
In summary, the current study aims to investigate whether stimuli and memory load influence the visual WM in patients with SZ. The results may help to elucidate the mechanism of such a deficit in SZ. Specifically speaking, if the results show nonequivalent WM impairments for different stimuli, it may imply that the visual processing of stimuli in WM is impaired in addition to a general deficit in WM ability of SZ.
Methods
Participants
A total of 18 patients with SZ and 18 age-matched HCs were recruited for this study. Each patient was diagnosed with SZ according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV). All patients were diagnosed with SZ for the first time and had been taking antipsychotics drugs or undergoing SZ treatment for no longer than 1 year before participating in the study. Only first-episode patients with SZ were included in the study in order to avoid confounding factors resulting from the combination of antipsychotic medication and illness chronicity. Participants were excluded if they had a history of severe neurological disorders, other serious physical illnesses, or substance-related disorders. Patients were evaluated using the Positive and Negative Syndrome Scale (PANSS) and the Personal and Social Performance (PSP) scale by a trained psychiatrist.26,27
The 18 age-matched healthy volunteers were determined based on the doctor’s detailed consultation with the volunteers and their family. They reported no clinically significant illnesses, current or previous histories of any psychiatric or neurological disorders, and substance-related disorders. Individuals with family histories of psychiatric illness among their first-degree relatives were also excluded from the HC group. All of these subjects also participated in another study utilizing a visual search task that was published elsewhere.20 The present study and the previous one investigated two independent cognitive abilities and had different implications regarding the cognitive deficits in patients with SZ. As it was difficult to integrate the two results, we reported them separately.
The present study was carried out in accordance with the ethical guidelines for Research on Human Subjects from the Institutional Review Board of Guangzhou Huiai Hospital, and written informed consent was obtained from all subjects in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board of Guangzhou Huiai Hospital. All participants received payments for their participation.
Stimuli and procedures
The subjects sat in a comfortable chair and were tested in a dimly lit room. The visual stimuli were seen from a distance of 85 cm, presented on a Dell 19-inch LCD monitor with a spatial resolution of 1,024×768 and a refresh rate of 60 Hz. Throughout the experiment, the subjects were asked to fixate on a small dot that was presented at the center of the monitor.
Face and house stimuli were utilized. In all, 64 faces (32 females) with a neutral expression were selected from the Chinese Facial Affective Picture System (CFAPS).28 The outer features (hair, ears, and face contour) were excluded, and the brightness and root mean square (RMS) contrast were matched via Photoshop. Finally, each face picture extended 1.93×2.10°. In all, 64 pictures of houses were selected from the Internet via search engines, such as Google. All pictures were converted to grayscale and stretched to the same size as the pictures of the faces. The brightness and contrast were also matched.
The experiment utilized a DMTS paradigm to examine two kinds of visual WM: face WM (Figure 1A) and house WM (Figure 1B). The tests were different only in terms of the stimuli used for memorizing and testing. In face WM experiment, each trial began with a fixation for a random duration between 1,000 and 2,000 ms. Then, one or two sample face(s) were presented on the screen for 600 ms at the four possible positions of top-left, top-right, bottom-left, and bottom-right relative to the fixation point. The center of each stimulus (face or house) was 2.47° away from the fixation point. Subsequently, another fixation was presented for 3,000 ms. The subjects were asked to try their best to retain the memory of the presented face(s) during this period. Following this, the probe face was presented for 600 ms, and the subjects were asked to press one of two keys to indicate if the probe matched one of the sample(s) (“n” for yes, “m” for no) as quickly and accurately as possible. There was no time limit for participants to respond. Each block contained 40 trials. Half of the trials contained one face in the sample stage (low load, easy task), and the other half contained two faces (high load, hard task). In each load condition, the probe in one-half of the trials matched the sample. The order of the four kinds of trials (low load or high load×matched or unmatched) was counterbalanced in each block. Each subject completed six blocks (three blocks of face stimuli and three blocks of house stimuli) of the experiment.
  | Figure 1 The match-to-sample task for visual WM measurement. |
Statistical analyses
Our experimental design was a 2 (stimulus: face/house)×2 (memory load: low/high)×2 (subject group: SZ/HC) mixed design. The stimuli and memory load were within-subject factors, while the subject group was a between-subject factor. ACC and reaction time (RT) were calculated for each subject. We first performed a 2×2×2 ANOVA for each dependent variable. Multiple t-tests with Bonferroni adjustment were performed when planned comparisons were needed. The effect size (ES) and statistical power were provided with each statistical test. The ES was the partial eta squared for the interaction effect and the main effect in ANOVA. In the independent samples t-test, the ES was the standardized mean difference (Cohen’s d). Finally, we performed Pearson correlation analysis on the behavioral performance and the characteristics of the patients including duration of illness, clinical dosage, and symptom scores. Bonferroni adjustment was also applied as multiple correlation analyses were conducted.
Results
Demographic and clinical characteristics
The demographic and clinical data from the 18 patients and 18 HCs are given in Table 1. There were no significant differences between the SZ group and the HC group in terms of gender, age (t=0.2, P=0.831), or education (t=0.2, P=0.823). Patients used antipsychotics including paliperidone, olanzpine, amisulpride, risperidone, and aripiprazole. The percentage of patients who used these antipsychotics was 44.44%, 27.78%, 22.22%, 11.11%, and 11.11% respectively. The dose of antipsychotics was converted to chlorpromazine dosage.
Behavioral performance of visual WM
In this WM experiment, the subjects were instructed to identify whether the target face (or house) was presented on the sample display. Trials with RTs outside mean±3 SD were excluded from further analysis. The averaged ACC and RT were calculated for each condition (Table 2; Figure 2). We then performed two 2 (stimulus: face/house)×2 (subject group: SZ/HC)×2 (memory load: high/low) ANOVAs to evaluate the ACC and RT results.
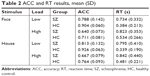  | Table 2 ACC and RT results, mean (SD) |
  | Figure 2 ACC (A) and RT (B) results for each task in people with SZ and HCs. |
Regarding the ACC results, we first determined that there was no significant interaction among the three factors, F(1,34)=1.500, P=0.229, ES=0.042, power=0.222. Furthermore, the interaction between stimuli and group, F(1,34)=0.524, P=0.474, ES=0.015, power=0.108, and the interaction between load and group, F(1,34)=2.247, P=0.143, ES=0.062, power=0.308, were both nonsignificant. The lack of significant interactions among these factors indicated that the WM deficit in SZ was not influenced by the stimulus or the memory load.
As the interaction effects were not statistically significant, we next examined the main effect of each factor. First, the main effect of the group was statistically significant, F(1,34)=12.242, P=0.001, ES=0.265, power=0.925, indicating a significant WM deficit in the SZ participants. Second, the main effect of load was significant, F(1,34)=255.61, P<0.001, ES=0.883, power=1.000, indicating that the participants performed worse when the memory load was higher. Third, the main effect of the stimuli was significant, F(1,34)=15.495, P<0.001, ES=0.313, power=0.969, indicating a lower ACC in the face WM than in the house WM.
We also performed a 2×2×2 ANOVA of RTs. The interaction among the three factors were not statistically significant, F(1,34)=0.747, P=0.394, ES=0.021, power=0.134. However, there were significant interactions between the factors of group and stimuli, F(1,34)=4.160, P=0.049, ES=0.109, power=0.509, and between the factors of group and memory load, F(1,34)=4.869, P=0.034, ES=0.125, power=0.573. The significant interactions between these factors might be attributed to the stimuli and load having an influence on the RTs of the HC group but not the RTs of the SZ group. Figure 2 shows that the RTs were generally longer in the SZ group, but they remained relatively constant across conditions.
To further examine the effects of the stimuli, we calculated the average ACC and RT for each stimulus and then performed a series of comparisons between the two stimuli in each group. We found that the HC subjects demonstrated lower ACC, t(17)=−3.559, P<0.05, Bonferroni corrected, ES=0.839, power=0.918, and longer RTs, t(17)=3.743, P<0.05, Bonferroni corrected, ES=0.882, power=0.941, in the face WM test, which indicated that the face WM test was harder than the house WM test. The SZ subjects did not show a significant difference in ACC, t(17)=−2.125, P=0.049, uncorrected, ES=0.501, power=0.518, and a non-significant difference in RT, t(17)=−0.968, P>0.05, Bonferroni corrected, ES=0.228, power=0.150, between the two stimuli.
Correlation between behavioral performance and the symptoms
By analyzing the behavioral performance mentioned earlier, we found that the SZ patients had deficits in both face WM and house WM. We next investigated whether these deficits correlated with the severity of SZ. A correlation analysis was performed to compare behavioral performance and the symptoms of the SZ patients. The results showed that there was no significant correlation between the performance and the duration of illness or the dose of antipsychotics that were converted to chlorpromazine dosage for both face WM and house WM (Table 3). Additionally, there was no significant correlation between the symptom scores and ACC or RT in each stimulus and load condition.
Discussion
Individuals with SZ showed lower ACC and longer RTs for both the face WM and the house WM than control participants, indicating a general deficit in visual WM among SZ patients. We calculated the ES of the WM deficit as the standardized mean difference of the ACC between SZ and HC in each condition (face/low load: ES=1.059; face/high load: ES=0.921; house/low load: ES=1.091; and house/high load: ES=1.121). The ESs of the WM deficits ranged from 0.92 to 1.12, a finding that was comparable to previous findings showing face WM deficits with an average ES of 0.82.3 The ACC results showed that there were no significant interactions between the group and the visual stimuli or between the group and memory load. The ESs and statistical powers of the two interactions were relatively small (ES=0.02 and 0.06, power=0.11 and 0.31). These ACC results suggested that visual stimuli and memory load may have small effects on WM deficits in SZ. Power analysis further showed that more than 32,400 and 188 subjects are needed to detect significant interaction effects for stimuli×group and load×group, respectively, with a power of 0.95. Detailed analyses did not show lower accuracies in the face WM than in the house WM, indicating that the face may not be a special stimulus in the WM deficits in SZ. Further correlation analyses revealed that the WM performance was not correlated with symptoms. Thus, the current ACC results suggest that the WM deficits in SZ are not influenced by visual stimuli. In addition, our study showed that load manipulation may also have little impact on ACC in WM tasks among patients with SZ.
Unlike the ACC results, RTs revealed a significant interaction between the group and the other factors. One possibility for this result might be that the SZ subjects reacted with a constant speed. First, as Figure 2 shows, the SZ subjects had much longer RTs in all the conditions than the HC subjects. Second, we performed a 2 (load)×2 (stimuli) repeated measures ANOVA on the RT data from the SZ subjects and found no significant interaction and no significant main effects. In other words, RTs were constant among SZ subjects. Of note, the difficulty of the present study was not high enough even in the high-load condition. The constant reaction speed may show that SZ patients tended to prioritize ACC in the ACC–RT trade-off when the memory load was within their capacity. Although such an explanation seems reasonable, we cannot deny the possibility that visual stimuli and memory load can influence the RTs of SZ subjects. A previous study showed a deficit in processing speed in SZ subjects, which may be affected by factors such as antipsychotic medication.29 We also found substantial processing speed deficits in the WM tasks in the SZ subjects, which was consistent with previous findings.29 Furthermore, our results indicated that such deficits were strong in the low-load house WM test and weak in the high-load face WM test. Additional studies are needed to investigate how stimuli and memory load affect processing speed deficits in WM and why the deficits are the weakest when face stimuli are used in a high-load WM task.
An important finding in the present study was that face WM ACC is not specifically impaired in SZ, as suggested by the nonsignificant interaction between stimuli and group. Comparisons between the ACC results for face and house stimuli showed a higher ACC in the house WM test in HC subjects, indicating that the test of memorizing houses was relatively easier. A marginally significant difference was found between the stimuli in the SZ subjects. The quantity of the difference between the two kinds of WM was equivalent for SZ (−0.026) and HC (−0.037), which contradicted our hypothesis that the ACC in the face WM task would be much lower among the SZ subjects. According to our hypothesis, face processing should be impaired in SZ subjects and lead to worse performance in the face WM. This hypothesis was proposed based on the inference that the patients with SZ suffer from face-related deficits in visual processing and such deficits might affect visual WM. First, previous studies have revealed a face-related deficit in visual processing, which might impact one or more processes of face WM. For example, MRI studies have found that the anatomical structure and activity of the FFA were abnormal in SZ patients, indicating impaired function in this area that processes face stimuli.21,22,25 Furthermore, event-related potential (ERP) studies have shown that the face-related N170 component is also impaired in SZ patients.30 More importantly, such an impairment in N170 was face specific, ie, other stimuli (eg, building or tree) induced N170 activity at normal or markedly less impaired rates than face stimuli.31,32 As SZ patients may suffer from face-related deficits in visual processing, the precision in encoding, maintaining, or retrieving face stimuli may be affected by these deficits, thus resulting in lower ACC in WM performance. However, we did not observe such a result in the present study. Therefore, our study results suggest that the precision of stimulus processing may not influence visual WM in SZ.
In addition to the effect of the stimuli on the WM deficit in SZ, our ACC results also showed little influence from memory load on the WM deficit. A previous study using a similar match-to-sample task found that the length of the delay period did not affect the WM deficit in SZ.33 In addition, a meta-analysis showed that increasing the delay did not influence the WM deficit in SZ.2 These studies manipulated the memory load by changing the delay, while we manipulated memory load by changing the number of items. Although all these results collectively indicate that the WM deficit in SZ is unaffected by memory load, it should be noted that the memory load was relatively low in the present study. Even in the high-load condition (two items), subjects performed well beyond chance for both stimuli (~0.65 in SZ and ~0.7 in HC). Therefore, the task appeared to be insufficiently difficult and within the WM capacity of SZ participants. Thus, additional studies are needed to better understand the effect of WM capacity on the WM deficit in SZ.
A previous meta-analysis found that there was no consistent association between the duration of illness, antipsychotic medications, or symptoms and WM in SZ,3 which was also found in the present study. The lack of an association between the severity of illness and WM indicates that the WM deficit is an early indicator of SZ. In addition, spatial WM has been found to be impaired in both SZ patients and their unaffected co-twins.34 WM deficits are, thus, a potential indicator of a high risk of SZ, even when the symptoms are not evident.
Although we did not find a stimulus-specific WM deficit based on the ACC results in the present study, it should be noted that the current design did not cover the whole range of WM load manipulation and that the results might change when three or more items are required to be memorized. Further studies are thus needed to examine the stimulus-related WM deficit in SZ patients using higher memory loads.
Conclusion
In summary, our ACC results demonstrated that the visual WM deficit in SZ patients may not be influenced by stimuli. In addition, the memory load may have little impact on the WM deficit when the load is relatively low. Furthermore, the WM deficit was not related to the duration of the illness, medication, or SZ symptoms. These findings reveal that the WM deficits in SZ might be a general impairment in WM ability and might not be related to the impaired visual processing of face stimuli in SZ. In the present study, only face and house stimuli were used. Additional stimuli should be examined in further studies. In addition, how the stimuli and load affect RTs in WM tasks among SZ patients requires further investigation.
Abbreviations
WM, working memory; PFC, prefrontal cortex; VLPFC, ventrolateral prefrontal cortex; MRI, magnetic resonance imaging; FFA, fusiform face area; ERP, event-related potential; DMTS, delayed match-to-sample; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance; CFAPS, Chinese Facial Affective Picture System; SZ, schizophrenia; HCs, healthy controls; RT, reaction time; ACC, accuracy; ES, effect size.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (81671334 to YZ) and the Medical Scientific Research Foundation of Guangdong Province of China (B2018063).
Disclosure
The authors report no conflicts of interest in this work.
References
Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15(2):73–95. | ||
Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114(4):599–611. | ||
Forbes NF, Carrick LA, Mcintosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. 2009;39(6):889–905. | ||
Johnson MK, Mcmahon RP, Robinson BM, et al. The relationship between working memory capacity and broad measures of cognitive ability in healthy adults and people with schizophrenia. Neuropsychology. 2013;27(2):220–229. | ||
Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160(10):1809–1816. | ||
Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–69. | ||
Eich TS, Nee DE, Insel C, Malapani C, Smith EE. Neural correlates of impaired cognitive control over working memory in schizophrenia. Biol Psychiatry. 2014;76(2):146–153. | ||
Lett TA, Voineskos AN, Kennedy JL, Levine B, Daskalakis ZJ. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biol Psychiatry. 2014;75(5):361–370. | ||
Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158(7):1105–1113. | ||
Magnussen S, Greenlee MW. The psychophysics of perceptual memory. Psychol Res. 1999;62(2–3):81–92. | ||
Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390(6657):279–281. | ||
Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428(6984):748–751. | ||
Ester EF, Anderson DE, Serences JT, Awh E. A neural measure of precision in visual working memory. J Cogn Neurosci. 2013;25(5):754–761. | ||
Christophel TB, Cichy RM, Hebart MN, Haynes JD. Parietal and early visual cortices encode working memory content across mental transformations. Neuroimage. 2015;106:198–206. | ||
Ester EF, Sprague TC, Serences JT. Parietal and frontal cortex encode stimulus-specific mnemonic representations during visual working memory. Neuron. 2015;87(4):893–905. | ||
Harrison SA, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature. 2009;458(7238):632–635. | ||
Yu Q, Shim WM. Occipital, parietal, and frontal cortices selectively maintain task-relevant features of multi-feature objects in visual working memory. Neuroimage. 2017;157:97–107. | ||
Bortolon C, Capdevielle D, Raffard S. Face recognition in schizophrenia disorder: a comprehensive review of behavioral, neuroimaging and neurophysiological studies. Neurosci Biobehav Rev. 2015;53:79–107. | ||
Martin F, Baudouin JY, Tiberghien G, Franck N. Processing emotional expression and facial identity in schizophrenia. Psychiatry Res. 2005;134(1):43–53. | ||
She S, Zhang B, Li X, et al. Face-related visual search deficits in first-episode schizophrenia. Psychiatry Res. 2017;256:144–149. | ||
Lee CU, Shenton ME, Salisbury DF, et al. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59(9):775–781. | ||
Onitsuka T, Niznikiewicz MA, Spencer KM, et al. Functional and structural deficits in brain regions subserving face perception in schizophrenia. Am J Psychiatry. 2006;163(3):455–462. | ||
Kanwisher N, Mcdermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–4311. | ||
Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2109–2128. | ||
Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol Psychiatry. 2003;53(12):1099–1112. | ||
Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. | ||
Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV social and occupational functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329. | ||
Gong X, Huang Y, Wang Y, Luo Y. Revision of the Chinese facial affective picture system. Chinese Mental Health Journal. 2011; 25(1): 40–46. | ||
Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167(7):828–835. | ||
Mccleery A, Lee J, Joshi A, Wynn JK, Hellemann GS, Green MF. Meta-analysis of face processing event-related potentials in schizophrenia. Biol Psychiatry. 2015;77(2):116–126. | ||
Herrmann MJ, Ellgring H, Fallgatter AJ. Early-stage face processing dysfunction in patients with schizophrenia. Am J Psychiatry. 2004;161(5):915–917. | ||
Maher S, Mashhoon Y, Ekstrom T, Lukas S, Chen Y. Deficient cortical face-sensitive N170 responses and basic visual processing in schizophrenia. Schizophr Res. 2016;170(1):87–94. | ||
Lencz T, Bilder RM, Turkel E, et al. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Arch Gen Psychiatry. 2003;60(3):238–243. | ||
Pirkola T, Tuulio-Henriksson A, Glahn D, et al. Spatial working memory function in twins with schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58(12):930–936. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


