Back to Journals » Lung Cancer: Targets and Therapy » Volume 9
Stereotactic body radiation therapy (SBRT) in the management of non-small-cell lung cancer: Clinical impact and patient perspectives
Authors Donovan EK, Swaminath A
Received 7 October 2017
Accepted for publication 26 January 2018
Published 16 March 2018 Volume 2018:9 Pages 13—23
DOI https://doi.org/10.2147/LCTT.S129833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Elysia K Donovan,1,2 Anand Swaminath1,2
1Department of Oncology, McMaster University, Hamilton, ON, Canada; 2Juravinski Cancer Centre at Hamilton Health Sciences, Hamilton, ON, Canada
Abstract: Stereotactic body radiation therapy (SBRT) has emerged as a new technology in radiotherapy delivery, allowing for potentially curative treatment in many patients previously felt not to be candidates for radical surgical resection of stage I non-small-cell lung cancer (NSCLC). Several studies have demonstrated very high local control rates using SBRT, and more recent data have suggested overall survival may approach that of surgery in operable patients. However, SBRT is not without unique toxicities, and the balance of toxicity, and effect on patient-reported quality of life need to be considered with respect to oncologic outcomes. We therefore aim to review SBRT in the context of important patient-related factors, including quality of life in several domains (and in comparison to other therapies such as conventional radiation, surgery, or no treatment). We will also describe scenarios in which SBRT may be reasonably offered (i.e. elderly patients and those with severe COPD), and where it may need to be approached with some caution due to increased risks of toxicity (i.e. tumor location, patients with interstitial lung disease). In total, we hope to characterize the physical, emotional, and functional consequences of SBRT, in relation to other management strategies, in order to aid the clinician in deciding whether SBRT is the optimal treatment choice for each patient with early stage NSCLC.
Keywords: non-small-cell lung cancer, early stage, stereotactic radiation, quality of life, toxicity
Introduction
Stereotactic body radiation therapy (SBRT) or stereotactic ablative body radiotherapy (SABR) has emerged as a new technology in radiotherapy delivery, allowing for potentially curative treatment in many patients previously felt not to be candidates for surgical resection of stage I non-small-cell lung cancer (NSCLC). While surgery has historically been the standard of care, many patients suffer from comorbid illnesses including COPD, cardiac disease, diabetes, etc. SBRT facilitates delivery of highly ablative doses of radiation while minimizing the amount of high-dose radiotherapy to other organs. Outcomes of patients treated with lung SBRT are consistently high, with <10% local recurrence at the treated site and ~80% control within the lung lobe and local lymph nodes.1,2 This is attributed to the fact that the lobe and nodes would otherwise be resected by standard surgery.3
Although there is increasing interest, supported by recent data suggesting similar local control and overall survival (OS) for SBRT in patients who are medically operable, lobectomy still remains the standard of care in surgical candidates. For those patients who are not surgical candidates, SBRT provides an opportunity for a more convenient, less invasive, and less morbid treatment. While SBRT provides nonoperable patients with a chance at improved disease control and life expectancy as compared with no treatment, it also has the potential to improve patient-reported physical symptoms and quality of life (QOL). In turn, it has been suggested that patient-reported QOL can be prognostic of clinical outcomes.4,5
In determining which treatment is the best choice for a patient with NSCLC, clinicians must consider both oncologic outcomes such as propensity for local control and OS, balanced with risks of toxicity, comorbid illness, and QOL. Patient preference must also be considered, and patients therefore must be guided in each of these outcomes in order to make informed decisions.
We therefore attempt to examine the literature pertaining to toxicity, and patient-reported QOL with respect to SBRT versus other available treatment options, including surgical resection or conventional radiotherapy for early stage NSCLC. We will also discuss groups of patients who may most benefit from SBRT, including patients with COPD and the elderly. Finally, we will review patients in whom SBRT should be approached with caution, such as those with interstitial lung disease or centrally located tumors. Ultimately, we hope to characterize the physical and functional consequences of SBRT treatment for NSCLC, in relation to other management strategies, to aid the clinician in deciding whether SBRT is the optimal treatment choice for each patient with early-stage NSCLC.
QOL in patients treated with SBRT
For the average inoperable patients, or those operable patients refusing surgery, studies have shown mixed reports of physical and functional symptoms following SBRT. A systematic review of clinical trials, reviews, and observational studies reporting on QOL following SBRT for early-stage lung cancer was recently completed, which identified 9 prospective studies.6 Clinically significant QOL changes were deemed to be >10% difference compared with baseline. Investigators found many of the studies reported stability of health-related QOL following SBRT, while two studies reported improvement in emotional domains. Other studies however reported mixed results, with two studies reporting worsening of dyspnea over time with SBRT (though clinically insignificant in one), and another reporting worsening fatigue, which was statistically significant. Finally, one of the largest studies in this review by Lagerwaard et al reported that poor baseline condition was also associated with decreased OS.5
Many qualitative measurement tools have been used to report physical, functional, and psychosocial aspects of QOL. The European Organization for Research and Treatment of Cancer Quality of Life Core 30 (EORTC QLQ-C30) and Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaires assess general or global QOL, and the EORTC Lung Cancer 13 (EORTC QLQ LC-13) questionnaire attempts to provide respiratory-specific measures of QOL. Many studies have assessed QOL using these scales without reporting significant declines following SBRT.6
Additionally, there are social and functional considerations in this group of patients, who often have significant comorbidities and poor baseline functional status. These patients may therefore have considerable difficulty tolerating and recovering from strenuous surgical resections or long conventional courses of radiotherapy in comparison.7 Investigators of prospective surgical cohort studies have reported variable QOL outcomes; however, in a majority of studies postsurgery physical scores and symptoms are worse than presurgery scores.8,9 Few studies report improvements in QOL scores at 2–3 years compared to immediately postsurgery.10 Patients undergoing laparoscopic surgeries also generally report better QOL scores than patients with open procedures.7 One study reported that 10% decreases in physical and mental QOL postsurgery were associated with an increased risk of death in 18% and 13% of patients, respectively, emphasizing the importance of considering QOL effects of treatment.9
However, SBRT is generally well tolerated in the short term, perhaps in part due to the shorter treatment course. Jain et al11 studied the optimal SBRT course by observing the difference in patient-reported QOL between a 4-day (daily) and 11-day (non-daily) regimen. Surprisingly, they found statistically significant differences in dyspnea and physical functioning scores, which worsened at 1 month with the shorter course compared to longer course.11 In medically frail patients, separation of the treatments from daily to non-daily schedules may be advantageous, as it allows time for rest and recovery between each treatment, and may be supported by clinical evidence of superior outcomes.12
A number of studies have examined the incidence of sub-acute (i.e. 3 or fewer months) effects following SBRT. For example, Sun et al13 completed a prospective study of 19 patients treated with SBRT on short-term patient-reported physical and psychosocial QOL domains. While baseline cognitive and activities of daily living scores were all quite good, scores for overall QOL were moderate, and social activities and functional QOL on FACT-L were very low. Baseline worry significantly decreased over 6 weeks, and baseline anxiety over 12 weeks (P<0.05). Symptom scores including pain, lack of energy, and cough were also more severe at the 12 week time point. While these side effects may occur secondary to radiotherapy, they may also reflect the baseline health of this population. Overall, the authors concluded that QOL scores remained generally stable over time, which may be considered positive given the competing risks of other comorbid illnesses. While attempting to obtain information on a breadth of domains, the large number of tests completed on a small number of patients may potentially make the results less valid.13
A prospective study from the Cleveland clinic found that of 21 patients receiving SBRT, there was no change in QOL on the FACT-L scale over the first year posttreatment. They also found, despite some worsening of pulmonary function testing (specifically the diffusion capacity of the lungs for carbon monoxide (DLCO) value posttreatment), there was no subjective decrease in respiratory function or symptoms overall.14
Long-term QOL is also essential to analyze as many toxicities of SBRT can occur months to years following treatment, including pneumonitis, pulmonary fibrosis, rib fracture, or pain.15,16 Investigators from Montreal reported QOL in 45 patients receiving SBRT for early-stage lung cancer in three to five fractions. Over time, the only clinically significant decrease in scores on the EORTC QLQ-C30 and EORTC LC-13 (defined as at least a 10-point reduction) were with respect to social functioning (11–12%). Interestingly, despite this, emotional scores increased by 14% (nonsignificant).17 Van der Voort van Zyp et al also reported an improvement in emotional domains using the EORTC QLQ C-30 and LC-13, which was statistically significant at a median of 17 months.18
Another large cohort of 382 Dutch patients treated with SBRT similarly found no difference in QOL on the EORTC QLQ C-30 scale over a 2-year time period. Fatigue and dyspnea were among the lowest baseline scores at study initiation; they therefore suggested that these patients referred with multiple comorbidities and related symptoms tolerate SBRT extremely well, particularly in comparison with that expected with more invasive treatments.5
While the results of these studies are quite variable and use a variety of assessment metrics, the patient population that is generally treated with SBRT is often quite fragile, and SBRT must be considered in the context of other alternatives. This includes no treatment, which may limit life expectancy significantly, as average survival for untreated early-stage lung cancer is ~12 months, and where population-based data have shown SBRT to be more effective than no therapy.19 Alternatively, invasive treatments may diminish quality and quantity of life due to short- and long-term treatment effects. In this setting, SBRT appears to be well-tolerated with minimal toxicity and acceptable QOL.
SBRT versus conventional radiotherapy: Convenience and safety for patients
Prior to the development of SBRT, the only nonsurgical option for patients with early NSCLC was 3D conventional radiotherapy (3D CRT). Intuitively, the shorter course of SBRT and less acute toxicity make it desirable in comparison with 3D CRT for eligible patients; however, the translation of these factors into measurable differences in QOL is not definitive. Table 1 describes the results of two studies comparing 3D CRT versus SBRT with respect to QOL.
Widder et al examined global QOL and physical functioning, and patient-rated dyspnea over time using the EORTC QLQ-C30 and QLQ-LC13 in a prospective cohort of patients receiving SBRT compared with a previous cohort of patients receiving 3D CRT.20 Patients with comorbidities had a statistical decrease in pulmonary function, while the group as a whole did not. Acute and subacute QOL change, and comparisons between groups prior to 1 year were not reported, where there may be differences owing to the length of conventional versus SBRT treatment regimens.
The primary outcome of the recent randomized SPACE trial of 3D CRT versus SBRT showed no significant difference in local control between the two arms, despite being possibly underpowered to detect a significant difference. Nonetheless, patients receiving 3D CRT suffered from increased rates of pneumonitis, thought to be secondary to increased irradiated lung due to the larger field sizes typically used in 3D CRT treatments,21 and as such, SBRT was recommended as compared to 3D CRT. Further randomized trials including the Australian CHISEL trial22 and Canadian LUSTRE trial23 will help further clarify the differences in QOL between 3D CRT and SBRT particularly in a modern radiotherapy planning era.
Despite concerns for toxicity and adverse QOL effects on patients using highly ablative radiotherapy regimens, SBRT appears safe in the long-term, and cancer outcomes are at least equivalent if not superior to 3D CRT. While less evidence is available to appreciate the short-term benefit, fewer visits and radiotherapy treatments are also intuitively more convenient for patients, and socioeconomically preferable from the patient perspective.
Tolerability and QOL of SBRT compared with surgery
Surgical resection remains the standard of care in operable patients, yet it may be challenging in some circumstances to offer surgery. Several factors determine operability, including assessment of comorbidities and baseline testing results. While previously postoperative FEV1 <30–40% was considered high risk for postsurgical complications, DLCO <30–40% has recently emerged as a more accurate predictor for postoperative complications.24 Further, some guidelines suggest that any patient with baseline DLCO values or FEV1 values <80% predicted could undergo exercise testing including peak V02 measurements (maximal volume of oxygen that can be utilized during peak exercise) to better define their surgical complication risk.24 Patients with V02 maximum <35% predicted or less than <10 mL/kg/min are considered high risk and surgery is not recommended.24 In those patients who are at high risk for surgical resection, SBRT should be seriously considered and discussed with patients.
In terms of clinical outcomes, OS in early stage nonoperable patients treated with SBRT were initially reported at ~50–70% at 3 years, substantially lower than surgery. This was likely due to non-cancer-related illness and death in a medically frail population.25 More recently, SBRT has been considered by some to have promising survival in for early-stage lung cancers in operable patients as well.3 A 2017 meta-analysis of eleven studies suggested similar if not improved OS and distant and local control advantage to SBRT compared with surgical resection in early stage non-small-cell patients. The applicability of these results is limited as the meta-analysis consisted mostly of retrospective studies, and limited direct comparisons between SBRT and surgery.
A pooled analysis of two randomized trials, STARS and ROSEL,26 which compared lobectomy versus SBRT for early-stage NSCLC showed promising rates of 3-year OS with SBRT (95% versus 78% for surgery). Given the difficulty of randomizing patients to two widely available treatments, both of these studies closed early due to poor accrual, and as such the pooled analysis was underpowered with respect to OS. Therefore, while intriguing, these results must be interpreted very cautiously, and until further randomized evidence is available, surgery still remains the standard of care for early stage patients.26
While it is important to ensure assessment of patients for surgical eligibility, it is also prudent to inform patients of acute surgical complications. Complications may arise secondary to general anesthesia, bleeding or infection, and prolonged hospital stay. In comparison, SBRT is a much less invasive treatment, with little to no mortality in the 30 days following treatment.27
Perhaps, more importantly, long-term toxicity risks also differ between surgical versus SBRT patients. In the STARS and ROSEL pooled analysis, 10% of SBRT patients experienced grade 3 or more toxicity including chest wall pain, cough, dyspnea, fatigue, and rib fracture, and there were no grade 4 events. In contrast, 44% of patients in the surgical arm experienced grade 3 or 4 dyspnea, chest pain, or infections, and there was one treatment-related death.26
The influence on QOL can also differ between surgery and SBRT. A systematic review of 19 studies of health-related (HR) QOL following resected stage I–III lung cancer was conducted to report on mental, physical, and emotional domains.7 The Short Form Health Survey-36 (SF-36), EORTC QLQ-C30, and EORTC LC-13 were the most commonly used scales. A majority of studies reporting on mental health found a small improvement in mean scores at 6 months, and 60% of patients’ improvement in scores by 2–3 years. A majority of studies acknowledged mental and emotional health QOL scores were still worse than scores in the general population. Eighty-five percent of studies reporting physical health observed significant declines over time, including a 63% decrease in scores 1 month postsurgery. Many studies reported symptoms were still present up to 3 years after surgery, and investigators found a significant increase in dyspnea in surgical patients at 2 years.7
Physical symptoms reported on QOL scales were also dependent on the type of surgery. For example, patients undergoing lobectomy experienced less dyspnea and pain than patients who had pneumonectomy. However, video-assisted thoracoscopic surgery (VATS) was compared to thoracotomy in two studies and had superior outcomes in reduced pain and physical and emotional HR QOL, and quicker recovery time. The review also found that patients requiring adjuvant therapy experienced worsening physical symptoms at 6 months after therapy, while mental QOL was not affected.7
In comparison to the increase in symptoms and decline in physical QOL scores postsurgery, after SBRT patients generally maintain or improve upon pretreatment QOL scores and symptoms.16 The level of recovery months to years after surgery is variable, while SBRT patients tend to have well-preserved QOL if healthy at baseline. A few studies report mild decreases in QOL scores over time following SBRT as well; however, given much of the SBRT literature is in nonsurgical candidates, baseline COPD and poor lung function may cause deteriorations in scores independent of SBRT effects.20,28
There is limited data directly comparing the impact of surgery versus SBRT on QOL. The ROSEL29 randomized trial of surgery versus SBRT, which closed early (n=22) due to poor accrual, did capture patient-reported QOL data using the EORTC QLQ-C30, EORTC QLQ LC-13, and EuroQoL questionnaires. Each was administered every 3 months for the first year, and 6 months thereafter to 3 years. The global health status was found to be significantly worse (P=0.038) for surgical versus SBRT patients at a median of 42 months. There were no other QOL differences reported, however the small number of patients and lack of power in this study must be considered.29 Current trials including STABLEMATES (NCT01622621) and VALOR (CSP #2005) will compare surgery versus SBRT in a randomized setting and will hopefully better delineate differences in QOL between these treatments.30,31
To investigate patient preference, Shaverdian et al contacted 102 early stage NSCLC patients who had undergone SBRT, or surgery followed by SBRT at a later date. They found that prior to meeting with oncologists, 56% of patients had not heard of SBRT. In reference to initial expectations, 92% of patients reported experiencing fewer symptoms, 87% reported less anxiety, and 59% found SBRT to be more convenient following treatment. In those 39 patients who received surgery and SBRT, 79.5% were more satisfied with SBRT, and all patients reported recovering more quickly, having less stress and less caregiver burden with SBRT.32
Overall, while surgery should be advocated for eligible patients, SBRT should be strongly considered in those who may be at high risk for complications postoperatively, or for patients wishing to minimize posttreatment morbidity. SBRT appears to be well tolerated with comparable if not improved recovery, symptoms, and QOL as compared to surgery.
Defining appropriate candidates for SBRT
Severe COPD
COPD is present in 50–70% of lung cancer cases and is an independent lung cancer risk factor while sharing a common risk factor of tobacco use. Patients with COPD range from asymptomatic or mildly dyspneic to requiring oxygen at end stages of disease. Generally, pulmonary function tests (PFTs) show FEV1 and DLCO of <60%.28 Severity of COPD may be expressed by the Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) criteria, which defines 4 classes of disease based on predicted FEV1 (stage I at least 80%, stage II between 50% and 80%, stage III 30–50%, or stage IV <30% or chronic respiratory failure).33
Patients with COPD often have other associated cardiac comorbidities, and a higher-than-expected annual incidence of mortality.34 Pulmonary function is generally decreased at baseline, and patients with the highest risk of lung cancer also tend to be those with the poorest lung function.33 With the advent of SBRT, however, patients with more severe COPD or emphysema may be considered suitable candidates for SBRT.35
Palma et al34 reported on 176 patients with COPD (median 38% predicted postoperative FEV1) and stage I NSCLC treated with SBRT. Three-year OS was 47%, likely lower than expected in stage I patients due to comorbidity given the excellent local control rates (89%) they observed at 3 years. Results of this study and two others regarding COPD patients are presented in Table 2, as well as results of a systematic review. In this particular cohort of 176 inoperable patients, they likely had poorer baseline characteristics than those patients reported in the surgical studies, yet they experienced less treatment-related morbidity and mortality.34
Elderly patients
Elderly patients, particularly those with comorbidities, may be particularly susceptible to physical and functional decline following surgery. A best evidence review of studies of surgical and nonsurgical treatment of NSCLC found higher rates of palliative care were required in those over age 70 undergoing surgery.36
These patients however seem to have comparable rates of control, toxicity, and tolerability following SBRT as compared with younger patients. Haasbeek et al reported 193 patients treated with SBRT for NSCLC and observed OS of 45.1% and local control of 89% at 3 years. Twenty-five percent had severe COPD. There was no difference in survival or local control between those greater or less than 75 years, and patients over age 75 actually had improved distant disease control compared with patients younger than 75 years. Less than 40% of patients experienced acute toxicities (75% of which were fatigue), and there were <10% grade 3 adverse events in these patients with only 2% requiring steroids for radiation pneumonitis (RP). These outcomes for control, survival, and toxicity in this group of elderly patients with moderate-to-severe COPD are therefore comparable to those expected in all-comers with early-stage lung cancers treated with SBRT.37
Louie et al published a Markov model analysis to predict the outcomes of patients aged 75 years or older with severe COPD, treated with SBRT versus best supportive care. The model was based on the outcomes of 247 prospectively followed patients treated with SBRT, compared with population-based outcomes for untreated stage I lung cancer patients with COPD. They also used health utility data from previous studies to calculate quality-adjusted survival time. The model predicted superior OS in patients receiving SBRT (6.8–47.2%) at 5 years versus those who were untreated (2.8–9%). They also predicted that improved quality-adjusted life months were greater in those patients receiving SBRT by 8 months.28
Approaching SBRT with caution
Central or apical tumor location
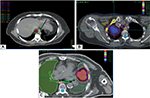
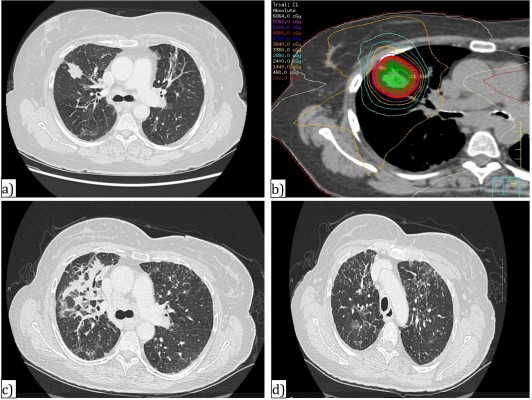
Not all patients benefit from the low rates of toxicity accompanied by SBRT. Tumor location may play a significant role in safety of SBRT delivery, and adverse events may result from attempts to deliver ablative doses to tumors in close proximity to organs at risk such as the central mediastinal structures and brachial plexus (Figure 1). While SBRT has promising outcomes and low toxicity in those patients with peripheral tumors, in contrast, treatment of central tumors (defined as being within 2 cm of the proximal airways, or within 1 cm of the central mediastinal structures) may have toxicity rates as high as 47% without dose adaptation.27 This includes damage to vascular structures, esophageal or tracheal ulceration, bleeding, and/or perforation. Timmerman et al27 reported six deaths in a cohort of 70 patients treated with SBRT to 60–66 Gy in three fractions, and 14 patients experienced grade 3 or higher toxicity. Freedom from severe toxicity was 83% in those with peripheral tumors versus 54% in those with central tumors at 2 years. Patients with ultra-central tumors where the lesion may be touching the proximal airway may be even more susceptible to risks of severe pulmonary toxicity post SBRT, despite the use of more fractionated regimens. Tekatli et al reported a 21% rate of death thought to be related to SBRT, including pulmonary hemorrhage in 15%,38 for ultra-central tumors. Other retrospective studies, however, support the use of SBRT for central and ultra-central tumors with good control and minimal toxicity with smaller doses per fraction.39,40 The recently completed phase I–II RTOG 0813 trial showed that for central tumors, safe dose escalation beyond 50 Gy in five fractions (up to 60 Gy in five fractions) could occur,41 but there was a small incidence of grade 3 or higher late toxicity in higher dose levels. Therefore, some variation still exists in determining the appropriate SBRT regimen in patients with centrally located lung cancers; results from the Canadian LUSTRE trial and European LungTech trial will attempt to further clarify the role of SBRT in these patients.42
Central cardiac and vascular structures may also be at risk. Stam et al conducted a multicenter retrospective review of SBRT plans for early stage NSCLC in the Netherlands. Thirty-three percent of patients were deceased at a median of 34.8 months, and of these only 26% of deaths were cancer related. Investigators found an increase in non-cancer death in those patients with high dose to the superior vena cava and left atrium, and in particular in those with poor performance status, impaired FEV1, and cardiac history.43 Other experiences have reported development of esophageal fistulas with high-point doses between 48 and 51 Gy in four fractions.44 In patients with tumors adjacent to or near the brachial plexus, there also appears to be a dose-dependent risk of brachial plexopathy; a study from Forquer et al45 in 37 patients with apical lesions reported a 19% risk of brachial plexopathy at a median of 2 years post treatment. The risk of plexopathy was significantly increased depending on the dose from 8% to 46% at 2 years for doses exceeding 26 Gy in three to four fractions.
As a result of this, adjustment and adaptation of SBRT dose and fractionation is necessary to reduce the risks of toxicity as much as possible; such strategies have resulted in safe SBRT delivery to central structures, with minimal or no grade 3 toxicity. If too extreme, however, dose reduction may result in a cost to local control, and total biologically equivalent SBRT dose of <100 Gy is thought to adversely affect outcomes.46,47 Sixty grays delivered in eight fractions may be used to achieve local control safely in the case of central tumors.42 Appropriate dose limitations should still be maintained when using this regimen, including the esophagus to 40 Gy maximum point dose or 20 Gy to 5 cc, and the heart, vessels, and trachea to 64 Gy maximum point dose and 60 Gy to 10 cc (or to 5 cc in the case of the trachea and proximal tree).23 In most cases, with careful planning and reduced or risk-adapted dose per fraction, SBRT can be delivered safely even in difficult tumor locations, without compromise in local control or survival.48
Interstitial lung disease
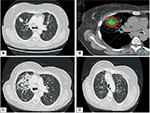
Interstitial lung disease (ILD) comprises a spectrum of diseases manifesting as inflammation and fibrosis of the pulmonary parenchyma. Clinical symptoms include fatigue, cough, hypoxia, and fever while radiologic findings of pulmonary infiltrates are seen. Patients may physically and functionally deteriorate with decreased ability to perform activities of daily living, and in severe circumstances, pulmonary hypertension, and oxygen dependence.49 There are over 200 subtypes of ILD, which for example may be associated with connective tissue disorder (i.e. scleroderma), medication induced or idiopathic.50 Cigarette smoking has increasingly been recognized as a risk factor, and may exacerbate severity of existing pneumonitis.51 Pulmonary function tests generally show a restrictive picture with a reduced diffusion capacity. While ILD itself can be managed through observation, symptom control or medically by treating the inciting cause some severe cases can significantly affect prognosis.
While ILD is not an established contraindication to SBRT treatment, patients are at much higher risk of radiation-toxicity than the average patient (Figure 2).52 Additionally, QOL in these patients has not been well documented, yet significant toxicity in these patients has been reported in a number of series.52-57
A recent systematic review reported on 122 patients across 13 studies with preexisting ILD treated with SBRT. Of these patients, treatment-related mortality was 15.6% and morbidity was 25%. This is in contrast to patients without ILD,26 where the risk of grade 3 toxicity including RP is generally <10%.
A number of patient and treatment-related factors may influence the toxicity risk from SBRT treatment for early stage NSCLC in patients with ILD. These factors must be very carefully considered prior to recommending SBRT, as development of side effects and adverse effects may negatively affect QOL. In patients with subclinical ILD, the risk of RP may be comparable to the general population.53 These patients are generally able to tolerate fractionated radiotherapy quite well. Studies have reported no correlation between toxicity development and retrospective identification of ILD.
The severity of radiologic findings may also correlate with the risk of developing SBRT related ILD in patients.54 Ueki et al. reviewed imaging of 157 patients who underwent SBRT for stage I lung cancer, and retrospectively found of 20 patients with preexisting ILD. The presence of radiologic ILD correlated significantly with incidence of both grade II (55% versus 13.3%) and III (10% versus 1.5%) RP compared those without ILD.55 While 50% of those with minimal radiologic disease had grade 2 or greater RP incidence, there was no correlation with grade 3 or greater RP in this lower severity ILD group. Yamashita et al also found preexisting radiologic ILD, seen as shadowing on CT scans, in 78% of patients who developed severe RP (grade 4–5) post-SBRT.56 Yet other studies found no increased risk of RP in those patients with only minimal ILD.57
Idiopathic pulmonary fibrosis (IPF) is among the most severe form of ILD, with a life expectancy of less than 5 years.58 The incidence of lung cancer in IPF patients is very high, at up to 15% at 5 and 50% by 10 years.59 While the exact reason for increased cancer pathogenesis in IPF patients is unknown, it has been acknowledged that IPF and lung cancers share up-regulation of common proteins and cytokines.44 Radiologic findings are usually severe.60
Patients with IPF are at higher risk of complications with biopsy and most of cancer treatment. Complications from RP and radiation fibrosis, particularly following SBRT, may be quite severe in ILD and IPF patients.61,62 This has been reported in many retrospective studies. One review of a small series of patients treated between 2009 and 2014 in Montreal found 21% developed grade 5 toxicity (death) secondary to RP following SBRT.61 Takeda et al. also described RP incidence in seven of 133 patients treated with SBRT, two of whom had IPF.62
Further, Onishi et al reported 6% grade 5 toxicity at 3 years in patients with IPF.63 In contrast, treatment-related death has been reported in all-comers treated with SBRT at 0.6%,59 suggesting a slightly higher risk of treatment-related mortality in patients with IPF.
Oncologists must also consider whether better tolerated cancer treatment options than SBRT exist for these patients with ILD. Other modalities of treatment reported for treatment of early stage lung cancers in patients include radiofrequency ablation (RFA) and minimally invasive surgery. As mentioned, Chen et al completed a systematic review of IPF patients treated for NSCLC. For those treated with SBRT, treatment-related mortality was 15.6% and morbidity was 25%. The rates of treatment-related mortality were significantly lower in SBRT studies with pathologic and radiologic review of ILD severity prior to treatment (7.3% versus 22%).52
Chen et al also found 30 surgical studies in ILD patients with an overall treatment-related mortality rate of 2.2% and morbidity rate of 12%. While these low toxicity rates observed with surgery are impressive, the proportion of patients deemed medically operable was 100% in the surgical studies reported, compared to 0–29% in the other modalities in this review (including SBRT and RFA). Most of these studies were retrospective in nature, with inconsistencies in reporting of ILD severity and proportion of patients with idiopathic pulmonary fibrosis. It is therefore hard to assess the safety of different modalities based on the available data.52
Overall survival rates also depend not only on treatment administered but also on the pretreatment severity of ILD, overall performance status, and comorbidities of the individual patient. In the same review, Chen et al report 3-year OS from four SBRT studies as 0–53.8% for patients with ILD versus 54–80% for those without ILD. Three-year OS for surgical series (extrapolated from 5-year OS using Kaplan–Meier curves) was 31.4–75% for ILD patients compared to 78.5–94.5% for patients without ILD. Again due to the bias in pretreatment status, it is difficult to draw firm conclusions from these numbers.
Dosimetric factors including radiotherapy dose and volume should also be considered while planning SBRT especially in ILD and IPF patients.55 Steroids, and now antifibrotic therapies including nintedanib and pirfenidone, may also play a role in minimizing the adverse events after SBRT.64
Given the risk of potential toxicities due to SBRT, and the poorer prognosis in those patients with baseline ILD, observation may be the most appropriate course in some patients. The expected median survival of early-stage lung cancer is ~12 months without treatment. Expected survival with ILD may be 1–5 years depending on disease severity. Some studies have suggested withholding SBRT with advanced radiologic grades of ILD prior to treatment.55 A standard threshold DLCO and FEV1 have not been determined on pulmonary function tests and may not truly indicate the likelihood of adverse reactions post treatment. The risks of pursuing active treatment must be communicated with the patient, including clinical worsening of dyspnea, oxygen requirement, or death. This must be balanced with the risks of tumor growth without treatment including but not limited to dyspnea, fistula, and lung collapse. In these cases, shared decision-making between patients and providers is of utmost importance.
Conclusion
SBRT has been established as an effective therapy for control and survival in NSCLC. It is desirable to patients given its convenience, minimal invasiveness, decreased morbidity, and tolerable side effects in most cases. Existing literature indicates that long-term toxicities and the effect on QOL appear minimal with SBRT. Patients who are the elderly or with underlying COPD in particular may benefit from SBRT, as it provides an opportunity for cure without exacerbating potentially poor baseline conditions. Tumor location is a factor, and clinicians must be aware of potential risks and be willing to provide risk-adapted SBRT doses that are still effective without increasing the risk of toxicity. Patients with ILD and IPF must be cautiously considered for SBRT and monitored following treatment as severe side effects may develop, inhibiting function and overall health. Further research into toxicity and QOL is warranted in these special patient populations as well.
Disclosure
The authors report no conflicts of interest in this work.
References
Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010 Mar 17;303(11):1070–1076. | ||
Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: A retrospective analysis. Lancet Oncol. 2012 Aug;13(8):802–809 | ||
Rusthoven C, Kavanagh B, Karam S. Improved survival with stereotactic ablative radiotherapy (SABR) over lobectomy for early stage non-small cell lung cancer (NSCLC): addressing the fallout of disruptive randomized data. Ann Transl Med. 2015;3(11):149. | ||
Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;(7):102. | ||
Lagerwaard FJ, Aaronson NK, Gundy CM, Haasbeek CJ, Slotman BJ, Senan S. Patient-reported quality of life after stereotactic ablative radiotherapy for early-stage lung cancer. J Thorac Oncol. 2012;7:1148–1154. | ||
Chen H, Louie AV, Boldt GR, Rodrigues GB, Palma DA, Senan S. Quality of life after stereotactic ablative radiotherapy for early-stage lung cancer: a systematic review. Clin Lung Cancer. 2016;17(5):141–149. | ||
Poghosyan H, Sheldon LK, Leveille SG, Cooley ME. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2013;81(1): 11–26. | ||
Möller A, Sartipy U. Long-term health-related quality of life following surgery for lung cancer. Eur J Cardiothorac Surg. 2012;41(2):362–367. | ||
Sartipy U. Prospective population-based study comparing quality of life after pneumonectomy and lobectomy. Eur J Cardiothorac Surg 2009;36(6):1069–1074. | ||
Aoki T, Tsuchida M, Hashimoto T, Saito M, Koike T, Hayashi J. Quality of life after lung cancer surgery: video-assisted thoracic surgery versus thoracotomy. Heart Lung Circ. 2007;16(4):285–289. | ||
Jain S, Poon I, Soliman H, et al. Lung stereotactic body radiotherapy (SBRT) delivered over 4 or 11 days: a comparison of acute toxicity and quality of life. Radiother Oncol. 2013;108 (2):320–325. | ||
Alite F, Stang K, Balasubramanian N, et al. Local control dependence on consecutive vs. nonconsecutive fractionation in lung stereotactic body radiation therapy. Radiother Oncol. 2016;121(1):9–14 | ||
Sun V, Kim J, Williams AK, Raz DJ, Sampath S, Ferrell B. Quality of life and symptoms following stereotactic body radiotherapy in early-stage lung cancer patients. J Community Support Oncol. 2014;12(11):407–414. | ||
Videtic GM, Reddy CA, Sorenson L. A prospective study of quality of life including fatigue and pulmonary function after stereotactic body radiotherapy for medically inoperable early-stage lung cancer. Support Care Cancer. 2013;21:211–218. | ||
Aoki M, Sato M, Hirose K, et al. Radiation-induced rib fracture after stereotactic body radiotherapy with a total dose of 54–56 Gy given in 9–7 fractions for patients with peripheral lung tumor: Impact of maximum dose and fraction size. Radiat Oncol. 2015;10:99. | ||
Nambu A, Onishi H, Aoki S, et al. Rib fracture after stereotactic radiotherapy for primary lung cancer: prevalence, degree of clinical symptoms, and risk factors. BMC Cancer. 2013;13:68. | ||
Mathieu D, Campeau MP, Bahig H, et al. Long-term quality of life in early-stage non-small cell lung cancer patients treated with robotic stereotactic ablative radiation therapy. Pract Radiat Oncol. 2015;5(4):365–373. | ||
Van der Voort van Zyp NC, Prevést J-B, van der Holt B, et al. Quality of life after stereotactic body radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Physics. 2010;77(1):31–37. | ||
Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;10(28):5153–5159. | ||
Widder J, Postmus D, Ubbels J, Wiegman EM, Langendijk JA. Survival and quality of life after stereotactic or 3D-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):291–297. | ||
Nyman J, Hallqvist A, Lund JA. SPACE—a randomized study of SBRT versus conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol 2016;121(1):1–8. | ||
Siva S, Shaw M, Chesson B, Gill S, Ball D. Analysis of the impact of chest wall constraints on eligibility for a randomized trial of stereotactic body radiotherapy of peripheral stage I non-small cell lung cancer. J Med Imaging Radiat Oncol. 2012;56(6):654–660. | ||
Swaminath A, Wierzbicki M, Parpia S, et al. Canadian phase III randomized trial of stereotactic body radiotherapy versus conventionally hypofractionated radiotherapy for stage I, medically inoperable non-small-cell lung cancer—rationale and protocol design for the Ontario Clinical Oncology Group (OCOG)—LUSTRE Trial. Clin Lung Cancer. 2017; 18(2):250–254. | ||
Brunelli, A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines for evaluating fitness for radical treatment (surgery and chemo-radiotherapy). Eur Respir J. 2009;34(1):17–41. | ||
Bahig H, Filion E, Vu T, et al. Excellent cancer outcomes following patient-adapted robotic lung SBRT but a case for caution in idiopathic pulmonary fibrosis. Technol Cancer Res Treat. 2015;14(6):667–676. | ||
Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637. | ||
Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006; 24(30):4833–4839. | ||
Louie AV, Rodrigues G, Hannouf M, et al. Withholding stereotactic radiotherapy in elderly patients with stage I non-small cell lung cancer and co-existing COPD is not justified: outcomes of a Markov model analysis. Radiother Oncol. 2011;99(2):161–165. | ||
Louie AV, van Werkhoven E, Chen H, et al. Patient reported outcomes following stereotactic ablative radiotherapy or surgery for stage IA non-small-cell lung cancer: results from the ROSEL multicenter randomized trial. Radiother Oncol. 2015;117(1):44–48. | ||
VA Office of Research and Development. Affairs UDoV. CSP #2005—veterans affairs lung cancer surgery or stereotactic radiotherapy trial (VALOR). Available from: https://clinicaltrials.gov/ct/show/NCT02984761?order=1. ClinicalTrials identifier: NCT02984761. Accessed January 19, 2018. | ||
ClinicalTrails.gov. JoLT-Ca A randomized phase III study of sublobar rsection (SR) versus stereotactic ablative radiotherapy (SABR) high risk patients with stage I non-small cell lung cancer (NSCLC). The STABLEMATES trials. Available from: https://clinicaltrials.gov/ct2/show/NCT01622621. ClinicalTrials identifier: NCT01622621. Accessed January 18, 2018. | ||
Shaverdian N, Wang PC, Steinberg M, Lee P. The patient’s perspective on stereotactic body radiation therapy (SBRT) vs. surgery for treatment of early stage non-small cell lung cancer (NSCLC). Lung Cancer. 2015;90(2):230–233. | ||
Kishi K, Gurney JW, Schroeder DR, Scanlon PD, Swenson SJ, Jett JR. The correlation of emphysema or airway obstruction with the risk of lung cancer: a matched case-controlled study. Eur Resp J. 2002;19:1093–1098. | ||
Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys. 2012;82(3):1149–1156. | ||
Baumann P, Nyman J, Hoyer M, et al. Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer—a first report of toxicity related to COPD/CVD in a non-randomized prospective phase II study. Radiother Oncol. 2008;88(3):359–367. | ||
Chambers A, Routledge T, Pilling J, Scarci M. In elderly patients with lung cancer is resection justified in terms of morbidity, mortality and residual quality of life? Interact Cardiovasc Thorac Surg. 2010;10(6):1015–1021. | ||
Haasbeek CJ, Lagerwaard FJ, Antonisse ME, Slotman BJ, Senan S. Stage I nonsmall cell lung cancer in patients age > or = 75 years: outcomes after stereotactic radiotherapy. Cancer. 2010;15:116(2):406–414. | ||
Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non-small cell lung cancer. J Thorac Oncol. 2016;11(7):1081–1089. | ||
Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer. 2015;89(1):50–56. | ||
Roesch J, Panje C, Sterzing F, Mantel F, Nestle U, Andratschke N, Guckenberger M. SBRT for centrally localized NSCLC—what is too central? Radiat Oncol. 2016;11(1):157. | ||
Bezjak A, Paulus R, Gaspar LE, Choy H. Primary study endpoint analysis for NRG Oncology/RTOG 0813 trial of stereotactic body radiotherapy (SBRT) for centrally located non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 2016;94(1):5–6. | ||
Adebahr S, Collette S, Shash E, et al. LungTech, an EORTC Phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. Br J Radiol. 2015;88(1051):20150036. | ||
Stam B, Peulen H, Guckenberger M, et al. Dose to heart substructures is associated with non-cancer death after SBRT in stage I–II NSCLC patients. Radiother Oncol. 2017;123(3):370–375. | ||
Stephans K, Djemil T, Diaconu C, et al. Esophageal dose tolerance to hypofractionated stereotactic body radiation therapy: risk factors for late toxicity. Int J Radiat Oncol Biol Phys. 2014;90(1):197–202. | ||
Forquer JA, Fakiris AJ, Timmerman RD, Lo SS, Perkins SM, McGarry RC, Johnstone PA. Brachial plexopathy from stereotactic body radiotherapy in early-stage NSCLC: dose-limiting toxicity in apical tumor sites. Radiother Oncol. 2009;93(3):408–413. | ||
Davis J, Medbery C, Sharma S, et al. Stereotactic body radiotherapy for centrally located early-stage non-small cell lung cancer or lung metastases from the RSSearch® patient registry. Radiat Oncol. 2015; 10:113. | ||
Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2011;81(4):305–316. | ||
Park H, Harder E, Mancini BR, Decker RH. Central versus peripheral tumor location: influence on survival, local control and toxicity following stereotactic body radiotherapy for primary non-small cell lung cancer. J Thorac Oncol. 2015;10(5):832–837. | ||
Gutsche M, Rosen GD, Swigris JJ. Connective tissue disease-associated interstitial lung disease: a review. Curr Respir Care Rep. 2012;21(1):224–232. | ||
Antoniou KM, Margaritopoulos GA, Tomassetti S, Bonella S, Costabel U, Poletti V. Interstitial lung disease. Eur Respir Rev. 2014;23:40–54. | ||
Troy L, Corte T. Interstitial lung disease in 2015: Where are we now? Thorax. 2015;44(8):546–552. | ||
Chen H, Senan S, Nossent E, Boldt RG, Warner A, Palmer DA, Louie AV. Treatment-related toxicity in patients with early-stage non-small cell lung cancer and coexisting interstitial lung disease: a systematic review. Int J Rad Oncol Biol Phys. 2017;98(3):622–631. | ||
Tsujino K, Hashimoto T, Shimada T, et al. Combined analysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9(7):983–990. | ||
Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol. 1997;169:977–983. | ||
Ueki N, Matsuo Y, Togashi Y, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiotherapy for lung cancer. J Thorac Oncol. 2015;10:116–125. | ||
Yamashita H, Kobayashi-Shibata S, Terahara A, et al. Prescreening based on the presence of CT-scan abnormalities and biomarkers (KL-6 and SP-D) may reduce severe radiation pneumonitis after stereotactic radiotherapy. Radiat Oncol. 2010;5:32. | ||
Yamaguchi S, Ohguri H, Ide S, et al. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: the potential risk of extensive radiation pneumonitis. Lung Cancer. 2013;82(2):260. | ||
Bargagli E, Bonti V, Ferrari K, et al. Lung cancer in patients with severe idiopathic pulmonary fibrosis. In Vivo. 2017;31(4):773–777. | ||
Ozawa Y, Takefumi A, Minako O, et al. Impact of preexisting interstitial lung disease on acute, extensive radiation pneumonitis: retrospective analysis of patients with lung cancer. PLoS One 2015;10(10): e0140437. | ||
Sverzellati, N. Highlights of HRCT imaging in IPF. Respir Res. 2013;14(1):S1–S3. | ||
Bahig H, Filion E, Vu T et al. Severe radiation pneumonitis after lung stereoractic ablative radiation therapy in patients with interstitial lung disease. Pract Radiat Oncol. 2016;6:367-374. | ||
Takeda A, Enomoto T, Sanuki N, Nakajima T, Takeda T, Sayama K, Kunieda E. Acute exacerbation of subclinical idiopathic pulmonary fibrosis triggered by hypofractionated stereotactic body radiotherapy in a patient with primary lung cancer and slightly focal honeycombing. Radiat Med. 2008;26(8):504–507. | ||
Onishi H, Marino K, Terahara A, et al. Case series study of 26 patients who developed fatal radiation pneumonitis (RP) after stereotactic body radiotherapy for lung cancer. Int J Radiat Oncol Biol Phys. 2009;75(3):S62. | ||
Linhas R, Machado D, Campainha S, Neves S, Barroso A. Concomitant lung cancer and interstitial lung disease: a challenge in clinical practice. Rev Port Pneumol. 2017;23(2):104–105. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.