Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 12
Stem-cell therapy for ovariectomy-induced osteoporosis in rats: a comparison of three treatment modalities
Authors Sadat-Ali M , Al-Dakheel DA, AlMousa SA, AlAnii FM, Ebrahim WY, AlOmar HK , AlSayed HN, Acharya S , AlHawaj H
Received 4 February 2019
Accepted for publication 2 April 2019
Published 14 June 2019 Volume 2019:12 Pages 17—25
DOI https://doi.org/10.2147/SCCAA.S204099
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Mir Sadat-Ali,1 Dakheel A Al-Dakheel,1 Sulaiman A AlMousa,1 Fawaz M AlAnii,1 Waleed Y Ebrahim,1 Hussain K AlOmar,1 Hasan N AlSayed,1 Sadananda Acharya,2 Hussain AlHawaj3
1Department of Orthopaedic Surgery; 2College of Public Health; 3Institute of Research and Medical Consultations, Imam Abdul Rahman Bin Faisal University, Dammam, Saudi Arabia
Background: Recent studies have shown that ovariectomy-induced osteoporosis in rats can be reversed by infusion of osteoblasts cultured from mesenchymal stem cells (MSCs). This study compares the influence of MSCs, osteoblasts, and exosomes derived from osteoblasts for the treatment of osteoporosis.
Methods: Osteoporosis was induced in 40 female Sprague Dawley rats by performing ovariectomy. After 12 weeks, bone marrow was harvested and MSCs separated from bone-marrow aspirate as described by Piao et al. After 15 days, autologous osteogenically differentiated cells from the MSCs were available. Exosomes were isolated from osteoblasts by modification of the technique described by Ge et al. MSCs and osteoblasts (106 cells in 0.5 mL normal saline) and exosomes (100 μg protein) were injected into the tail veins of the animals. Animals were euthanized after 12 weeks and femurs and lumbar spines dissected and analyzed using high-resolution peripheral quantitative computed tomography.
Results: When compared to the control group, osteoblast-treated animals showed significant differences in all parameters compared, with P-values ranging between <0.002 and <0.0001. Comparison among osteoblasts, MSCs, and exosomes, showed that osteoblasts had positive and statistically significant new-bone formation. The comparison for the spine was similar to the distal femur for osteoblasts.
Conclusion: This study showed robust positive bone-forming changes after osteoblast injection in the distal femur and the spine when compared to controls, MSCs, and exosomes.
Keywords: osteoporosis, ovariectomy, osteoblasts, mesenchymal stem cells (MSCs), exosomes
Introduction
Osteoporosis is a chronic and debilitating disease of aging. Postmenopausal osteoporosis has come close to becoming an epidemic in the developed and developing world, with increased morbidity and mortality. The “silent disease", as it is rightfully termed, presents with a fracture due to minimal trauma its first indication.5 The true cost of managing osteoporosis-related fractures in Saudi Arabia is still unknown, but a recent study indicated that by 2050, the lifetime cost of treating osteoporosis-related femoral fractures in Saudi Arabia may reach over SR35 billion annually (US$9.33 billion).6 The cost of managing osteoporosis is increasing exponentially, but new modalities of treatment are lagging behind. We still are dependent on antiresorptives, such as bisphosphonates, anabolic agents like synthetic parathyroid hormone, and the newer monoclonal antibody denosumab, and all have their limitations and complications of use.7–12
Preclinical studies have shown promising results in the management of ovariectomy-induced osteoporosis. Kiernan et al2 showed that mesenchymal stem cells (MSCs) injected into a mouse model led to improved bone formation and reversed microarchitecture, suggesting that MSC injections may be used to combat osteoporosis, whereas Sadat-Ali et al1 reported that culture-expanded osteoblasts, when injected in ovariectomized (Ovx) rats, were effective in significantly increasing bone formation.
Exosomes, which are bioactive microvesicles, are secreted by MSCs, and osteoblasts are nanoparticles of 30–100 nm in size and carry proteins and RNAs.13 MSC-derived exosomes have been found to mediate in promoting healing of tissue and repair of acute and chronic injuries.14
With the increasing number of aged people around the world and increase in the longevity of the human race, osteoporosis is bound to trouble health-care economics, added to the fact that research and development of new osteoporosis drugs are at a standstill. Stem-cell therapy opens a new avenue in the treatment of osteoporosis if the reversal of osteoporosis in animal studies is confirmed.
The objective of this study was to compare the efficacy of culture expanded MSCs, osteoblasts, and osteoblast-derived exosomes on the effect of Ovx-induced osteoporosis in rats.
Methods
Ethical approval was obtained from Imam AbdulRahman Bin Faisal University, Dammam, Saudi Arabia (vide number 2015116/2017), 40 Sprague Dawley female rats were used as our model to study reversal of osteoporosis. The rats were housed and handled in accordance with the National Advisory Committee for Laboratory Animal Research guidelines. Animals were accommodated with total mobility, fed a standard diet, and the room maintained at 26°C. Ovariectomy was done at 1 month of age to cause osteoporosis. At 12 weeks, a bone biopsy was performed to look at the quality of bone. Bone marrow was aspirated at the time of the biopsy. From the bone-marrow aspirate of the individual rats, using the technique described by Piao et al,3 MSCs were separated. In this study, no MSCs from other species, such as human or BALB/c mouse were used. For each individual rat, MSCs obtained from the bone-marrow aspirate and osteoblasts cultured from the cell suspension, as descrbed earlier.2 After 2 weeks, osteoblasts were ready to be injected.
Isolation of exosomes from rat bone-marrow MSC–derived osteoblasts
MSC-derived osteoblasts were seeded onto T75 cell-culture flasks (Nalge Nunc International, New York, NY, USA) at a rate of 103 cells/cm2 alongside 20 mL basal DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% exosome-depleted FBS (Thermo Fisher Scientific), 5 mM L-glutamine (Thermo Fisher Scientific) , 100 IU/mL penicillin (Sigma-Aldrich, St Louis, MO, USA) and 100 μg/mL streptomycin (Sigma-Aldrich). Cells were allowed to attach and grow up to 4 days in the same medium at 37°C at 5% CO2 and 95% humidity in a CO2 incubator. Culture supernatant containing osteoblast exosomes was aseptically collected from five of such flasks into two 5 mL cenTrifuge tubes. Adhered osteoblast cells were harvested and frozen for further use. The culture supernatant was further subjected to ultracentrifugation to pelletize exosomes. If present, any cells were removed by spinning the supernatant at 300 g for 10 minutes at 4°C. Dead cells in the supernatant were removed by centrifugation at 2,000 g for 10 minutes at 4°C. Further cenTrifugation at 10,000 g for 30 minutes at 4°C was done to pelletize and remove cell debris. Finally, the supernatant was ultracentrifuged (Sorvall WX) at 100,000 g for 70 minutes at 4°C under vacuum to pelletize exosomes. Contaminated protein in the pelletized exosomes was removed by repeated washing in normal saline solution (0.85% NaCl) before final pelletization at the same speed. Pelletized exosomes were resuspended in normal saline solution and concentration measured (as 100 µg/mLprotein) and aliquoted for further use.
Animals were divided into four groups: group 1 (control) received injected normal saline, group 2 MSCs, group 3 osteoblasts, and group 4 exosomes. MSCs and osteoblasts of 106 cells in 0.5 mL normal saline and (for exosomes) 100 µg protein were injected into the tail veins of the animals.
Rats were killed after 8 weeks of MSC, osteoblast, and exosome infusion by overdose of ketamine mixed with xylazine. The study bones were dissected, stored in 1% formalin, and grouped. The specimens were shipped to B-Cube , Brüttisellen, Switzerland. New-bone formation and bone-strength parameters were measured and calculated as reported earlier.2
Statistical analysis
Each sample was measured three times and an average taken to have acceptable precision.2 Data were analyzed using SPSS version 21 with the level of statistical significance set at <0.05.
Results
There were no deaths in any of the groups. When compared to the control group for distal femur, osteoblast-treated animals had significant differences in most of the parameters compared, with P-values ranging between <0.002 and <0.0001 (Table 1). Trabecular number and thickness were significantly higher in the osteoblast group than the other groups: 3.695±1.300–1.562±0.297 (MSC group) and 1.610±0.184 (exosome group). Trabecular spacing was 0.240±0.087 in the osteoblast group, 0.590±0.119 in the MSCs group, 0.566±0.079 in the exosome group, and 0.062±0.002 in the control group.
 | Table 1 Structural indices of distal femur in the four groups analyzed by high-resolution peripheral quantitative computed tomography |
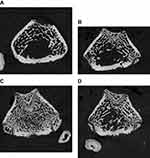
Figure 1 shows high-resolution peripheral quantitative computed tomography scans of the distal femur in the four groups. The osteoblast group showed more bone formation than the other two groups (MSC and exosomes). Figure 2, A shows saggital sections of the distal femur, showing a dense trabecular pattern in the osteoblast group compared to the other groups.
For control versus MSC groups, the later was significant only in total volume, thickness of trabeculae, and connectivity density (P<0.09, 0.04, and 0.05, respectively; Table 2), whereas in the exosome group significant parameters were trabecular thickness (P<0.002), trabecular number (P<0.01), connective density (P<0.007), and trabecular spacing (P<0.002; Table 3). Table 4 compares the control and exosome groups, showing better index values for the latter.
 | Table 2 Comparison between control versus MSCs for distal femur |
 | Table 3 Comparison between control versus osteoblasts for distal femur |
 | Table 4 Comparison between control versus exosomes for distal femur |
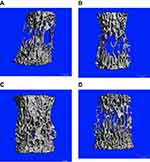
Table 5 shows ththat the four groups of spine showed similarity with regard to the distal femur. Trabecular numbers (1/mm) in the osteoblast group were 4.644±0.378, 2.643±0.237 (MSC group), and 3.018±0.279 (exosome group), while trabecular spacing was the lowest in the osteoblast group (0.147±0.029), with 0.269±0.036 in the exosome group and 0.314±0.032 in the the MSC group. Tables 6–8 highlight comparisons among the groups. Figure 3A shows scans of the fourth lumbar vertebra in the four groups, depicting wide spaces in the control and MSC groups when compared to the osteoblast and exosome groups.
 | Table 5 Structural indices of spine in the four groups as analyzed by HRpQCT |
 | Table 6 Comparison between control versus MSCs for spinal indices |
 | Table 7 Comparison between control versus osteoblasts for spinal indices |
 | Table 8 Comparison between control versus exosomes for spinal indices |
Discussion
Our study shows that Ovx-induced osteoporosis in rats can be reversed and the efficacy of osteoblasts is far superior to MSCs and osteoblast-extracted exosomes. Earlier studies have shown that MSCs influence bone formation in Ovx-induced osteoporosis, but this comparative study shows that MSCs does have positive effects, but not to the extent of osteoblasts. Osteoblast-derived exosomes also had a significant impact on some analyzed parameters, such as MSCs and osteoblasts.
Bone strength comes from bone volume, trabecular number, thickness, and connectivity, density and lack of any of these deprives the bone of mechanical strength.13 Both MSCs and exosomes increased number and thickness with connectivity density, but showed no significant increase in bone volume. The increase in trabecular number and thickness without increasing actual bone volume does not help in reversing the low bone mass related to osteoporosis.
It has been reported that 200 million people suffer from osteoporosis and that it causes 8.9 million fractures,14 with expected cost of treatment of osteoporosis fractures in the US to surpass $25 billion.15 Assessment from China reported that by 2050, the cost of fractures due to osteoporosis will reach 1 million fractures, costing $25.43 billion.16 These high figures are present despite the preventive measures and awareness of osteoporosis and its complications. There are a couple of reasons for this. As the population is aging and people are living longer, they suffer from osteoporosis for longer periods.
Physicians are looking for new therapies because of complications in presently available potent drugs. However, emerging therapies for the management of osteoporosis got halted when two promising drugs were discontinued due to a higher risk of complications. Romosozumab was reported to have more cardiovascular events compared with alendronate.17 Odanacatib, a selective inhibitor of cathespin K to decrease bone resorption, was discontinued due to an increased risk of stroke.18 Many researchers believe that medications with novel mechanisms to treat osteoporosis can be expected in the future, but they do not know when.19–21 Our preclinical study has shown that stem-cell therapy could be one of the novel treatment modalities. In line with this, we have decided to replicate the study in a larger animal.
In conclusion, this study shows that osteoblasts are potent terminal cells that could be used in the reversal of osteoporosis, while MSCs and exosomes showed positive changes that were partial. The efficacy of exosomes needs to be further evaluated by giving a higher µg/mL protein to see the effect on bone formation.
Acknowledgment
The authors thank Dr Fares Uddin for his tireless support to this project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sadat-Ali M, Al-Turki HA, Acharya S, Al-Dakheel DA. Bone marrow-derived osteoblasts in the management of ovariectomy induced osteoporosis in rats. J Stem Cells Regen Med. 2018;14(2):1–6.
2. Kiernan J, Hu S, Grynpas MD, Davies JE, Stanford WL. Systemic mesenchymal stromal cell transplantation prevents functional bone loss in a mouse model of age-related osteoporosis. Stem Cells Transl Med. 2016;5(5):683–693. doi:10.5966/sctm.2015-0231
3. Piao H, Youn T-J, Kwon J-S, et al. Effects of bone marrow derived mesenchymal stem cells transplantation in acutely infarcting myocardium. Eur J Heart Fail. 2005;7(5):730–738.
4. Ge M, Ke R, Cai T, Yang J, Mu X. Identification and proteomic analysis of osteoblast-derived exosomes. Biochem Biophys Res Commun. 2015;467(1):27–32. doi:10.1016/j.bbrc.2015.09.135
5. Chopra A. Osteoporosis: A new understanding of its impact and pathogenesis. J Am Osteopath Assoc. 2000;100(1 suppl):S1–4.
6. Sadat-Ali M, Al-Dakheel DA, Azam MQ, et al. Reassessment of osteoporosis-related femoral fractures and economic burden in Saudi Arabia. Arch Osteoporos. 2015;10:37.PMID:26494131. 10.1007/s11657-015-0240-5
7. Graham DY. What the gastroenterologists should know about the gastrointestinal safety profiles of bisphosphonates. Dig Dis Sci. 2002;47:1665–1678.
8. de Groen PC, Lubbe DF, Hirsch LJ, et al. Esophagitis associated with the use of alendronate. N Engl J Med. 1996;335:1016–1021. doi:10.1056/NEJM199610033351403
9. Rupel K, Ottaviani G, Gobbo M, et al. A systematic review of therapeutical approaches in bisphosphonates-related osteonecrosis of the jaw (BRONJ). Oral Oncol. 2014;50:1049–1057; pii:S1368-8375(14)00250-4. 10.1016/j.oraloncology.2014.08.016
10. Holzinger D, Seemann R, Matoni N, Ewers R, Millesi W, Wutzl A. Effect of dental implants on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2014;72(10):
11. Kharazmi M, Hallberg P. Bisphosphonate-associated atypical femoral fractures and one-year mortality. Ups J Med Sci. 2014;119(4):357–358.
12. Bhadada SK, Sridhar S, Muthukrishnan J, et al. Predictors of atypical femoral fractures during long term bisphosphonate therapy: A case series & review of literature. Indian J Med Res. 2014;140(1):46–54.
13. Thompson D. On Growth and Form. New York, NY: Macmillan Co.; 1945:1116.
14. Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8:136. doi:10.1007/s11657-013-0136-1
15. Burge R, Dawson-Hughes B, Solomon D, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–475. doi:10.1359/jbmr.061113
16. Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of osteoporosis-related fractures and costs in China: 2010–2050. Osteoporos Int. 2015;26:1929–1937.
17.
18. Mullard A. Merck & Co. drops osteoporosis drug odanacatib. Nat Rev Drug Discov. 2016;15(10):669–671.
19. Camacho PM, Petak SM, Binkley N, et al. American association of clinical endocrinologists and American College of Endocrinology: clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2016;22(suppl 4):S1–S42. doi:10.4158/1934-2403-22.12.e1
20. Cosman F, de Beur S, LeBoff M, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. Erratum in: Osteoporos Int. 2015;26(7):2045–2047.
21. Buckley L, Guyatt G, Fink HA, et al. American College of Rheumatology guideline for the prevention and treatment of gluco-corticoid-induced osteoporosis. Arthritis Care Res (Hoboken). 2017;69(8):1095–1110. doi:10.1002/acr.22950
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.