Back to Journals » International Journal of General Medicine » Volume 14
STC1 is a Novel Biomarker Associated with Immune Characteristics and Prognosis of Bladder Cancer
Authors Sun J , Wei X, You J, Yue W, Ouyang J, Ling Z, Hou J
Received 21 July 2021
Accepted for publication 1 September 2021
Published 11 September 2021 Volume 2021:14 Pages 5505—5516
DOI https://doi.org/10.2147/IJGM.S329723
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jiale Sun,1,* Xuedong Wei,1,* Jiawei You,1 Wenchang Yue,1 Jun Ouyang,1 Zhixin Ling,1 Jianquan Hou1,2
1Department of Urology, The First Affiliated Hospital of Soochow University, Suzhou, People’s Republic of China; 2Department of Urology, Dushu Lake Hospital Affiliated to Soochow University, Suzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhixin Ling; Jianquan Hou
Department of Urology, The First Affiliated Hospital of Soochow University, 188 Shizi Street, Suzhou, 215006, Jiangsu, People’s Republic of China
Email [email protected]; [email protected]
Background: Stanniocalcin-1 (STC1) is a well-studied oncogene that promotes different types of cancer progression. However, the expression status of STC1, the values of STC1 on prognosis, and its immune characteristic in bladder cancer (BLCA) have not been well examined.
Methods: The expression of STC1 and its clinicopathological as well as immune characteristics in BLCA samples were firstly identified in The Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus (GEO) databases. Immunohistochemistry (IHC) performed on the tissue microarray (TMA) slide was further used to validate the expression of STC1 and its relationship with immune features in 16 non-muscle invasive bladder cancer (NMIBC) samples and 42 muscle invasive bladder cancer (MIBC) samples.
Results: The expression of STC1 was upregulated in higher stage BLCA. High STC1 expression also predicted poor prognosis in BLCA. Subsequently, the TMA validated the expression and prognostic value of STC1 in BLCA. Bioinformatics analysis demonstrated that STC1 and common immune checkpoints as well as immune markers of various immune cells were positively correlated in TCGA. In addition, IHC data from the TMA further validated that tumor cells with higher STC1 level tended to express higher PDL1 as well as increased infiltration of CD3+ T cells.
Conclusion: To our knowledge, this is the first comprehensive study that investigates the clinical and immune characteristics of STC1 in BLCA. It may provide new insight into the function of STC1 in regulating tumor immune microenvironment. Further studies are warranted to uncover the potential mechanisms that mediate STC1 expression and tumor immunity in BLCA.
Keywords: STC1, bladder cancer, tissue microarray, immune characteristics, immune checkpoints
Introduction
Bladder cancer (BLCA) is the second most frequently diagnosed urinary cancer, with an estimated 573,000 new cases and 213,000 deaths worldwide in 2020.1 Although non-muscle invasive bladder cancer (NMIBC) accounts for approximately 75% of all BLCA,2 the major concern is its high rates of recurrence, progression and even metastasis after appropriate primary curative treatment or sequential intravesical therapy.3 Subsequently, cisplatin-based combination chemotherapy is widely used as the standard first-line systemic treatment for patients who present with muscle-invasive bladder cancer (MIBC) or metastatic disease.4 Nevertheless, even with this kind of therapy, the median overall survival is still poor, which is estimated to be about 12–14 months.5 In the recent few years, second-line immunotherapy with immune checkpoint inhibitors (ICIs) has been established and is still being studied in BLCA treatment.6 Several multi-center randomized controlled trials have demonstrated that these ICIs such as PD1/PDL1 inhibitors showed superior clinical benefits over chemotherapy.7–10 Unfortunately, these studies also found that only a minority of patients could respond to common ICIs. Therefore, it could be of great significance to discover more novel immune-related biomarkers and targets for the assessment and treatment of BLCA.
Stanniocalcin-1 (STC1) is a 56kDa homodimeric glycoprotein hormone that was first identified in bony fish and was then identified in human in 1995.11 Both fish and human STC are involved in plasma calcium and phosphate homeostasis. However, in contrast to fish STC, which is expressed mainly in the corpuscles of Stannius, human STC1 is expressed in multiple tissues and is not detected in the circulation under normal circumstances.12 It means that human STC1 could possibly be associated with cellular immortalization, a key feature of the cancer cell phenotype. In recent years, accumulating evidence has proved that STC1 plays a crucial role in different types of cancer13 and is involved in multiple cancer-related signaling pathways, such as NOTCH1-SOX2,14 NF‐kB15 and JNK signaling pathways.16 Of them, NF-kB was previously reported to suppress apoptosis and promotes BLCA cell proliferation by upregulating survivin expression.17 JNK was also proved to play an important role BLCA cell invasion.18 In addition, several clinical studies have also revealed that STC1 may have a negative correlation with prognosis.19,20 However, more detailed and comprehensive reports of STC1 and its association with clinicopathological feature in BLCA are still lacking.
Furthermore, the expression of tumor STC1 correlates with immunotherapy efficacy and is negatively associated with patient survival across diverse cancer types, which is recently reported.21 STC1 could mediate tumor immunity by functioning as an intracellular “eat-me” signal blocker, indicating that STC1 could possibly be used as a significant target for immunotherapy. In addition, among all the genitourinary cancer, BLCA has received the most extensive tumor immunotherapy in clinical practice. Tumor immunology has been proven to play a vital role in BLCA progression and prognosis.22 Given the fact that BLCA is a hot spot in tumor immunotherapy, we conducted this study to identify the relationship between STC1 and BLCA.
Therefore, in the present research, we comprehensively investigated the clinical relevance of STC1 in BLCA specimens, explored its associations with clinical characteristics and tumor immunity in BLCA by analyzing RNA sequencing (RNA-seq) data from The Cancer Genome Atlas (TCGA) database and the Gene Expression Omnibus (GEO) database. Besides, the protein level of STC1 and its correlations with PDL1 as well as tumor infiltrating lymphocytes (TIL) in BLCA was validated by immunohistochemistry (IHC) staining via tumor tissue microarray (TMA). Our results revealed the correlation of STC1 expression with clinical characteristics and immunoregulatory factors in BLCA and we expect that our findings will give a more comprehensive insight into the role of STC1 in BLCA.
Methods and Materials
Acquisition and Preprocess of Public Data
The data of RNA-seq (FPKM value) and clinical information in TCGA-BLCA database were downloaded from UCSC Xena data portal.23 Data on RNA-seq were log2 transformed. Besides, two BLCA GEO cohorts with detailed clinical data were downloaded, namely GSE13507 and GSE128959. For further analysis, a total of 430 samples from TCGA database and 456 samples from two GEO cohorts, which contained both gene expression and clinical data were extracted.
Screen of Co-Expressed Immune Genes and Enrichment Analysis
To identify the STC1 associated immune signature in BLCA, immune-related genes defined by the ImmPort database were selected.24 Immune-related genes that correlated with STC1 expression (Pearson |R| ≥ 0.2) in TCGA database were further extracted. Totally, 377 immune-related genes in TCGA-BLCA database were eligible for subsequent analysis. Next, the Database for Annotation, Visualization and Integrated Discovery (DAVID)25 was employed to perform Gene Ontology (GO) and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis of STC1 co-expression immune genes. Top 10 enrichment terms were exhibited in this research.
Tissue Microarray and Immunohistochemistry
The TMA slide (HBlaU079Su01) was obtained from Outdo Biotech Co. Ltd. (Shanghai, China). The slide contained samples from 63 patients with stage I–IV BLCA. Next, IHC staining was performed directly on this slide. The primary antibodies were as following: anti-STC1 (ab229477, Abcam), anti-CD3 (ab16669, Abcam), anti-PDL1 (PA167, Abcarta). Antibody staining was visualized with DAB and hematoxylin counterstain (ZSGB-Bio). Ethical approval for the study of TMA was granted by the Clinical Research Ethics Committee, Outdo Biotech (Shanghai, China) in accordance with the Declaration of Helsinki.
Evaluation of IHC Staining
The TMA slides were digitally scanned using Aperio Digital Pathology Slide Scanners. All results of IHC were evaluated using an established semi-quantitative approach by two independent pathologists in a blind manner. According to the intensity of the staining, the positive reaction of STC1 was scored into four grades: 0 (negative), 1 (low), 2 (moderate) and 3 (high). The percentages of STC1 positive cells were also scored into five grades: 0 (0%), 1 (<10%), 2 (10–50%), 3 (51–80%) and 4 (>80%). The immunoreactive score (IRS) gives a range of 0–12 as a product of multiplication between the intensity and percentage scores. PDL1 expression was assessed by tumor proportion score (TPS), which is defined as the number of positive tumor cells divided by the total number of viable tumor cells multiplied by 100%. CD3 is the surface marker of mature T cells and is used to detect T helper and cytotoxic T cells. Therefore, tumor infiltrating lymphocytes (TIL) infiltration was assessed as a continuous variable based on the percentage of stromal tissue area occupied by CD3+ T cells.
Statistical Analysis
All statistical analysis and figure exhibitions were implemented using R 4.0.3 (R Foundation, Vienna, Austria) and GraphPad Prism 9.0 (GraphPad Inc, San Diego, CA, USA). Continuous variables fitting a normal distribution between binary groups were compared using a t-test. Otherwise, the Mann–Whitney U-test was applied. Correlation analysis was evaluated by Pearson method if variables fit a normal distribution. Otherwise, Spearman correlation analysis was applied. Log rank test was performed to assess the difference between the survival curves. All tests with two-sided P < 0.05 were considered statistically significant.
Results
The Association of STC1 Expression with the Clinicopathological Characteristics and Prognosis of Patients with BLCA
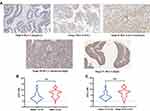
We firstly analyzed the expression of STC1 in BLCA according to the clinicopathological characteristics in TCGA database and two BLCA GEO cohorts. In TCGA database, STC1 mRNA levels in stage II BLCA was significantly lower than that in stage III and stage IV BLCA (Figure 1A). In addition, STC1 mRNA levels were higher in advanced BLCA (pT3~T4) than in early stage of BLCA (pT0~T2) (Figure 1B). However, the difference of STC1 expression between low- and high-grade BLCA in TCGA database was not significant (P=0.07) (Figure 1C). Kaplan–Meier analysis of overall survival (OS) illustrated that BLCA patients with higher STC1 expression exhibited significantly shorter OS compared with that with lower STC1 expression (Figure 1D). In GSE13507, STC1 mRNA levels in NMIBC were significantly lower than that in MIBC (Figure 1E). In GSE128959, the results also showed that STC1 expression was statistically positively correlated with BLCA T stage (Figure 1F).
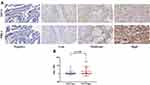
Next, IHC staining were performed to clarify the STC1 expression in our BLCA cohort. The detailed information of patients and TMA were shown in Supplementary 1–4. Representative images showed the samples with different stage stained with STC1 (Figure 2A). Compared with NMIBC, samples with MIBC expressed higher STC1 (Figure 2B). The IRS of STC1 were higher in stage III~IV BLCA than in stage I~II BLCA (Figure 2C). We also divided BLCA patients into low- and high-expression groups based on the median IRS of STC1 to evaluate its prognostic value. As shown in Figure 3A, patients with higher STC1 expression exhibited significantly shorter OS compared with those with lower STC1 expression in our recruited cohort. Moreover, IRS of STC1 in BLCA samples was negatively correlated with OS (Figure 3B) and dead patients expressed higher STC1 compared with the alive patients (Figure 3C).
STC1 Was Correlated with Common Immune Checkpoints Expressions in BLCA
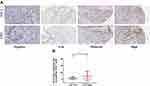
Recent evidence showed that STC1 played a crucial role in mediating tumor immunity.21 Therefore, we examined the potential correlations between STC1 and immune-related molecules in BLCA. First, we examined the relationships between STC1 and common immune checkpoints, and the results showed that positive correlations between STC1 and PDL1, PD-L2, OX40L, TIM3, OX40, FOXP3, CTLA4, B7H3 in TCGA database (Figure 4). Given the significant role of PDL1 in immunotherapy, we further validated the positive correlation between STC1 and PDL1 using IHC staining in our cohort. Representative images showed the samples stained with STC1 and PDL1 (Figure 5A), and tumor with higher STC1 expression tended to express higher PDL1 (P=0.055) (Figure 5B).
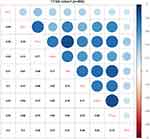 |
Figure 4 Association between STC1 and common immune checkpoints expressions in TCGA database. |
STC1 Expression is Related to Density of CD3+ T Cells Infiltration in BLCA
The correlation between STC1 and CD3+ T cells infiltrating in BLCA was also validated using IHC staining in our cohort. Representative images showed the samples stained with STC1 and CD3+ T cells (Figure 6A). As were also shown in Figure 6B, tumor with higher STC1 expression tended to express higher density of stromal CD3+ T cells.
STC1 Was Associated with Various Immune Cells in BLCA
Subsequently, we analyzed the correlations between STC1 and representative immune markers of various immune cells, including general T cells, CD8+ T cells, CD4+ T cells, T helper 1 (Th1) cells, Th2 cells, Th17 cells, Treg cells, follicular helper T (Tfh) cells, B cells, M1 macrophages, M2 macrophages, tumor-associated macrophages, neutrophils and dendritic cells in BLCA (Table S1). Interestingly, the STC1 expression level showed significantly positive correlations with the expression of specific markers of immune cells in TCGA database, namely, general T cell markers (CD3D, CD3E, CD2, CD7), CD8+ T cell markers (CD8A, CD8B, CD45R), CD4+ T markers (CD4, IL7RA, BTLA, PDCD1), Th1 cell markers (STAT1, STAT4, CXCR3, CCR5), Th2 cell markers (CXCR6, CCR3), Th17 cell markers (STAT3, IL17RA), Treg cell markers (FOXP3, TGFB, ITGA4, CCR8), Tfh cell markers (CD84, CD83), B cell markers (CD20, CD79A, CD79B), M1 macrophage markers (FCGR3A, CD86, NOS2), M2 macrophage markers (CD163, VSIG4, ARG1), tumor-associated macrophage markers (IL10, CD206, CCL2, CCL5), neutrophil markers (CD11b, CXCR1) and dendritic cell markers (TLR7, HLA-DPB1, CD1c, CD1e). These results indicated that higher STC1 expression in the BLCA microenvironment resulted in greater immune cell infiltration compared with BLCA with lower STC1 expression.
GO and KEGG Analysis of STC1 Co-Expression Immune Genes
Lastly, to further explore the STC1-associated immune signatures in BLCA, 377 immune-related genes that correlated with STC1 expression (Pearson |R| ≥ 0.2) were identified in TCGA database. As were shown in the heatmap, the majority of genes (318 genes) were positively correlated with STC1 expression, while the minority of genes (59 genes) were inversely correlated with STC1 expression (Figure 7, Supplementary 5). DAVID was next used to perform GO and KEGG pathway analysis to clarify the biological processes, molecular functions, cellular components and pathways of these genes. As shown in Figure 8, these results also suggested that STC1 could play a crucial role in immune functions in BLCA.
Discussion
For the past decade, a number of ICIs have been approved as first-line therapy for cisplatin-ineligible BLCA or as second-line therapy for metastatic BLCA. However, only 30% of these patients could respond to common ICIs immunotherapy.26 In order to improve the prognosis of patients with BLCA, uncovering novel personalized immunotherapeutic strategies are extreme urgencies that need to be well addressed. In this study, the clinical significance of STC1 in BLCA was systematically analyzed. Moreover, we found that the expression of STC1 was closely associated with immune checkpoints and immune cell infiltration levels. In short, our study provides a new understanding of the role of STC1 in BLCA.
Several research groups have tried to define the clinical value of STC1 expression in cancer. In gastric cancer, Fang et al27 demonstrated that the high/moderate of STC1 protein was significantly associated with lymph metastasis, clinical stage and adverse 3-year progression-free survival. In hepatocellular carcinoma, Chan et al16 reported that a higher serum STC1 level was correlated with larger tumor size and poorer 5-year disease-free survival. Another study also reported that the level of STC‑1 expression was directly associated with the relapse‑free and OS of basal‑type breast cancer patients.28 However, the role of STC1 in BLCA has so far not been available yet. Here, we firstly took advantage of bioinformatics analysis and found that STC1 was notably overexpressed in advanced BLCA, upregulated in MIBC compared with NMIBC and could predict poor survival rate, suggesting that STC1 expression was correlated with a more aggressive biological process. Furthermore, to acquire a more convinced conclusion, we performed IHC on the BLCA tissue via TMA technique. The results were highly consistent with that acquired from the bioinformatics analysis.
Although no relevant literature has reported the potential carcinogenic mechanism of STC1 in BLCA so far, Bai et al29 previously reported that STC1 promoted prostate cancer cell proliferation and correlated with the expression levels of cell cycle-related proteins, cyclin E1/CDK2. In line with their results, another study also reported that STC1 could promote cell cycle progression, accelerate G1/S transition through elevating the expression of cyclin D1, Cdk4 and Cdk6, and suppressing p21 in clear cell renal cell carcinoma.30 Therefore, STC1 may possibly promote BLCA cell proliferation via a mechanism through which several cyclins and CDKs are recruited.
Our study also demonstrated that the expression of STC1 and some common immune checkpoints in BLCA were positively correlated. The positive correlation between STC1 and PDL1 was further validated using IHC staining in our cohort, suggesting that targeting STC1 might be a strategy to improve the efficiency of immunotherapy. In fact, a number of former studies have demonstrated that a combination of PDL1/PD-1 and some immune-related molecules may enhance antitumor immunity. A preclinical study showed that combined blockade of PD-1/PDL1 and IL-6 signals resulted in synergistic anti-tumor effects.31 Zeng et al32 reported that targeting the CXCR4-CXCL12 axis in combination with PD-1/PDL1 could inhibit tumor growth and prevent multifaceted immunosuppression in ovarian cancer. In melanoma, the result of a double-blind, Phase 3 clinical study suggested that nivolumab (a PD-1 checkpoint inhibitor) combined with ipilimumab (a CTLA-4 checkpoint inhibitor) resulted in significantly longer progression-free survival than ipilimumab alone.33 Therefore, based on our study, STC1 might be as a novel immune-related molecule to enhance immunotherapy efficiency in combination with PDL1 in the future.
Moreover, our findings were in line with the result of a former study, which mechanistically clarified the immunoregulatory role of STC1 in cancer.21 It is known that BLCA is an immunosensitive tumor, which is infiltrated by T cells, macrophages, dendritic cells, neutrophils and mast cells. Furthermore, among all types of cancer, BLCA is associated with one of the highest mutation burdens, which is a known predictor of treatment response to checkpoint. These mutations could also add more complexity to the tumor microenvironment.34 Meanwhile, it is known that calreticulin translocation to the cell membrane serves as an “eat me” signal, thereby promoting efferocytosis of cancer cell.35 However, tumor STC1 interacts with calreticulin and minimizes calreticulin membrane exposure. Thus, this impairs membrane calreticulin-directed antigen-presenting cells (macrophages and dendritic cells) phagocytosis and T cell activation. Interestingly, our study also revealed the tight correlation between STC1 and immune features in BLCA, suggesting that STC1 exactly plays a prominent role in regulating tumor immune microenvironment.
The immune cell infiltration has been verified to play critical roles in the development and progression of various cancers.36 In our study, the relationship between the expression of STC1 and immune features in BLCA was comprehensively analyzed by the public datasets and our TMA samples. It showed that the expression of STC1 was positively correlated with various immune infiltrating cells. Generally speaking, a dense TIL is a common characteristic of tumors that have a favorable prognosis. However, a significant correlation between dense TIL and poor prognosis has also been reported in several solid tumors, including BLCA.37–41 Krpina et al37 found that the number of CD3+ and CD8+ lymphocytes observed in the non-recurrent group of BLCA patients was lower than that in recurrent patients. Patschan et al38 assessed CD3+ T cells infiltration in a cohort of primary T1 BLCA using TMA and the result showed that high levels of CD3+ T cells were significantly associated with poor prognosis and progressive disease. Rouanne et al39 also reported that the density of stromal TILs was not associated with a better clinical outcome in BLCA. This dual role of TIL in promoting or suppressing tumor growth is still one of the most challenging questions in immunology.42 Based on our study, a scenario that high STC1 expression is positively related to dense TIL, which causes poor clinical outcomes in BLCA can be envisioned. With the development of BLCA, tumor-specific adaptive immune response is enhanced via propagating the expansion of immune cells. Simultaneously, more tumor STC1 interacts with calreticulin, thereby abrogating membrane calreticulin-directed phagocytosis by antigen-presenting cells. Although the immune response is still being strengthened, as the “eat-me” signal is blocked, tumor cells still acquire the ability to escape STC1-related immune recognition.
The study has several limitations. Firstly, since cancer tissues could show high heterogeneity, TMA sections, as a part of the whole tumor tissue, may not be representative of the entire tumor insofar as STC1 expression. Secondly, participants involved in this study had no history of immunotherapy, which means the prognostic value of STC1 in BLCA after immunotherapy is not studied. Lastly, a relatively small number of BLCA samples were included in our study. Future studies with a larger patient sample size are warranted.
Conclusion
To our knowledge, this is the first comprehensive study that investigates the associations between STC1 expression and clinicopathological characteristic of BLCA. More importantly, via bioinformatics, we found that STC1 was potentially positively correlated with a variety of immune-related genes and well-known immune checkpoints in BLCA. Subsequently, the results of TMA identified the relationship between STC1 and PDL1 as well as CD3+ T cells. Further studies are warranted to uncover the potential mechanisms that mediate STC1 expression and tumor immunity in BLCA.
Abbreviations
BLCA, bladder cancer; NMIBC, non-muscle invasive bladder cancer; MIBC, muscle-invasive bladder cancer; ICIs, immune checkpoint inhibitors; STC1, Stanniocalcin-1; RNA-seq, RNA sequencing; TCGA, The Cancer Genome Atlas; GEO, Gene Expression Omnibus; TIL, tumor infiltrating lymphocytes; IHC, immunohistochemistry; TMA, tissue microarray; DAVID, Database for Annotation, Visualization and Integrated Discovery; KEGG, Kyoto Encyclopedia of Genes and Genomes; GO, Gene Ontology; IRS, immunoreactive score; TPS, tumor proportion score; OS, overall survival.
Data Sharing Statement
The RNA sequencing data and related clinical information were obtained from TCGA (http://cancergenome.nih.gov/) databases and GEO databases (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE128959) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE13507). All other data and materials are available from the corresponding authors upon reasonable request.
Funding
This work was supported by grants from National Natural Science Foundation of China (NO.82002715, NO.81472401, NO. 81772708), Natural Science Foundation of Jiangsu Province (BK20190170), Science and Technology Program of Suzhou City (No. SLJ201906) and Programs for Recruitment of Clinical Medical Top Team of Suzhou.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249.
2. Tan WS, Kelly JD. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat Rev Urol. 2018;15(11):667–685. doi:10.1038/s41585-018-0092-z
3. Kufukihara R, Kikuchi E, Ogihara K, et al. Role of Previous Malignancy History in Clinical Outcomes in Patients with Initially Diagnosed Non-Muscle Invasive Bladder Cancer. Ann Surg Oncol. 2021;28(9):5349–5359. doi:10.1245/s10434-021-09750-0
4. Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82–104. doi:10.1016/j.eururo.2020.03.055
5. Bellmunt J, Petrylak DP. New therapeutic challenges in advanced bladder cancer. Semin Oncol. 2012;39(5):598–607. doi:10.1053/j.seminoncol.2012.08.007
6. Stenehjem DD, Tran D, Nkrumah MA, Gupta S. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco Targets Ther. 2018;11:5973–5989. doi:10.2147/OTT.S135157
7. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi:10.1056/NEJMoa1613683
8. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–322. doi:10.1016/S1470-2045(17)30065-7
9. Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized Phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30(6):970–976. doi:10.1093/annonc/mdz127
10. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. doi:10.1016/S0140-6736(16)32455-2
11. Chang AC, Janosi J, Hulsbeek M, et al. A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol Cell Endocrinol. 1995;112(2):241–247. doi:10.1016/0303-7207(95)03601-3
12. Yoshiko Y, Aubin JE. Stanniocalcin 1 as a pleiotropic factor in mammals. Peptides. 2004;25(10):1663–1669. doi:10.1016/j.peptides.2004.04.015
13. Zhao F, Yang G, Feng M, et al. Expression, function and clinical application of stanniocalcin-1 in cancer. J Cell Mol Med. 2020;24(14):7686–7696. doi:10.1111/jcmm.15348
14. Li Y, He ZC, Zhang XN, et al. Stanniocalcin-1 augments stem-like traits of glioblastoma cells through binding and activating NOTCH1. Cancer Lett. 2018;416:66–74. doi:10.1016/j.canlet.2017.11.033
15. Jeon M, Han J, Nam SJ, Lee JE, Kim S. STC-1 expression is upregulated through an Akt/NF-κB-dependent pathway in triple-negative breast cancer cells. Oncol Rep. 2016;36(3):1717–1722. doi:10.3892/or.2016.4972
16. Chan KK, Leung CO, Wong CC, et al. Secretory Stanniocalcin 1 promotes metastasis of hepatocellular carcinoma through activation of JNK signaling pathway. Cancer Lett. 2017;403:330–338. doi:10.1016/j.canlet.2017.06.034
17. Cui X, Shen D, Kong C, et al. NF-κB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci Rep. 2017;7:40723. doi:10.1038/srep40723
18. Shen KH, Li CF, Chien LH, et al. Role of galectin-1 in urinary bladder urothelial carcinoma cell invasion through the JNK pathway. Cancer Sci. 2016;107(10):1390–1398. doi:10.1111/cas.13016
19. Brantley KD, Kjaersgaard A, Cronin-Fenton D, et al. Stanniocalcin expression as a predictor of late breast cancer recurrence. Cancer Epidemiol Biomarkers Prev. 2018;27(6):653–659. doi:10.1158/1055-9965.EPI-17-0905
20. Shirakawa M, Fujiwara Y, Sugita Y, et al. Assessment of stanniocalcin-1 as a prognostic marker in human esophageal squamous cell carcinoma. Oncol Rep. 2012;27(4):940–946. doi:10.3892/or.2011.1607
21. Lin H, Kryczek I, Li S, et al. Stanniocalcin 1 is a phagocytosis checkpoint driving tumor immune resistance. Cancer Cell. 2021;39:480–493.
22. Schneider AK, Chevalier MF, Derre L. The multifaceted immune regulation of bladder cancer. Nat Rev Urol. 2019;16(10):613–630. doi:10.1038/s41585-019-0226-y
23. Goldman MJ, Craft B, Hastie M, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–678. doi:10.1038/s41587-020-0546-8
24. Bhattacharya S, Dunn P, Thomas CG, et al. ImmPort, toward repurposing of open access immunological assay data for translational and clinical research. Sci Data. 2018;5:180015. doi:10.1038/sdata.2018.15
25. Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi:10.1038/nprot.2008.211
26. Lopez-Beltran A, Cimadamore A, Blanca A, et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers. 2021;13(1):131. doi:10.3390/cancers13010131
27. Fang Z, Tian Z, Luo K, Song H, Yi J. Clinical significance of stanniocalcin expression in tissue and serum of gastric cancer patients. Chin J Cancer Res. 2014;26(5):602–610.
28. Han J, Jeon M, Shin I, Kim S. Elevated STC1 augments the invasiveness of triple‑negative breast cancer cells through activation of the JNK/cJun signaling pathway. Oncol Rep. 2016;36(3):1764–1771. doi:10.3892/or.2016.4977
29. Bai Y, Xiao Y, Dai Y, et al. Stanniocalcin 1 promotes cell proliferation via cyclin E1/cyclin‑dependent kinase 2 in human prostate carcinoma. Oncol Rep. 2017;37(4):2465–2471. doi:10.3892/or.2017.5501
30. Ma X, Gu L, Li H, et al. Hypoxia-induced overexpression of stanniocalcin-1 is associated with the metastasis of early stage clear cell renal cell carcinoma. J Transl Med. 2015;13:56. doi:10.1186/s12967-015-0421-4
31. Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011–5022. doi:10.1158/0008-5472.CAN-18-0118
32. Zeng Y, Li B, Liang Y, et al. Dual blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment. FASEB J. 2019;33(5):6596–6608. doi:10.1096/fj.201802067RR
33. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi:10.1056/NEJMoa1504030
34. Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322. doi:10.1038/nature12965
35. Schcolnik-Cabrera A, Oldak B, Juarez M, Cruz-Rivera M, Flisser A, Mendlovic F. Calreticulin in phagocytosis and cancer: opposite roles in immune response outcomes. Apoptosis. 2019;24(3–4):245–255. doi:10.1007/s10495-019-01532-0
36. Lei X, Lei Y, Li JK, et al. Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–133. doi:10.1016/j.canlet.2019.11.009
37. Krpina K, Babarovic E, Jonjic N. Correlation of tumor-infiltrating lymphocytes with bladder cancer recurrence in patients with solitary low-grade urothelial carcinoma. Virchows Arch. 2015;467(4):443–448. doi:10.1007/s00428-015-1808-6
38. Patschan O, Sjodahl G, Chebil G, et al. A molecular pathologic framework for risk stratification of stage T1 urothelial carcinoma. Eur Urol. 2015;68(5):824–832. doi:10.1016/j.eururo.2015.02.021
39. Rouanne M, Betari R, Radulescu C, et al. Stromal lymphocyte infiltration is associated with tumour invasion depth but is not prognostic in high-grade T1 bladder cancer. Eur J Cancer. 2019;108:111–119. doi:10.1016/j.ejca.2018.12.010
40. Tille JC, Vieira AF, Saint-Martin C, et al. Tumor-infiltrating lymphocytes are associated with poor prognosis in invasive lobular breast carcinoma. Mod Pathol. 2020;33(11):2198–2207. doi:10.1038/s41379-020-0561-9
41. Petitprez F, Fossati N, Vano Y, et al. PD-L1 expression and CD8(+) T-cell infiltrate are associated with clinical progression in patients with node-positive prostate cancer. Eur Urol Focus. 2019;5(2):192–196. doi:10.1016/j.euf.2017.05.013
42. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi:10.1126/science.1203486
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.