Back to Journals » Research and Reports in Urology » Volume 12
Statins Prevent Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy: A Single-center Retrospective Study with a Median Follow-up of 51.20 Months
Authors Jarimba R , Lima JP , Eliseu M, Carvalho J, Antunes H, Tavares da Silva E , Moreira P, Figueiredo A
Received 23 April 2020
Accepted for publication 4 August 2020
Published 28 September 2020 Volume 2020:12 Pages 439—446
DOI https://doi.org/10.2147/RRU.S258267
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Roberto Jarimba,1 João Pedroso Lima,1 Miguel Eliseu,1 João Carvalho,1 Hugo Antunes,1,2 Edgar Tavares da Silva,1,2 Pedro Moreira,1 Arnaldo Figueiredo1,2
1Urology and Renal Transplantation Department, Centro Hospitalar e Universitário de Coimbra, Coimbra, Portugal; 2Faculty of Medicine, University of Coimbra, Coimbra, Portugal
Correspondence: Roberto Jarimba
Urology and Renal Transplantation Department, Centro Hospitalar e Universitário de Coimbra, Rua Professor Mota Pinto, Coimbra 3004-561, Portugal
Tel +351968209250
Email [email protected]
Introduction: Prostate cancer is the most commonly diagnosed cancer in men. Radical prostatectomy is a potentially curative alternative for localized disease, although a significant percentage of these patients will suffer a biochemical recurrence with associated mortality. A wide spectrum of anticancer properties of statins has been demonstrated and the role of these drugs in prevention and treatment of other types of cancer is being increasingly studied.
Objective: The aim of this study was to investigate whether the use of statins is associated with reduced risk of biochemical recurrence among patients submitted to radical prostatectomy.
Patients and Methods: We retrospectively reviewed 875 patients submitted to radical prostatectomy between January 2009 and December 2018. Approximately 45.7% of the patients were on medication with statins at the time of surgery. We evaluated a possible association between statin use and biochemical recurrence and which patients would benefit the most with statin treatment.
Results: Overall, statins were associated with an approximately 40% reduction in risk of biochemical recurrence at a median follow-up time of 51.2 months (HR 0.599, p< 0.05). Patients with pT2c staging (HR 0.486, p=0.017) and ISUP ≥ 3 (HR 0.61, p=0.011) seem to have benefited more from statin use.
Conclusion: In this cohort, use of statins proved beneficial in reducing the risk of biochemical recurrence among patients submitted to radical prostatectomy. Prospective studies are required to confirm this result and to evaluate its safety profile in those patients.
Keywords: prevention, prostate cancer, radical prostatectomy, recurrence, statins
Introduction
Prostate cancer is the most common type of cancer diagnosed in men, accounting for 15% of all cancer diagnosed.1 Early diagnosis provided by PSA (prostate specific antigen) testing allows detection of localized disease in the majority of patients, which increases the probability of a cure. Although radical prostatectomy is one of the main treatment modalities used, a significant proportion of patients suffer from a biochemical recurrence with a high risk of progression to distant metastasis and death.2
Statins are cholesterol-lowering drugs and are among the most prescribed medications.3 Their anticancer properties, such as cell cycle progression arrest,4 apoptosis induction,5 inflammation response decrease, and angiogenesis impairment,6 have been studied in many cancers, including lung,7 colorectal,8 breast,9 melanoma,10 bladder,11 and prostate.12
There are conflicting data about the beneficial effect of statins on biochemical recurrence rate after radical prostatectomy, but there is a plausible trend toward the decreasing biochemical recurrence incidence rate among statin users after curative therapy.13
With this work we tried to shed more light onto the question of whether the continuous use of statins reduces the biochemical recurrence rate after radical prostatectomy.
Objectives
To investigate whether the use of statins is associated with a reduction of biochemical recurrence in patients submitted to radical prostatectomy and to select the patients who may benefit the most from its usage.
Patients and Methods
Patients eligible for this study were submitted to radical prostatectomy between January 1, 2009 and December 31, 2018. The follow-up extended from January 1, 2009 until February 28, 2019. In 2019, our center treated 358 patients diagnosed with prostate cancer, representing roughly 5.5% cases nationwide.
The database used was anonymized and unstructured. Data were originally extracted from an electronic medical records database. Demographic and clinicopathological (T stage, N stage, ISUP score, preoperative PSA level, and positive surgical margin) features, statin and metformin use and adjuvant treatment (radiotherapy and/or androgen deprivation therapy) were available at baseline. Use of statins was accessed by GPI and generic name. All hMG-CoA reductase inhibitors (including combination therapies such as ezetimibe/simvastatin) were considered, but dosing, preoperative duration or indication were not. Those who had initiated the drug after the surgery and the exposition time, defined as the period between statin prescription and the end of follow-up period, were accessed. Only patients using statins at the date of surgery and that did not suspend the drug afterward were included in the statin arm.
Per local protocol, patients with prostate cancer submitted to radical prostatectomy are followed with PSA measurements at three, six, and 12 months after prostatectomy, and then, every six months up to three years, and then annually thereafter. The occurrence of biochemical recurrence (defined as two consecutive PSA measurements >0.2 ng/mL after an undetectable PSA <0.1 ng/mL), metastasis (diagnosed either by CT scan, bone scintigraphy or G68 PSMA-PET) and/or death and the time of their occurrence were available.
Subjects who met the following exclusion criteria were not included in the study: (1) patients submitted to adjuvant radiotherapy or adjuvant androgen deprivation therapy in the context of multimodal therapy; (2) patients who failed to achieve an undetectable PSA (<0.1 ng/mL) after radical prostatectomy.
The primary endpoint of this study was biochemical recurrence.
This study received approval by Comissão de Ética do Centro Hospitalar e Universitário de Coimbra. The individual written informed consent was dismissed by the Ethical Committee based on the dimension and anonymity of the database, ensuring patient data confidentiality and compliance with the Declaration of Helsinki.
Statistical Analysis
Descriptive statistics and pathological characteristics were calculated for all patients included in the present study, as well as by statin use status.
Cox proportional hazards model was used to examine the effect of statin use, controlling for clinical and pathological features. The Kaplan–Meier method was used to evaluate the risk of biochemical recurrence. In order to access the potential effect of statin use in biochemical recurrence in different settings we performed two different analyses: (1) a Cox regression excluding patients who started statin use during follow-up, to eliminate the misclassification (exposure) bias; (2) a Cox regression including only patients who were not in statin treatment at the time of surgery in order to access the association of statin use and risk of biochemical recurrence in this particular set of patients. All analyses were adjusted for age, preoperative PSA level, T stage, N stage, surgical margins status and metformin use.
Nine posthoc subgroup analyses were conducted for the biochemical recurrence outcome for the following subgroups: by pathological T stage, ISUP ≤2 vs ISUP ≥3 and by surgical margin status. Patients who started statin use during follow-up were excluded from this analysis.
All analyses were conducted using IBM SPSS statistics version 23. All comparisons were made using two-sided tests, with p<0.05 considered statistically significant.
Results
A total of 875 patients submitted to radical prostatectomy performed between 2009 and 2018 were assessed for inclusion. After applying the exclusion criteria, 702 were included in the study. 45.7% of men had a history of statin use. Of patients who were not using statin at time of surgery 8.1% started statin use during the follow-up, with a median exposure time of 39.45 months, 9.7% of them suffered biochemical recurrence.
Mean age of the cohort was 63.66 (±6.631) years, while the mean preoperative PSA level was 8.51 (±7.54) ng/mL and mean follow-up time was 51.20 (±31.258) months.
Statin users were older than nonstatin users (64.43 vs 62.95 years old, p<0.05). Other covariates, such as age, preoperative PSA level, T stage, N stage, ISUP score and surgical margin status were similar between the groups. The baseline demographic and clinicopathological characteristics are shown in Table 1.
 |
Table 1 Baseline Demographic and Clinicopathological Characteristics by Prostate Statin Use Status |
Biochemical Recurrence
A total of 145 patients (20.6% of total subjects) suffered biochemical recurrence during a mean follow-up of 51.20 months.
In the Cox regression, HR was 0.599, (95%CI: 0.420–0.855, p<0.05) for biochemical recurrence concerning the statin group vs nonstatin group, adjusted for baseline demographic and clinical features. T stage, ISUP score and surgical margin status were associated with biochemical recurrence. Patients who started statin treatment during the follow-up were excluded from this analysis.
In order to access the association of postoperative statin use with biochemical recurrence we performed a different Cox regression as described in the methods. We accessed the association of postoperative statin use and biochemical recurrence, only in patient subgroup who were not using statins at the time of surgery: postoperative statin exposure was an independent factor associated with a reduced risk of biochemical recurrence in this subgroup of patients (HR 0.165, 95%CI: 0.051–0.533, p<0.05).
The Figure 1 represents the Kaplan–Meier chart of biochemical recurrence free-survival by statin user status.
 |
Figure 1 Biochemical recurrence based on statin use. |
In the subgroup analyses, we considered subgroups based on T stage, surgical margin status and ISUP score. Effect of statins seemed significant in the patients with pT2c and ISUP ≥3. HRs for pT2c and ISUP ISUP ≥3 for statin users vs nonstatin were 0.486 (95%CI: 0.268–0.881, p<0.05) and 0.261 (95%CI: 0.492–0.740, p<0.05), respectively, as represented in Table 2. The Figure 2 represents the Kaplan-Meier chart of biochemical recurrence free-survival by statin user status for pT2c, ISUP ≤2 and ISUP ≥3 patients subgroup.
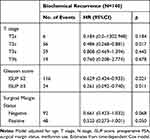 |
Table 2 Adjusted Hazard Ratio of Statin Use on Prostate Cancer Recurrence |
 |
Figure 2 Kaplan–Meier survival chart by subgroups. |
Discussion
Statins work by inhibiting HMG-CoA, the key enzyme of the mevalonate pathway, impairing the activation of important cell cycle regulators such as the Ras and Rho family. Deregulation of these cell cycle regulators has been linked to prostate carcinogenesis process.14,15 However, regulation of androgen production,16 pro-apoptotic,5 anti-inflammatory17,18 and anti-angiogenic actions6 have been identified as possible beneficial effects of statins.
Some epidemiological studies have demonstrated a decreased risk in prostate cancer diagnosis and statin use.19,20 However, five recent prospective studies showed no association between statins and cancer risk, although it seems to exist an association between the use of statins and the decreased risk of advanced prostate cancer.21–24 Other studies found a decreased level of PSA among statin users.25,26
The effects of statins in biochemical recurrence has not been extensively studied. Among men submitted to a radical prostatectomy, between 27 and 53% will develop rising PSA and are at risk of progression to clinical recurrence and increased prostate cancer mortality.27 If a protective effect of statin against biochemical recurrence was found, it would be a significant step forward in the management of patients with localized prostate cancer.
To our knowledge, only a few studies have addressed the role of statin use in patients submitted to radical prostatectomy as the only treatment. Ku et al,28 Krane et al,29 Mondul et al,30 and Rieken et al31 did not find a significant reduction of the risk of biochemical response among statin users vs nonstatin users.
In the Krane et al29 study, the main subject studied was the relationship between statin use and pathological features of the tumor, showing that men under statin treatment had a lower PSA level, a more aggressive distribution of Gleason's score and a slightly higher proportion of Gleason 7 detected on final pathology. However, a decreased risk of biochemical recurrence in statin user was not found,29 even with a mean follow-up of 26 months.
Ku et al28 used biochemical recurrence as a secondary endpoint and failed to find a protective effect of statins against biochemical recurrence, but the statin users and follow-up period were low, with only 84 patients and 38 months, respectively.
Mondul et al30 also did not find any association between statin use and reduction of biochemical recurrence,30 but patients that started statins after the surgery were included in statin arm and the interval between surgery and the beginning of medication was not considered.
Mass et al found a lower preoperative PSA level and a slightly higher pathological Gleason score 7–10 among statin users, but failed to uncover a relation between statins and biochemical recurrence.32
In Rieken et al,31 the study with a larger cohort that has been published, failed to show any difference between statin users and nonstatin users regarding biochemical recurrence-free survival. The median follow-up period was relatively short (25 months), the rate of biochemical recurrence was smaller than reported in the current literature (11.8% vs 10.5% in nonstatin and statin users, respectively), the number of positive surgical margins was significantly higher between statin users (14.3% in nonstatin users group vs 16.3% in statin users group) and patients with nodal metastasis in whose androgen deprivation therapy was initiated postoperatively were included in the analysis.
In a cohort of 1319 patients submitted to radical prostatectomy, Hamilton et al33 did find approximately a 30% reduction in the risk of biochemical recurrence in statin users,33 but the study included patients submitted to adjuvant radiotherapy and/or deprivation hormone therapy. This cohort also had other limitations, including a high positive margin rate of 44% (which probably accounted for an unexpectedly high rate of BCR), a low number of evaluable patients (57%), short follow-up time and differences between statin users and nonusers at baseline.
Song et al34 found an association between postoperative statin use until 36 months decreased the risk of BCR independently especially in patients with high-risk disease. The effect was limited to medication up to 36 months and did not sustain beyond 36 months.
In contrast, Ritch et al35 showed that statin users were at 50% higher risk for BCR after radical prostatectomy compared to nonusers after adjusting for race, pathological stage, Gleason score, margin status and preoperative PSA. In this study, statin users were at higher risk for BCR despite lower baseline risk characteristics,35 but the results should be viewed with caution as only 39% of patients were evaluated consecutively and follow-up was relatively short at 36 months.
In the present study, we found an association between the statin use and a 40% decreased risk of biochemical recurrence at median follow-up of 51.20 months. The major benefits seem to happen in patients with no extraprostatic extension and ISUP ≥3. Postoperative statin exposure was a independent factor associated with a reduced risk of biochemical recurrence in this subgroup of patients (HR 0.165, 95%CI: 0.051–0.533, p<0.05). Statin users were slightly older, but other baseline clinicopathological features were similar between the groups.
Our study has some strengths: a relatively large sample that allowed us to assess the mains effects, similar groups regarding clinicopathological characteristics, a longer follow-up (51.20 months) compared to previous studies and an almost even distribution between statin users and nonusers (45.7% vs 54.3%).
Some limitations of this study must however be noted. Specific drug, dose, preoperative duration and indication were not assessed. Data on some possible confounders (as race, aspirin, race or disease condition) were not available. Furthermore, the retrospective character did not allow a normalized protocol of medical evaluations during therapy.
Based on our results, use of statins is associated with better oncological outcomes after radical prostatectomy, this effect being most noticeable in pT2c and ISUP ≥3 cases. Well designed prospective studies are needed to elucidate the role of statins in adjuvant treatment of prostate cancer and whether statins can be used as a protective measure against biochemical recurrence.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol. 2008;15(1):3866.
2. Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28(3):555–565. doi:10.1016/S0094-0143(05)70163-4
3. Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev. 2012;64(1):102–146.
4. Carlberg M, Dricu A, Blegen H, et al. Mevalonic acid is limiting for n-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface evidence for a new link between 3-hydroxy-3-methylglutaryl-coenzyme a reductase and cell growth. J Biol Chem. 1996;271(29):17453–17462. doi:10.1074/jbc.271.29.17453
5. Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16(4):508. doi:10.1038/sj.leu.2402476
6. Dulak J, Józkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr Cancer Drug Targets. 2005;5(8):579–594. doi:10.2174/156800905774932824
7. Khurana V, Bejjanki HR, Caldito G, Owens MW. Statins reduce the risk of lung cancer in humans: a large case-control study of US veterans. Chest. 2007;131(5):1282–1288. doi:10.1378/chest.06-0931
8. Poynter JN, Gruber SB, Higgins PDR, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352(21):2184–2192. doi:10.1056/NEJMoa043792
9. Kochhar R, Khurana V, Bejjanki H, Caldito G, Fort C. Statins to reduce breast cancer risk: a case control study in US female veterans. J Clin Oncol. 2005;23(16_suppl):514. doi:10.1200/jco.2005.23.16_suppl.514
10. Demierre M-F, Nathanson L. Chemoprevention of melanoma: an unexplored strategy. J Clin Oncol. 2003;21(1):158–165. doi:10.1200/JCO.2003.07.173
11. Parada B, Reis F, Pinto Â, et al. Chemopreventive efficacy of atorvastatin against nitrosamine-induced rat bladder cancer: antioxidant, anti-proliferative and anti-inflammatory properties. Int J Mol Sci. 2012;13(7):8482–8499. doi:10.3390/ijms13078482
12. Kumar A, Riviere P, Luterstein E, et al. Associations among statins, preventive care, and prostate cancer mortality. Prostate Cancer Prostatic Dis. 2020;1–11.
13. Tan P, Wei S, Yang L, et al. The effect of statins on prostate cancer recurrence and mortality after definitive therapy: a systematic review and meta-analysis. Sci Rep. 2016;6(1):29106. doi:10.1038/srep29106
14. Ericsson J, Edwards PA. Signaling Molecules Derived from the Cholesterol Biosynthetic Pathway. In: Bittman R, editor. Cholesterol. Subcellular Biochemistry. Boston, MA: Springer; 1997:1–21.
15. Benitah SA, Espina C, Valerón PF, Lacal JC. Rho GTPases in human carcinogenesis: a tale of excess. Rev Oncol. 2003;5(2):70–78.
16. Dillard PR, Lin M-F, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295(1–2):115–120. doi:10.1016/j.mce.2008.08.013
17. Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4(12):977. doi:10.1038/nrd1901
18. Forrester JS, Libby P. The inflammation hypothesis and its potential relevance to statin therapy. Am J Cardiol. 2007;99(5):732–738. doi:10.1016/j.amjcard.2006.09.125
19. Shannon J, Tewoderos S, Garzotto M, et al. Statins and prostate cancer risk: a case-control study. Am J Epidemiol. 2005;162(4):318–325. doi:10.1093/aje/kwi203
20. Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar H-J. The risk of cancer in users of statins. J Clin Oncol. 2004;22(12):2388–2394. doi:10.1200/JCO.2004.02.027
21. Platz EA, Leitzmann MF, Visvanathan K, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–1825. doi:10.1093/jnci/djj499
22. Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Prev Biomark. 2007;16(11):2213–2217. doi:10.1158/1055-9965.EPI-07-0448
23. Flick ED, Habel LA, Chan KA, et al. Statin use and risk of prostate cancer in the California men’s health study cohort. Cancer Epidemiol Prev Biomark. 2007;16(11):2218–2225. doi:10.1158/1055-9965.EPI-07-0197
24. Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Prev Biomark. 2007;16(11):2226–2232. doi:10.1158/1055-9965.EPI-07-0599
25. Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. J Natl Cancer Inst. 2008;100(21):1511–1518. doi:10.1093/jnci/djn362
26. Cyrus-David Mfon S, Armin W, Timothy T, Kadmon D. The effect of statins on serum prostate specific antigen levels in a cohort of airline pilots: a preliminary report. J Urol. 2005;173(6):1923–1925. doi:10.1097/01.ju.0000158044.94188.88
27. Heidenreich A, Bastian PJ, Bellmunt J, et al; EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. doi:10.1016/j.eururo.2013.11.002
28. Ku JH, Jeong CW, Park YH, Cho MC, Kwak C, Kim HH. Relationship of statins to clinical presentation and biochemical outcomes after radical prostatectomy in Korean patients. Prostate Cancer Prostatic Dis. 2011;14(1):63. doi:10.1038/pcan.2010.39
29. Krane LS, Kaul SA, Stricker HJ, Peabody JO, Menon M, Agarwal PK. Men presenting for radical prostatectomy on preoperative statin therapy have reduced serum prostate specific antigen. J Urol. 2010;183(1):118–124. doi:10.1016/j.juro.2009.08.151
30. Mondul AM, Han M, Humphreys EB, Meinhold CL, Walsh PC, Platz EA. Association of statin use with pathological tumor characteristics and prostate cancer recurrence after surgery. J Urol. 2011;185(4):1268–1273. doi:10.1016/j.juro.2010.11.089
31. Rieken M, Kluth LA, Xylinas E, et al. Impact of statin use on biochemical recurrence in patients treated with radical prostatectomy. Prostate Cancer Prostatic Dis. 2013;16(4):367–371. doi:10.1038/pcan.2013.31
32. Mass AY, Agalliu I, Laze J, Lepor H. Preoperative statin therapy is not associated with biochemical recurrence after radical prostatectomy: our experience and meta-analysis. J Urol. 2012;188(3):786–791. doi:10.1016/j.juro.2012.05.011
33. Hamilton RJ, Banez LL, Aronson WJ, et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) database. Cancer. 2010;116(14):3389–3398. doi:10.1002/cncr.25308
34. Song C, Park S, Park J, et al. Statin use after radical prostatectomy reduces biochemical recurrence in men with prostate cancer: impact of statin use on prostate cancer recurrence. Prostate. 2015;75(2):211–217. doi:10.1002/pros.22907
35. Ritch CR, Hruby G, Badani KK, Benson MC, McKiernan JM. Effect of statin use on biochemical outcome following radical prostatectomy. BJU Int. 2011;108(8b):E211–E216. doi:10.1111/j.1464-410X.2011.10159.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
