Back to Journals » Cancer Management and Research » Volume 11
Statin use and its potential therapeutic role in esophageal cancer: a systematic review and meta-analysis
Authors Zhou C, Zhong X, Gao P, Wu Z, Shi J, Guo Z, Wang Z, Song Y
Received 8 November 2018
Accepted for publication 30 April 2019
Published 19 June 2019 Volume 2019:11 Pages 5655—5663
DOI https://doi.org/10.2147/CMAR.S193945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Cen Zhou,* Xi Zhong,* Peng Gao, Zhonghua Wu, Jinxin Shi, Zhexu Guo, Zhenning Wang, Yongxi Song
Department of Surgical Oncology and General Surgery, The First Hospital of China Medical University, Shenyang 110001, People’s Republic of China
*These authors contributed equally to this work
Purpose: Statins, known as inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductases, are designed to treat lipid disorders, especially hypercholesterolemia. Apart from their role in preventing heart diseases in patients with high cholesterol, recent evidence suggests that statins have anti-tumor properties. However, studies assessing the association between statin use and esophageal cancer survival outcomes have provided controversial results.
Methods: We conducted a systematic review and meta-analysis focusing on studies evaluating associations between statin use and survival outcomes for esophageal cancer patients.
Results: A total of five cohort studies comprising 24,576 patients were included. Statin use associated with improved overall survival (OS: HR 0.84, 95% CI, 0.75–0.94) and disease-free survival (DFS: HR 0.84, 95% CI, 0.75–0.96) of esophageal cancer patients. The improved survival outcomes were consistent in the esophageal adenocarcinoma subgroup and the esophageal squamous cell cancer subgroup.
Conclusion: A potential therapeutic role of statins in esophageal cancer has been demonstrated in our study, however, the results should be interpreted cautiously and need further confirmation by future studies.
Keywords: statins, esophageal cancer, survival outcome, drug repositioning
Introduction
Esophageal cancer, the sixth most common cause of cancer-related death worldwide, has become a serious public health concern.1 It was recently estimated to account for over 16,940 new cases and 15,690 cancer-related deaths in America alone in 2017.2 Although improvements in early diagnosis have marginally reduced the mortality of esophageal cancer, the prognosis of esophageal cancer patients remains unsatisfactory due to lymph node metastasis and high risk of tumor recurrence in situ after resection.3
Drug repositioning, defined as finding new indications for existing drugs, was first introduced to the public in a landmark article written by Ashburn and Thor in 2004.4 Drug repositioning has been proposed as a way to partly solve the gap between low productivity and ever-increasing pharmaceutical research and development spending faced by the biopharmaceutical industry.4 Several successful examples of drug repositioning have inspired extensive efforts to identify existing drugs with new potential unexpected benefits in diseases like cancer.5 A famous example of drug repositioning is thalidomide which has proven promising therapeutic effects on multiple myeloma and prostate cancer.6,7 Other well-known examples of drug repositioning include aspirin and metformin, which are reported to exert anti-cancer effects on colorectal cancer and endometrial cancer, respectively,.8,9 Statins, known as inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductases, are designed to treat lipid disorders, especially hypercholesterolemia.10 Apart from their role in preventing heart diseases in patients with high cholesterol, recent evidence suggests that statins have anti-tumor properties.11,12 Preclinical studies have shown that statin use has a direct restrictive effect on the growth of human esophageal adenocarcinoma (EAC) cells.13,14 Additional studies demonstrated a potential preventive role of statin use on esophageal cancer.15 However, contrasting results were seen in studies assessing the association between statin use and esophageal cancer survival outcomes.
Since reviewing existing evidence can provide more comprehensive insights for further research to explore potential therapeutic effects of statins in treating esophageal cancer, we conducted a systematic meta-analysis to thoroughly investigate whether statin use exerts therapeutic effects in esophageal cancer patients.
Methods
Design
This systematic review and meta-analysis were conducted in accordance with the provisions of the Cochrane Handbook, and the results were reported following the Preferred Reporting Items of Systematic reviews and Meta-Analyses (PRISMA) guidelines.16 The 27-point PRISMA Checklist was presented in
A systematic literature search was performed of the electronic databases PubMed, Embase, and Web of Science from inception through July 22, 2018, and then updated with two additional databases (Cochrane library and clinicaltrials.gov) on March 3, 2019, without consideration of language or publication year, to include all studies investigating associations between statin use and survival outcomes for esophageal cancer patients. The databases were searched using the following strategy: (HMG-CoA reductase inhibitor* OR statin* OR atorvastatin OR fluvastatin OR lovastatin OR pravastatin OR rosuvastatin OR pitavastatin OR simvastatin) AND (cancer OR neoplasm OR tumor OR malignan*) (“*” stands for truncation searching). A manual screen of reference lists cited in the retrieved articles was also conducted to identify additional related articles.
Study selection
Studies that met the following criteria were included: 1) studies clearly enrolled patients who were adults diagnosed with esophageal cancer, 2) studies clearly defined comparison of statin use, whether to placebo or no statin use, regardless of type, dosage, or frequency, and 3) outcomes of interest were overall survival (OS), cancer-specific survival, disease-free survival (DFS), and progression-free survival (PFS). Non-original studies, such as reviews, systematic reviews and meta-analyses, case reports, editorials, and letters to editors, were excluded. When cohorts overlapped in two or more studies, only the most recent publication was included.
The title and abstract of all identified studies were independently reviewed by two reviewers (CZ and XZ) to exclude studies that clearly did not meet the inclusion criteria. The full texts of the remaining studies were further reviewed by the same two reviewers prior to final inclusion. Any discrepancies were resolved through discussions.
Data extraction
The following details were extracted from the selected studies: first author of the study, publication year, study country, study design, histological type, follow-up period, sample size, initial treatment for esophageal cancer, hazard ratios (HRs) with corresponding 95% confidence intervals (CIs), and adjustment variables. The Newcastle-Ottawa quality assessment scale (NOS) was used to evaluate the quality of eligible articles, with articles categorized as low (0–3), moderate (4–6), or high quality (7–9) according to their scores.17
Statistical analyses
Statistical analyses were conducted using STATA version 12.0 (StataCorp. LLC, College Station, TX, USA). A random-effects model was used to conduct quantitative synthesis to provide more conservative estimates,18,19 considering that even when the estimate of I2 equals 0, the 95% CIs around I2 can be wide and the upper 95% CI often exceeds the 50% threshold.20 Heterogeneity among the included studies was estimated using the Cochran Q (X2) statistic and I2 statistic, with I2>50% indicating substantial heterogeneity.21 Subgroup analyses were performed based on study country of origin, study design, tumor site, or treatment pattern to explore possible sources of heterogeneity. Potential publication biases were assessed using funnel plots, Begg’s and Egger’s tests.22,23 The 95% prediction interval (PI) was calculated to predict the potential effect of statin use in an individual study setting and was more conservative than the average effect indicated by the 95% CI.24
Results
Study selection and the characteristics of included studies
Of the 11,825 eligible publications in the initial database search and manual reference screening, 11,697 remained after removing duplicates. After excluding 11,579 records based on reviewing the title and abstract, 118 potentially relevant records remained for further review. A total of five cohort studies comprising 24,576 patients met the inclusion criteria after excluding 113 investigations identified as inadequate (
 | Table 1 Baseline characteristics of included studies |
 | Table 2 The NOS quality of included studies |
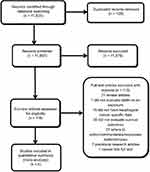 | Figure 1 The selection process for the included studies. |
Statin use and OS
The pooled estimate of OS was 0.87(95% CI, 0.79–0.96, I2=78.1%, Phet<0.001) for unadjusted HR, and 0.84 (95% CI, 0.75–0.94, I2=68.0%, Phet=0.005) for adjusted HR (Figure 2A and B). No evidence of publication bias was found via funnel plot, Begg’s test (P=0.71 for unadjusted HR, P=0.55 for adjusted HR), or Egger’s test (P=0.73 for unadjusted HR, P=0.41 for adjusted HR). We performed a subgroup analysis based on the two main histological subtypes of esophageal cancer. In the EAC subgroup, the pooled estimate of OS was 0.82 (95% CI 0.77–0.87, I2=0.0%, Phet=0.486) for unadjusted HR, and 0.74 (95% CI 0.57–0.95, I2=52.7%, Phet=0.146) for adjusted HR. In the esophageal squamous cell cancer (ESCC) subgroup, the pooled estimate of OS was 0.88 (95% CI 0.61–1.26, I2=69.0%, Phet=0.073) for unadjusted HR, and 0.83 (95% CI 0.73–0.94, I2=0.0%, Phet=0.854) for adjusted HR.
Statin use and DFS
The pooled estimate of DFS was 0.84 (95% CI, 0.77–0.92, I2=55.9%, Phet=0.034) for unadjusted HR, and 0.84 (95% CI 0.75–0.96, I2=59%, Phet=0.017) for adjusted HR (Figure 2C and D). No evidence of publication bias was found via funnel plot, Begg’s test (P=1.00 for unadjusted HR, P=0.71 for adjusted HR), or Egger’s test (P=0.65 for unadjusted HR, P=0.47 for adjusted HR). The subgroup analysis showed that the pooled estimate of DFS was 0.83 (95% CI 0.70–0.99, I2=67.5%, Phet=0.046) for unadjusted HR, and 0.81 (95% CI 0.71–0.93, I2=31.2%, Phet=0.234) for adjusted HR in the EAC subgroup, and 0.87 (95% CI 0.66–1.15, I2=72.7%, Phet=0.026) for unadjusted HR, 0.82 (95% CI 0.71–0.95, I2=0.0%, Phet=0.394) for adjusted HR in the ESCC subgroup.
Due to insufficient data provided in the included studies, we were unable to investigate associations between statin use and risks of progression, recurrence, or metastasis.
Discussion
Main findings and interpretation in light of the evidence
Statins are commonly prescribed cholesterol-lowering agents with an established human safety profile. Statins were recently reported to have anti-cancer activity, making them good candidates for drug repositioning. A series of basic studies have shown that statins inhibit the proliferation of EAC and ESCC cell lines by promoting apoptosis.14,30 A recent systematic review and meta-analysis also reported that statins may play a preventive role against developing esophageal cancer in subjects with or without Barrett’s esophagus.31 Results from epidemiologic studies have demonstrated that statin use after diagnosis is associated with reduced mortality from a range of malignant tumors, including breast, colorectal, and prostate carcinomas.32,33 However, controversies remain between studies exploring whether statin use improves the survival outcomes of esophageal cancer patients.
In this meta-analysis, we systematically and comprehensively assessed all studies that we could access that investigated the associations between statin use and survival outcomes for esophageal cancer patients. Among the five included studies comprising 24,576 esophageal cancer patients, we found that statin use statistically significantly reduced both all-cause mortality and cancer-specific mortality by 16%. It is worth addressing that we chose a random-effects model to provide more conservative results than a fixed-effects model could, accounting for the significant heterogeneity shown in our quantitative syntheses. Furthermore, we performed subgroup analyses based on histological subtypes to explore the origin of the observed heterogeneity. The statistically significant heterogeneity no longer persisted in EAC and ESCC subgroups in the meta-analyses of adjusted or unadjusted HR for OS and adjusted HR for DFS, but it persisted in the meta-analysis of unadjusted HR for DFS, suggesting that histological subtype may partially account for the observed heterogeneity. Meanwhile, subgroup analysis demonstrated a 19% reduced risk in DFS rates and 26% reduced risk in OS rates in the EAC subgroup, with 18% and 17% reductions, respectively, in the ESCC subgroup. All results of overall synthesis and subgroup analysis reached statistical significance, indicating that statins improve OS and DFS in esophageal cancer patients irrespective of histological subtype. The funnel plots, Begg’s test, and Egger’s test showed no indication of publication bias, strengthening the validity of this work. It is worth mentioning that George et al,26 which only provided adjusted HRs for OS and DFS, had a sample size (222) much smaller than the other four studies (1,921, 4,445, 11,750, and 6,238), and caused substantial variance considering the broad 95% CI. When we re-performed the quantitative syntheses of adjusted HR and 95% CI excluding George et al, the overall effect of statin uses on improving OS (adjusted HR:0.83, 95% CI 0.78–0.89, P<0.001) and DFS (adjusted HR:083, 95% CI 0.78–0.89, P<0.001) was even more significant, suggesting that potential therapeutic effects of statins for esophageal cancer patients may be confirmed by acquiring sufficient data and decreasing heterogeneity. Our results indicate a potential overall therapeutic effect of statins on esophageal cancer, regardless of histological subtype. To better probe this therapeutic role of statins in clinical practice, we calculated the 95% PI to explore the therapeutic effects on an individual study level. The 95% PI for OS (0.46–1.45) and DFS (0.43–1.51) both crossed the value of one, possibly because we only included five studies of relatively small sample sizes and with substantial heterogeneity. Thus, the results of our study require further confirmation with more large-scale studies and should currently be interpreted with caution. Nonetheless, this study demonstrates a potential therapeutic effect of statins and a promising future for further recommendation of statins as an alternative option for current treatment strategies.
Strengths and limitations
This is the first meta-analysis to address the association between statin use and survival outcomes of esophageal cancer patients. Furthermore, we calculated the 95% PI in our study which has rarely been included in previous meta-analyses studying the association between statin use and survival outcomes in other cancers.24 In contrast to CI, PI predicts the potential therapeutic effect of statin use on esophageal cancer patients in an individual study setting and is more applicable to translating meta-analysis results into clinical practice.24
Still, our study has several limitations. First, the number of included studies is limited, so the statistically-significant results found here need further confirmation by relevant future studies. Second, due to insufficient data provided in the included studies, we focused mainly on two survival outcomes, OS and DFS, and were unable to investigate associations between statin use and risk of progression, recurrence or metastasis, which are all critical indicators of the prognosis of esophageal cancer patients. Third, substantial heterogeneity exists among the included studies due to the divergent type, dose, duration, and pattern of statin use and the innate differences of research centers and populations. These limitations are largely caused by the insufficient data available. Our team will update this systematic review and meta-analysis when more data are provided in future studies.
Conclusion
In conclusion, this study demonstrates that statins may improve OS and DFS in esophageal cancer patients. However, the potential therapeutic roles of statins in esophageal cancer should be interpreted cautiously and need further confirmation by future studies.
Abbreviation list
HMG CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; RCT, randomized controlled trials; OS, overall survival; RFS, recurrence-free survival; CSS, cancer-specific survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; PI, prediction interval; PRISMA, The Preferred Reporting Items of Systematic reviews and Meta-Analyses; NOS, the Newcastle-Ottawa quality assessment scale.
Acknowledgments
This work was supported by the Natural Science Foundation of Liaoning Province (No. 20180550582), the Natural Science Foundation of China Medical University (YQ20160001), and the Science and Technology Program of Shenyang (18-014-4-07).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors have completed the ICMJE uniform disclosure form at
References
1. Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21(26):7933–7943. doi:10.3748/wjg.v21.i26.7933
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387
3. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–2396. doi:10.1016/S0140-6736(17)31462-9
4. Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nature Rev Drug Discovery. 2004;3(8):673–683. doi:10.1038/nrd1468
5. Shim JS, Liu JO. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int J Biol Sci. 2014;10(7):654–663. doi:10.7150/ijbs.9224
6. Stadtmauer EA. Tailoring initial treatment for newly diagnosed, transplantation-eligible multiple myeloma. Oncology. 2010;24(3 Suppl 2):7–13.
7. Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol. 2004;22(13):2532–2539. doi:10.1200/JCO.2004.05.074
8. Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. doi:10.1016/S0140-6736(10)61543-7
9. Nevadunsky NS, Van Arsdale A, Strickler HD, et al. Metformin use and endometrial cancer survival. Gynecol Oncol. 2014;132(1):236–240. doi:10.1016/j.ygyno.2013.10.026
10. Sirtori CR. The pharmacology of statins. Pharmacol Res. 2014;88:3–11. doi:10.1016/j.phrs.2014.03.002
11. Miraglia E, Hogberg J, Stenius U. Statins exhibit anticancer effects through modifications of the pAkt signaling pathway. Int J Oncol. 2012;40(3):867–875. doi:10.3892/ijo.2011.1223
12. Pisanti S, Picardi P, Ciaglia E, D’Alessandro A, Bifulco M. Novel prospects of statins as therapeutic agents in cancer. Pharmacol Res. 2014;88:84–98. doi:10.1016/j.phrs.2014.06.013
13. Ye F, Zhang GH, Guan BX, Xu XC. Suppression of esophageal cancer cell growth using curcumin, (-)-epigallocatechin-3-gallate and lovastatin. World J Gastroenterol. 2012;18(2):126–135. doi:10.3748/wjg.v18.i2.126
14. Sadaria MR, Reppert AE, Yu JA, et al. Statin therapy attenuates growth and malignant potential of human esophageal adenocarcinoma cells. J Thorac Cardiovasc Surg. 2011;142(5):1152–1160. doi:10.1016/j.jtcvs.2011.08.004
15. Singh S, Singh AG, Singh PP, Murad MH, Iyer PG. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11(6):620–629. doi:10.1016/j.cgh.2012.12.036
16.
17. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi:10.1007/s10654-010-9491-z
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
20. Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914–916. doi:10.1136/bmj.39343.408449.80
21. Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–129. doi:10.1111/1469-0691.12494
22. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
24. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi:10.1136/bmj.d549
25. Nguyen T, Khan A, Liu Y, El-Serag HB, Thrift AP. The association between statin use after diagnosis and mortality risk in patients with esophageal cancer: a retrospective cohort study of United States veterans. Am J Gastroenterol. 2018. doi:10.1038/s41395-018-0169-6
26. Nimako GK, Wintrob ZA, Sulik DA, Donato JL, Ceacareanu AC. Synergistic benefit of statin and metformin in gastrointestinal malignancies. J Pharm Pract. 2017;30(2):185–194. doi:10.1177/0897190015627255
27. Cardwell CR, Spence AD, Hughes CM, Murray LJ. Statin use after esophageal cancer diagnosis and survival: a population based cohort study. Cancer Epidemiol. 2017;48:124–130. doi:10.1016/j.canep.2017.04.015
28. Alexandre L, Clark AB, Bhutta HY, Chan SS, Lewis MP, Hart AR. Association between statin use after diagnosis of esophageal cancer and survival: a population-based cohort study. Gastroenterology. 2016;150(4):
29. Lacroix O, Couttenier A, Vaes E, Cardwell CR, De Schutter H, Robert A. Statin use after diagnosis is associated with an increased survival in esophageal cancer patients: a Belgian population-based study. Cancer Causes Control. 2019. doi:10.1007/s10552-019-01149-3
30. Ogunwobi OO, Beales IL. Statins inhibit proliferation and induce apoptosis in Barrett’s esophageal adenocarcinoma cells. Am J Gastroenterol. 2008;103(4):825–837. doi:10.1111/j.1572-0241.2007.01773.x
31. Thomas T, Loke Y, Beales ILP. Systematic review and meta-analysis: use of statins is associated with a reduced incidence of oesophageal adenocarcinoma. J Gastrointest Cancer. 2018;49(4):442–454. doi: 10.1007/s12029-017-9983-0
32. Mei Z, Liang M, Li L, Zhang Y, Wang Q, Yang W. Effects of statins on cancer mortality and progression: a systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int J Cancer. 2017;140(5):1068–1081. doi:10.1002/ijc.30526
33. Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367(19):1792–1802. doi:10.1056/NEJMoa1201735
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

