Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Stated Preferences for Attributes of a CYP2C19 Pharmacogenetic Test Among the General Population Presented with a Hypothetical Acute Coronary Syndrome Scenario
Authors Bereza BG, Coyle D, So DY , Kadziola Z, Wells G, Grootendorst P, Papadimitropoulos EA
Received 24 October 2019
Accepted for publication 19 February 2020
Published 19 March 2020 Volume 2020:12 Pages 167—175
DOI https://doi.org/10.2147/CEOR.S234298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Basil G Bereza, 1 Doug Coyle, 2 Derek Y So, 3 Zbigniew Kadziola, 4 George Wells, 2 Paul Grootendorst, 1 Emmanuel A Papadimitropoulos 1, 4
1University of Toronto Leslie Dan Faculty of Pharmacy, Toronto, ON, Canada; 2University of Ottawa School of Epidemiology and Public Health, Toronto, ON, Canada; 3University of Ottawa Heart Institute, Ottawa, ON, Canada; 4Eli Lilly & Company, Toronto, ON, Canada
Correspondence: Basil G Bereza
Leslie Dan Faculty of Pharmacy, 144 College Street, Toronto, ON M5S 3M2, Canada
Tel +1 416-978-6989
Email [email protected]
Background: Pharmacogenetic (PGx) testing identifies pharmacotherapeutic risks to permit personalized therapy. Identifying the genetic profile of patients with acute coronary syndrome (ACS) who are considered for therapy with clopidogrel (P2Y 12 receptor blockers) and acetylsalicylic acid (ASA) contributes to the treatment paradigm. Patient preferences would inform a collaborative framework and by extension inform healthcare policy formulation.
Purpose: To quantify stated preferences (willingness to pay) for attributes of a novel point-of-care PGx (CYP2C19) test using a discrete choice experiment (DCE) from the general public in Ontario, Canada, and to identify starting point bias of the cost attribute.
Methods: A web survey was created and included a questionnaire, decision board, and a DCE. DCE choice sets include the following attributes (levels): sample collection (blood, finger prick, and cheek swab), turnaround time for results (1 hr, 3 days, and 1 week), and cost in additional insurance premiums. The presence of starting point bias (cost attribute levels of $0, $1, $5 or $0, $2, $10) in the estimation of willingness to pay (WTP) was tested.
Results: Estimates for turnaround time and cost attributes were statistically significant. Coefficients related to the starting point bias were also significant. Approximately 67% of survey participants chose the PGx test compared to status quo treatment options. WTP for a 1 hr turnaround time compared to a 1-week turnaround time was $10.77 (95% CI 9.58 -12.25).
Conclusion: This translational study shows preference for a point of care PGx test.
Keywords: discrete choice experiment, pharmacogenetic test, patient preference, starting
point bias
Introduction
The United States Food and Drug Administration (FDA) had issued a warning related to the safety of clopidogrel prescribed to patients with a reduced or loss-of-function (LOF) CYP2C19 allele; stating that
poor metabolizers treated with (clopidogrel) at recommended doses exhibit higher cardiovascular event rates following acute coronary syndrome (ACS) or percutaneous coronary intervention (PCI) than patients with normal CYP2C19 function.1
In a 2011 publication, the Canadian Cardiovascular Society (CCS) recommended combination therapy with clopidogrel (P2Y12 receptor blockers) and acetylsalicylic acid (ASA) to prevent ischemic complications in patients suffering an ACS and to those undergoing PCI.2 In an updated guideline, the CCS recommended that dual antiplatelet therapy with ASA 81 mg daily and either ticagrelor 90 mg twice daily or prasugrel 10 mg once daily over clopidogrel 75 mg once daily for 1 year be prescribed to patients suffering an ACS and to those undergoing PCI.3
Absent from these recommendations is the option of using pharmacogenetic (PGx) testing for either the wild type CYP2C19 allele or the CYP2C19 heterozygous and homozygous *2 and *3 reduced function allele to determine a patient’s genetic profile and thus help determine the appropriate treatment option. Over the past few years, several trials have been designed to evaluate whether selecting antiplatelet therapy (clopidogrel, prasugrel, or ticagrelor) based on patient’s genetic characteristics leads to better clinical outcomes compared with the standard of care.4,5 In one of these trials, for example, the primary objective of the Tailored Antiplatelet Therapy Following PCI (TAILOR-PCI) trial was to measure the occurrence of major adverse cardiovascular events (MACE) after PCI. The estimated study completion date is March 2020.4
PGx tests have evolved over the last several years. Specifically, a next-generation PGx test analyzes patient samples at the point of care (POC);6 thus, eliminating the need to batch and send the sample to a laboratory for analysis. Moreover, results from the novel test are available to the clinician within 1 hr, compared to the 2–7 days required using other CYP2C19 tests. Finally, the newer PGx test enables health-care personnel with no previous training in genetic laboratory techniques to undertake genotyping.6
A proof of concept study of a novel point-of-care genetic test was carried out to accurately identify carriers of the CYP2C19*2 allele, which subsequently permitted carriers to be switched to the appropriate P2Y12 receptor blocker drug; thus enabling a pharmacogenetic approach to dual antiplatelet treatment after PCI. The study generated results with a sensitivity of 100% (95% CI 92.3–100%) and a specificity of 99.3% (95% CI 96.2–100%).7 This novel point-of-care genetic test was used as a therapeutic option in our study.
Collaborative (ie, patient, health-care provider) decision-making frameworks have been developed to help facilitate the delivery of care.8 Patient preferences may help inform this framework and by extension inform healthcare policy formulation. In health care, patient preferences may be elicited through stated preference techniques such as discrete choice experiments (DCE).9 In a DCE, participants are asked to choose between two or more scenarios. If a cost component is included in the choice set, the ensuing data may be used to calculate a willingness to pay (WTP) value and in turn inform economic evaluations.
Survey studies that contain a cost component are subject to starting point bias. Starting bias suggests that respondents to the survey are influenced by the initial cost point they are asked to consider within the survey. This study sought to quantify the elicited preferences and evidence of starting point bias for the genetic test cost attributes. A WTP was derived from stated preferences.
Methods
The complete design of the DCE survey (including screenshots) was submitted for ethics approval. This study was approved by the Office of Research Ethics at the University of Toronto (Protocol reference # 31225). A web-based survey was created by the authors, then distributed through a private market research firm (EKOS Research Associates) by emailing invitations to potential respondents.10 EKOS Research Associates was asked to stratify the target population to age, gender, and education based on the Canadian National Household survey (2011). Stratification was requested to align similar demographic attributes of the Ontario population to that of the survey respondents. The target population included both potential “users” and “non-users” of the PGx test from the general population in Ontario (Canada). The potential “user” population were individuals who would be informed by their physician that they would be at risk for ACS. “Non-users” were individuals who may want the product available even though they have no intention of purchasing it now or currently do not exhibit any risk factors listed for ACS.
In addition to a consent form and a demographic questionnaire, the electronic survey included descriptions of clinical conditions, a decision board, and a DCE. The clinical descriptions (ACS, MACE, and genetic testing) were based on patient advocacy web sites and patient information sheets provided by the University of Ottawa Heart Institute.11
A decision board was created to inform participants about the risks associated with three treatment options. (Figure 1). The adverse events communicated to the participant were MACE and bleeding. After reviewing the decision board, participants were asked to choose between the three generically labelled treatment options. Treatment A reflected the clinical outcomes of the status quo option (ie, universal clopidogrel). Treatment B represented the clinical outcomes of treatment with either prasugrel or ticagrelor. Treatment C represented the clinical outcomes of a PGx option where subjects who are non-carriers of loss of function (LOF) allelesare prescribed clopidogrel and LOF carriers are prescribed either prasugrel or ticagrelor.
 |
Figure 1 Screenshot: Decision board highlighting the available treatment options. Abbreviation: MACE, major adverse cardiovascular event. |
Three rates of MACE and bleeds were required to inform the Decision Board: Treatment option A, Treatment option B, and Treatment option C. Rates for treatment option A and B were retrieved from two head-to-head randomized controlled studies (TRITON-TIMI-3812 study and PLATO13). Rates for Treatment option C were taken from genetic sub-studies14–16 from the respective trials.
The TRITON-TIMI-38 compared efficacy and safety endpoints between clopidogrel and prasugrel; while the PLATO trial compared clopidogrel with ticagrelor. The rates of MACE are similar for clopidogrel compared to either prasugrel (12.10% vs 9.90%; p<0.001) or ticagrelor (11.70% vs 9.80%; p<0.001) trials. Since MACE and bleed rates were represented using face emojis (Figure 1), whole numbers needed to be used to represent the respective efficacy and safety rates. As such that the numbers were rounded to 12% (Treatment A) and 10% (Treatment B).
Genetic sub-studies reported the rates of MACE by CYP polymorphism. Under the PGx option in the Decision Board (Treatment C), clopidogrel was prescribed to subjects with fully functional CYP2C19*2 allele, and either prasugrel or ticagrelor was prescribed to patients who carry a LOF CYP2C19*2 allele. The rate of MACE for Treatment C was based on the MACE rates by polymorphism for each drug and weighed by the proportion of either fully functional or LOF carriers to the total study population. The proportion of LOF carriers was similar in both the TRITON and PLATO genetic sub-studies (28%). As such, the rate of subjects with fully functional alleles was 72%. Therefore, MACE rate for the PGx option is 8.14% (28%*8.50% + 72%*8.00%) using the TRITON genetic sub-study and slightly higher at 8.60% (28%*9.40% + 72%*8.30%) based on the PLATO sub-study. Conservatively, the rate of MACE under the PGx option will be rounded up to 9.
Through inference, survey participants who chose Treatment option A or B rejected the PGx treatment option. (By extension, these participants would not be willing to pay any price for the PGx treatment option, this proportional value was incorporated into quantifying the population level WTP). Alternatively, participants who chose Treatment C inferred that they were hypothetically, willing to consider a PGx test in their treatment algorithm, presumably to avoid the MACE and bleeding. As such, only participants choosing Treatment option “C” were directed to complete the DCE. The purpose of the DCE was to elicit preference values for attributes of a PGx test.
Participants in the DCE were presented with 8 choice sets. Each choice set described two hypothetical PGx tests, labelled generically “Choice A” or “Choice B” (Figure 2). Each choice set represented a different combination of attributes for a PGx test. Attributes of a PGx test was derived from a description of the next generation pharmacogenetic test6 and a literature review. In addition, an expert panel including a cardiologist, pharmacists, and health economist was convened to validate the attributes and attribute levels. It is likely that the DCE did not include every attribute important to every participant. However, the literature review and the face validation process (as provided by the convened expert panel) likely captured attributes that were relevant to most of the survey participants.
 |
Figure 2 Screenshot: Choice set example. |
Attributes (attribute levels) for each choice set were sample extraction (blood draw, finger prick, cheek swab), turnaround time for results (1 hr, 3 days, and 1 week) and cost. In Ontario, Canada, the payment mechanism for most health-care services at the point of consumption is through an insurance system. As such, the cost attribute was asked in the context of hypothetical insurance purchasing.17 Two versions of choice sets were created that reflected additional insurance premiums. Version 1 showed cost levels of $0, $1, and $5 and Version 2 showed cost levels of $0, $2, and $10. (Additional insurance premium values were chosen based on expert opinion, personal communication with Prof. Doug Coyle, University of Ottawa) Survey participants were randomly allocated to one of these versions. The purpose of this process was to measure starting point bias within a DCE.
Given three attributes, each with three levels; the total number of possible scenarios is 27 (33) each representing a unique product. Once each of the 27 unique choices were identified, choice sets were constructed by grouping two of the 27 unique choices to make up one set. This was accomplished using a random generator in Excel.18 Eight choice sets were randomly selected for each survey participant. A full factorial design was used which allows for estimation of all main effects (effect of each attributes) and interaction effects (effect of interaction between two or more attributes) independently of one another.9
Regression analysis was generated using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Specifically, a BCHOICE procedure was used within SAS software to run a Bayesian regression analysis for the discrete choice model. A conditional logit method was used to estimate the frequentist fixed effects regression. The logit model with random effects was also specified to consider the multiple observations from each participant. Inter-participant variation was dealt within the design of the DCE, as each participant was presented with eight randomly generated choice sets.
Willingness to pay (WTP) for each level of a given attribute was calculated by dividing the negative of the estimated β coefficient for each attribute by the coefficient of cost.19 Income effect on WTP was also estimated. The maximum WTP for a change in turnaround time from 0 to 1 in terms of money (cost) was quantified by forming the ratio of the marginal utilities where the marginal utility of Turnaround time is βTurnaround time and the marginal utility of cost = - βCost thus the maximum willingness to pay for a turnaround time of 1 hr instead of 1 week is - βTurnaround time/βCost. Similarly, the marginal utility of the sample is βSample and the marginal utility of cost = -β3 thus the maximum willingness to pay for a specific sample method is – βSample/βCost.
In effect, survey participants were asked to consider trading off attribute levels for money. For example, we quantified a WTP value that reflects the incremental amount individuals would be willing to pay between a blood draw and a cheek swab or between a finger prick and a cheek swab. WTP was also quantified for how much more individuals would be willing to pay between getting result in 1 hr compared to 1 week; or between 1 hr or 3 days.
An interaction term was added to the regression model with the purpose of determining the starting point bias in the DCE. For the cost attribute, approximately half of the study sample was allotted to the version where the cost attribute levels were $0, $1, $5 and the other half to the version where the cost attribute levels were $0, $2, and $10. The interaction term would help determine whether an interaction effect exists between the group that was provided the lower cost attribute levels in the DCE and the group that was provided higher levels in the DCE. A significant interaction effect would mean that there are significant differences between the two groups.
Sample size was dictated by numbers of choice sets and number of versions.19 Empirical evidence suggests that 20 participants per group of choice sets are sufficient to estimate reliable models.19 Given this assumption, and based on 8 choice sets, the sample size was estimated to be 8X20 = 160 participants. Given that we are also measuring starting point bias with two sets of costs the final sample size target was 320 participants.
Results
E-mail invitations were sent to 4,234 panel members registered with EKOS. Of these, 387 initiated and 329 individuals completed the survey. Table 1 presents the demographic characteristics of the DCE survey participants and compares the survey demographic to that of the province of Ontario in 2011. The survey sample size under-represented the under 25 years of age category and those with a bachelor’s degree and over-represented those with a graduate degree and households with an income of over $100,000 (CDN). The male:female proportion of the participants in this survey is similar to that of the 2011 Statistics Canada Census figures for Ontario. With the exception of the under 25-year-old age bracket, proportion of other age brackets is also similar to the 2011 Census.
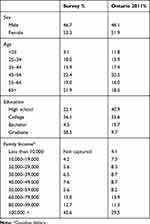 |
Table 1 Survey Demographics and Comparison to Ontario Population |
Of the 329 who completed the survey, 66.8% of participants chose the PGx treatment option. The one-hour attribute level was preferred to longer wait times (statistically significant). However, the cheek swab method was preferred to both the finger prick or blood draw (though this was not statistically significant). Results from regression analysis are presented in Table 2.
 |
Table 2 Summary of Coefficient Estimates Using: Bayesian Fixed Effects, Bayesian Random Effects, and Frequentist Approaches |
Coefficients for the starting point bias were statistically significant. This coefficient (standard deviation) [95% Highest posterior density interval] using Bayesian random effects was 0.2268 (0.0354) [0.1557–0.2936] and 0.5481 (0.1483)[0.2583–0.8386] for the fixed effect. The frequentist result (standard error) was 0.2254 (0.0357) and was significant (<0.0001). These values suggest that starting point bias exists for this DCE.
The odds that a participant chose the 1 hr turnaround time was 14 times that of the 1-week turnaround time (23 times for Version 1: with cost values of $0, $1, $5; 12 times for Version 2: with cost values of $0, $2, $10).
Table 3 presents possible predictors of choosing a specific cost level by sex, having private insurance, history with side effects, previous genetic testing, and income. Results are presented using the entire sample and from the frequentist, fixed and random effects approaches used for analysis. Predictors of cost levels chosen were having insurance, having experience with reacting badly to medication, having a family income of between $50,000 and $149,999 and having history of genetic testing. Sex and having an income of less than $50,000 or over $150,000 were not predictors of cost levels chosen.
 |
Table 3 Demographic Predictors of Choosing a Cost Attribute (Full Sample Set) |
Willingness to pay values for all attributes are presented in Table 4. From a frequentist approach, WTP (95% confidence interval) for a 1 hr turnaround time compared to a 1 week turnaround time was $10.77 (95%CI 9.58-12.25), $6.77 (95%CI 5.82-7.91), and 12.72 (95%CI 10.82-15.01) for the entire data set, Version 1 and Version 2, respectively. WTP values were slightly lower when using coefficients from the random effects approach for the entire data set, as well as for the $0,2,10 set. For the $0,1,5 set, WTP values were similar to slightly higher for turnaround time. As expected, the value for the incremental cost of choosing a 1 hr turnaround time was lower when the 3-day turnaround time was presented than the 1-week turnaround time.
 |
Table 4 Summary of Willingness to Pay Estimates: Bayesian Fixed Effects, Bayesian Random Effects, and Frequentist Approaches |
Discussion
The primary focus of this study was to quantify preferences for attributes of a PGx test and identify whether a starting point bias exists in this DCE. The secondary objective was to determine a WTP for a PGx test, whose value could be used to inform economic evaluations.
Approximately two-thirds of survey participants chose the PGx treatment option. Furthermore, those surveyed were 14 times more likely to choose shorter turnaround times and the cheek swab method of sample extraction over either the finger prick method or blood draw. The direction of the coefficients from the regression analysis was as expected, regardless of the method approach chosen. The direction of the coefficients indicated that survey participants understood the urgency of the possible adverse events and the importance of being treated with the appropriate medication given their genetic (CYP2C19) status.
The survey invited both potential “users” and “non-users” of the POC PGx test to participate in the survey. By doing so, the survey may have captured possible ‘family spillover benefits. Spillover benefits are an effect where “non-users” would deem a benefit from someone else (family member, friend) using the PgX test.
Of note is that there is a possibility that survey responses did not consider the constraints of their own budgets or incomes. The initial survey questionnaire did not capture participants’ ability to pay for their choices or whether the cost attribute fit into their budget. The question that was asked of the participant is how much more they are willing to spend in annual health insurance premiums in exchange for coverage of the PGx test. This question pre-supposes that participants were aware of their current level of health insurance premium expenditure. It should be noted that no studies have been located that shed light on this particular aspect of consumer awareness. However, providing a national average of individual expenditures for health insurance premiums prior to the survey may have been helpful for the participant. Furthermore, knowing this amount would have provided the basis of creating the attribute levels for the cost component of the DCE.
It should be noted that not all possible attributes were included in the survey. For example, the attribute of offering a separate cohort of trained technicians to interpret the results was not included in the choice sets. The novel PGx POC test would have required a brief training of clinicians and not a separate resource to interpret test results. This particular attribute may have been more relevant if the DCE was created to generate preferences from hospital administrators rather than the public. This was a survey sought to elicit preferences from the general population. As such, this attribute was left out.
While attributes other than cost were based on literature and input from co-investigators, determining what values to place on cost levels for the DCE was challenging. There was little guidance available from literature to determine cost attribute levels. This sentiment is shared by some in recent literature.20 Furthermore, it has been pointed out that coming up with cost attribute levels straddle a fine line between being too low (and thus irrelevant to the survey participants) or too high (thus being prohibitive).21 However, the concept of what is “too low” or “too high” has not been elucidated in literature. It should be noted that the Canadian market value for the PGx POC test used as the basis for this study was approximately C$125–150 per patient. Since the framework of the research question was in terms of additional insurance premiums in Canada, we reached out to insurance companies to get a sense of what the market level would be for novel technologies as they pertain to ACS. No response was provided. As such, we relied on expert opinion from academic sources familiar with DCE content. (personal communication). While it can be argued that the cost levels were “too low” in this study to be relevant, the existence of a starting point bias suggests that survey participants were influenced by the cost levels that were provided.
When identifying predictors of choosing a cost level, there were several consistencies between the analytic approaches. First, sex was not a predictor of choosing a cost level. Furthermore, lower and upper bands of the income levels were also not predictive of the cost levels chosen. However, as expected, having insurance was a predictor as well as previous exposure to adverse events.
Furthermore, analysis suggests that starting bias is present. Researchers must make a viable case for the cost values to be included in their DCEs. Erroneous cost levels in a DCE may result in biased values for the willingness to pay, rendering the resulting economic evaluations moot.
Another limitation is related to the attributes included in the DCE. For example, from the initial questionnaire, we know that some participants were concerned about confidentiality and privacy issues related to the DCE. Had there been an attribute that reflected privacy issues, the resulting WTP may have varied from the reported results. Furthermore, the novel POC PGx test would not have needed additional training to operate and as such may have provided some value to hospital administrators. This feature was not measured for preference since hospital staff were not surveyed.
Finally, no exit questions were asked of participants. A question related to whether the increased cost was affordable to their respective budget would have been informative. Furthermore, open-ended question regarding the ease of completing the survey, ease of understanding the content, and the ease of completing the DCE would have been informative for future DCE studies.
Despite the limitations, PGx testing is widely accepted as an alternative treatment algorithm for ACS of PCI. Under the collaborative decision framework, formulations of health-care policy should take into consideration stated preference studies. It should be noted that the results reported here are specific to the attributes of this POC PGx test described above and the underlying conditions and adverse events described in the decision board.
Conclusions
Pharmacogenetic technology is a complex intervention (ie, comprised of a number of related characteristics involving a number of stakeholders). This study shows that survey participants favoured rapid turnaround time for results from PGx tests and were willing to pay for the incremental safety and convenience.
Acknowledgments
The authors would like to thank Dr. Christopher J. Longo for his guidance and Mr. Peter Woods for the web platform creation.
Disclosure
Dr. So has received unrestricted grant support (physician-initiated grant) from Eli Lilly Canada; is a member of the advisory board and has received honoraria from AstraZeneca Canada; is a member of the advisory board for Bayer Canada; has received unrestricted grant support (physician-initiated grant) from Spartan Biosciences; has received unrestricted grant support (physician-initiated grant) from Aggredyne; has received unrestricted grant support (physician-initiated grant) from Diapharma/Roche Diagnostics; and has received honoraria from Abbott Vascular, Canada. Dr. Emmanuel Papadimitropoulos is an employee of Eli Lilly Canada Inc, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. United States Food and Drug Administration. Available from: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm.
2. Bell AD, Roussin A, Cartier R, et al. The use of antiplatelet therapy in the outpatient setting: Canadian Cardiovascular Society Guidelines. Can J Cardiol. 2011;27:208–221. doi:10.1016/j.cjca.2010.12.033
3. Mehta SR, Bainey KR, Cantor WJ, et al. Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol. 2018;34:214–233. doi:10.1016/j.cjca.2017.12.012
4. ClinicalTrials.Gov. Tailored antiplatelet therapy following PCI (TAILOR-PCI). Available from: https://clinicaltrials.gov/ct2/show/NCT01742117.
5. ClinicalTrials.Gov. Pharmacogenetics of Clopidogrel in Acute Coronary Syndromes (PHARMCLO). Available from: https://clinicaltrialsgov/ct2/show/NCT03347435?term=ardissino&rank=1.
6. BioScience Spartan Rx CYP2C19. Available from: https://www.spartanbio.com/.
7. Roberts JD, Wells GA, LeMay MR, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012;379:1705–1711. doi:10.1016/S0140-6736(12)60161-5
8. Kamal RN, Lindsay SE, Eppler SL. Patients should define value in health care: a conceptual framework. J Hand Surg Am. 2018;
9. Louviere J, Hensher D, Swait J. Stated Choice Methods. Cambridge: Cambridge University Press; 2000.
10. EKOS Research. Available from: www.ekos.com.
11. University of Ottawa. Available from: https://www.ottawaheart.ca/heart-condition/acute-coronary-syndrome.
12. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi:10.1056/NEJMoa0706482
13. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi:10.1056/NEJMoa0904327
14. Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi:10.1056/NEJMoa0809171
15. Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel. Relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–2560. doi:10.1161/CIRCULATIONAHA.109.851949
16. Wallentin L, James S, Storey RF, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphism on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi:10.1016/S0140-6736(10)61274-3
17. Gafni A. Willingness to pay as a measure fo benefits. Relevant questions in the context of public decisionmaking about healthcare programs. Med Care. 1991;29:1246–1252. doi:10.1097/00005650-199112000-00007
18. Microsoft Corporation. Microsoft excel. 2013.
19. Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making. A user’s guide. Pharmacoeconomics. 2008;26:661–677. doi:10.2165/00019053-200826080-00004
20. Rowen D, Stevens K, Labeit A, et al. Using a discrete-choice experiment involving cost to value a classification system measuring the quality-of-life impact of self-management for diabetes. Value Health. 2018;21:69–77. doi:10.1016/j.jval.2017.06.016
21. Ratcliffe J. The use of conjoint analysis to elicit willingness-to-pay values: Proceed with caution? Int J Technol Assess Health Care. 2000;16:270–275. doi:10.1017/S0266462300161227
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
