Back to Journals » Journal of Asthma and Allergy » Volume 15
Staph’s Toxins IgE Antibodies and Its Relation to the Severity of Allergic Rhinitis
Authors Guzmán-Avilán RI, González-Díaz SN , Guzmán-Avilán KD, De la Cruz-De la Cruz C , de León-Gutiérrez H, Guzmán-López S
Received 30 December 2021
Accepted for publication 4 April 2022
Published 17 May 2022 Volume 2022:15 Pages 665—671
DOI https://doi.org/10.2147/JAA.S356419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Luis Garcia-Marcos
Rosa Ivett Guzmán-Avilán,1,* Sandra Nora González-Díaz,1,* Katia Denisse Guzmán-Avilán,1 Carlos De la Cruz-De la Cruz,2 Humberto de León-Gutiérrez,3 Santos Guzmán-López3
1Centro Regional de Alergia e Inmunología Clínica (CRAIC), Hospital Universitario “Dr. José E. González”, Universidad Autónoma de Nuevo León, Monterrey, 64460, Nuevo León, México; 2Escuela de medicina y Hospital Universitario “Dr. José E. González”, Universidad Autónoma de Nuevo León, Monterrey, 64460, Nuevo León, México; 3Departamento de Anatomía Humana, Facultad de Medicina y Hospital Universitario “Dr. José E. González”, Universidad Autónoma de Nuevo León, Monterrey, 64460, Nuevo León, México
*These authors contributed equally to this work
Correspondence: Santos Guzmán-López, Departamento de Anatomía Humana, Facultad de Medicina y Hospital Universitario “Dr. José E. González”, Universidad Autónoma de Nuevo León, Av. Madero y Gonzalitos s/n, Colonia Mitras Centro, Monterrey, 64460, Nuevo León, México, Email [email protected]
Background: Specific IgE against Staphylococcus can be found in approximately 40% of patients with allergies, also in patients without allergies because they may be sensitized. These antibodies are functional, and they can induce histamine release contributing to chronic pruritus which can worsen disease severity. The objective of this study was to compare levels of specific IgE against S. aureus toxins in those populations.
Methods: A cross-sectional, comparative non-blinded survey was made at the Regional Center for Allergy and Clinical Immunology. Ninety-nine adults between 18 and 70 years of age with allergic rhinitis (AR) and without allergic rhinitis (wAR) were recruited. A clinical history and demographic data, and allergic sensitization patterns to 35 aeroallergens were obtained, and participants were classified according to their severity using the Allergic Rhinitis and Its Impact on Asthma (ARIA) classification. Specific IgE levels were determined using ImmunoCAP™ 100 platform.
Results: The median age (IQR) of the participants was 23 (20– 33.7); 56.2% were women. The most frequent comorbidities were asthma and obesity. Of the patients with AR, 46.7% were classified as mild intermittent and 25% as moderate persistent. IgE levels against staph toxins A, B, and TSST were significantly higher in the AR group vs the wAR group [median IQR 0.01 (0.01– 0.03) vs. 0.01 (0– 0.02), p = 0.01; 0.02 (0.01– 0.03) vs. 0.01 (0– 0.02), p= 0.02; 0.04 (0.02– 0.09) vs. 0.01 (0– 0.04), p=0.002, respectably]. A significant difference was found in serum IgE levels against Staph B toxin between severity subgroups.
Conclusion: People with AR have higher IgE levels against staph toxins A, B and TSST than wAR subjects. However, it is not possible declare that the IgE titers were related to disease severity.
Keywords: allergic rhinitis, ARIA, IgE, Staphylococcus aureus, staphylococcal superantigens
Introduction
A significant increase in allergic diseases has been observed globally in the last two decades. According to the World Health Organization, these conditions are among the most frequent diseases in pediatric and adult populations.1,2 It is estimated that 30 to 50% of the world population lives with one or more allergic diseases, of which 10–25% suffer from allergic rhinitis (AR).3 There is evidence that these diseases cause significant personal deterioration such as emotional problems, sleep disorders, and dysfunction in social activities.4 They also have a negative impact on the socioeconomic wellness of society.5,6
Staphylococcus aureus is considered a bacterium of the human microbiota. Approximately 25% of the population are S. aureus carriers.7 However, although S. aureus infection can be a symbiosis, it can also be a life-threatening condition at the expense of staphylococcal superantigens (SS) that induce an inflammatory immune response.8
Specific IgE against S. aureus can be found in approximately 40% of patients with allergies, also in patients without allergies because they may be sensitized.9–11 Specific antibodies against S. aureus are functional as they bind with high affinity to their respective receptors on mast cell membranes, inducing histamine release and contributing to chronic pruritus.9,10,12 In allergic disease, SS increase antigen sensitivity and decrease the T-cell response to steroids, which can worsen disease severity.8,13 Nevertheless, it remains elusive if in individuals with and without AR differ the levels of specific IgE against S. aureus toxins, and its possible relation between the production of specific IgE and allergic respiratory severity. Our study aims to find the relationship, if any, between the IgE against S. aureus toxins levels and allergic respiratory severity.
Materials and Methods
Study Design
The present cross-sectional comparative survey recruited 99 Mexican patients with (n=64) and without (n=35) AR. All subjects, including healthy volunteers, were subjected to skin “prick” test (SPT) for aeroallergens. Diagnosis of AR was based on ARIA guidelines.14 Patients without AR were recruited through preventive campaigns and were referred to the Regional Center for Allergy and Clinical Immunology clinic (CRAIC) at the Hospital Universitario “Dr. José Eleuterio González”.
For study inclusion, subjects had to be over 18 years of age. Subjects with uncontrolled arterial hypertension, pregnant and/or breastfeeding, subjects who had suffered an upper respiratory tract infection four weeks before study entry, and patients who were taking antibiotics for any reason were excluded. Similarly, subjects who did not complete the evaluations and who withdrew informed consent were eliminated. Same exclusion criteria were applicated to control group, additionally if the skin prick test was positive, the subject was excluded.
A 2:1 sample size calculation (cases: control) was performed, ensuring a power of 80% and a bilateral 95% confidence, to detect an elevation difference in IgE levels of 27%9 between the groups of patients without allergic rhinitis (control) and those with allergic rhinitis. A minimum sample of 60:30 participants respectively per group was needed.
Measurements
A complete medical history with an emphasis on personal and family history of allergic diseases was obtained. Demographic information for each patient and the skin prick tests to 35 aeroallergens (intramural and extramural) prevalent in our population were collected.
ARIA Classification
The severity of allergic rhinitis was determined according to the Allergic Rhinitis and its Impact on Asthma (ARIA) classification determining AR as mild and moderate and subdivided into persistent or intermittent, according to the level of symptoms.14
IgE Measurements
Serum measurements of specific IgE against S. aureus toxins, Staph A, Staph B, Staph C and Toxic Shock syndrome toxin (TSST) were performed using a fluor-enzymatic immunoassay autoanalyzer, the ImmunoCAP 100 platform (ThermoFisher), according to the manufacturer’s instructions. The measurements were carried out with a peripheral blood sample from the anterior fossa of the forearm.15,16
Quality of Life
The self-applicable Spanish version of the rhinoconjunctivitis quality of life questionnaire (RQLQ) was used with all patients.17 This questionnaire assesses the domains of activity limitations, sleep problems, nose symptoms, eye symptoms, non-nose/eye symptoms, practical problems, and emotional function.
Ethical Considerations
This study was approved by the Research Ethics and the Research Committees of the Facultad de Medicina and Hospital Universitario, Universidad Autonoma de Nuevo León (Registration number AL14-003). All patients were informed of the objectives of the study and signed written informed consent.
Statistical Analysis
Descriptive statistics were applied to determine the frequency and proportions of demographic (gender) and clinical variables (allergic diseases and aeroallergens). For continuous quantitative variables (age and anti-Staphylococcus IgE levels) after a normality assessment (measured by asymmetry and kurtosis and the Kolmogorov Smirnov test), the median and the interquartile range (IQR) were used as a measure of central tendency and dispersion. IgE levels of the AR participants vs the controls were compared using the Mann Whitney U-test. The Kruskal–Wallis test was used to compare the severity subgroups. Significant differences between groups were represented by p <0.05. P value thresholds were Bonferroni-corrected for multiple comparisons.18 SPSS version 23.0 statistical software (IBM Corp. Armonk, NY) was used for data processing.
Results
Demographic Data
Ninety-nine adults between 18 and 70 years of age with and without allergic rhinitis were recruited. Of these, 10 were eliminated for not having responded to the evaluation or withdrawing their consent (Figure 1). The median age and interquartile range of all participants was 23 years (20–33.7), 31.5 (22.4–40.7) for the allergic rhinitis group, and 20 (18 −20.5) for the control group. Women represented 56.2%, of which 51.7% and 65.5% were in the AR and control groups, respectively (Table 1).
 |
Table 1 Demographic Characteristics |
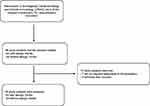 |
Figure 1 Study flow diagram. |
The most frequent comorbidities in the AR group were asthma, obesity, gastroesophageal reflux disease (GERD), and food allergy. On the other hand, the main allergens demonstrated by the skin “prick” tests in the AR population were Dermatophagoides pteronyssinus (71.7%), Dermatophagoides farinae (68.3%), Fraxinus americana (26.7%), Cynodon dactylon (23.3%), Periplaneta americana (21.7%), and Phleum pratense (13.3%). All healthy volunteers (control) showed no reaction in response to these allergens (Table 2).
 |
Table 2 Intramural and/or Extramural Aeroallergens Evaluated and Its Proportion in AR Patients |
Proportion of Positive Specific IgE
The proportion of specific IgE against any of the 4 staphylococcal toxins, 79.3% for the control group and 83.3% for the AR group is compared in Table 3. When comparing positivity for toxins in isolation between these groups, no significant difference was found.
 |
Table 3 Presence of IgE Antibodies |
ARIA Classification and Specific IgE Levels
Of the 60 patients with AR, 46.6% were classified as mild intermittent (MiIAR), 20% mild persistent (PMiAR), 8.3% moderate intermittent (MoIAR), and 25% moderate persistent (PMoAR). The IgE levels against toxins Staph A, Staph B, and TSST were significantly different, with higher values in subjects from the AR group compared to the control group. No difference was found between the IgE levels vs staph C toxin between the groups (Table 4).
 |
Table 4 Comparison of IgE Levels Against Staphylococcal Toxins Between the Allergic Rhinitis Group and the Control Group |
When comparing the specific IgE serum levels against staphylococcal toxins in patients with AR classified by severity, a significant difference was found in serum IgE levels just against Staph B toxin between the subgroups. This difference was found between MiIAR and PMiAR [median IQR 0.02 (0.0125–0.0575) vs. 0.01 (0–0.01), p = 0.029] (Table 5).
 |
Table 5 Comparison of IgE Levels Against Staphylococcal Toxins in Patients with RA Between ARIA Classification Groups |
A comparison was made between the severity classifications of AR and the different domains evaluated by the RQLQ. In the seven domains of the RQLQ, the 28 patients classified with MiIAR indicated less involvement compared to the rest of the subgroups. Statistically, a difference was found between the groups in 4 out of 7 domains: sleep, practical problems, nasal, and emotional symptoms. However, in the post hoc analysis with the Bonferroni correction just the ‘nasal symptoms’ domain was statistically significant between MiIAR and MoPAR [median IQR 1.25 (0.56–1.75) vs. 3.25 (1.75–4.25), p = 0.11] (Table 6).
 |
Table 6 Comparison of AR Classifications Between the Dimensions Evaluated by the RQLQ |
Discussion
In this study, we sought to correlate the sensitivity of IgE against Staphylococcus aureus in patients diagnosed with allergic rhinitis and compare the severity with the RQLQ developed by Junipper et al. A difference was found in the presence of IgE antibodies to staphylococcal toxins between the AR and control groups. Additionally, there was a difference between IgE immunoglobulins against Staph B toxin when subdivided, based on severity. On the other hand, in the different domains of the RQLQ, lower values were found in the mild intermittent allergy rhinitis group.
This leads us to infer that there is a relationship between the presence of antibodies and the presence of AR, but our data is not enough to support that this relationship were associated on severity. Meanwhile, the RQLQ reinforce that a classification of greater symptom severity has a greater impact on the different aspects of quality of life.
Some studies, as Rossi et al19 found an association between S. aureus superantigens and exacerbations in patients with allergic diseases. Interestingly, we could not be corroborated this finding; the specific IgE serum levels against staphylococcal toxins in patients with AR classified by severity did not differ. On the other hand, Shiomori et al20 reported that allergic rhinitis makes it easier for patients to be S. aureus nasal carriers and that this factor seems to be detrimental in the disease. In our study, we did find a major proportion of S. aureus in AR patients but we could not associate it with disease severity.
The present study is subject to some limitations. Although participants of different ages and sexes were included to maintain representativeness, the control group was significantly younger. However, as stated by John Bradley in his review,21 the presence of IgE antibodies once exposed to S. aureus does not differ significantly once adulthood is reached, so we consider that it does not generate a major problem in our patients´ results. In addition, each of the study participants underwent a skin prick test, ensuring that the control participants did not present reactivity to the 35 most common aeroallergens in our community, which, although in a small control group, ensures comparability between the groups.
With respect to IgE sensibilization in patients with AR, some consideration should be highlighted. Future studies, with longer follow up periods should investigate the correlation between IgE sensibilization with number of exacerbations and poor symptoms control despite treatment. If this association can be proven, clinicians could implement a more intense and individualized treatment approach, focused on antigen-IgE desensitization in order to reach an adequate symptom control.
Conclusions
The present study reinforces the hypothesis that subjects diagnosed with allergic diseases, in our case with AR, have higher specific IgE immunoglobulin levels against Staph A, B and TSST toxins compared to healthy subjects. However, we did not have enough evidence to declare that IgE titles are related to the level of disease severity. More studies with a different methodological design are needed to assess whether there is an association between IgE levels and the occurrence of allergic diseases such as AR.
Disclosure
Rosa Ivett Guzmán-Avilán and Sandra Nora González-Díaz are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Pearce N, Aït-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax. 2007;62(9):757–765. doi:10.1136/thx.2006.070169
2. WHO. Prevention of allergy and allergic asthma. In: Based on the WHO/WAO Meeting on the Prevention of Allergy and Allergic Asthma. WHO; 2003:3–7.
3. Brama SS. The global burden of asthma. Chest. 2011;130(1):4S–12S. doi:10.1378/chest.130.1
4. Aït-Khaled N, Pearce N, Anderson HR, et al. Global map of the prevalence of symptoms of rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Allergy. 2009;64(1):123–148. doi:10.1111/j.1398-9995.2008.01884.x
5. Bosuquet J, Khaltaev N, Cruz AA, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;43(7):552–557.
6. Thomas M. Allergic rhinitis: evidence for impact on asthma. BMC Pulm Med. 2006;6(SUPPL. 1):1–7. doi:10.1186/1471-2466-6-S1-S4
7. Hyun DW, Min HJ, Kim MS, et al. Dysbiosis of inferior turbinate microbiota is associated with high total IgE levels in patients with allergic rhinitis. Infect Immun. 2018;86(4):1–14. doi:10.1128/IAI.00934-17
8. Survarnsit K, Kiratisin P, Chaweewan B, Pongsakorn T. Prevalence of nasal carriage of Staphylococcus aureus in allergic rhinitis patients and healthy controls in Thailand. Asian Pacific J Allerg Immunol. 2022;9–13. doi:10.12932/ap-080719-0598
9. Bachert C, van Steen K, Zhang N, et al. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol. 2012;130(2):376–381. doi:10.1016/j.jaci.2012.05.012
10. Cardona ID, Sang HC, Leung DYM. Role of bacterial superantigens in atopic dermatitis: implications for future therapeutic strategies. Am J Clin Dermatol. 2006;7(5):273–279. doi:10.2165/00128071-200607050-00001
11. Vroling AB, Fokkens WJ, van Drunen CM. How epithelial cells detect danger: aiding the immune response. Allergy. 2008;63(9):1110–1123. doi:10.1111/j.1398-9995.2008.01785.x
12. Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18(1):21–26. doi:10.1097/MOO.0b013e3283350053
13. Kareem N, Alchalabi R, Suleiman AA. Isolation and identification of Staphylococcus aureus produce super-antigen which trigger IgE production from Iraqi patients with rhinitis. EurAsian J BioSci. 2020;14:5399–5404.
14. Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines—2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
15. Eigenmann PA, Kuenzli M, D’Apuzzo V, et al. The ImmunoCAP® rapid Wheeze/Rhinitis child test is useful in the initial allergy diagnosis of children with respiratory symptoms. Pediatr Allerg Immunol. 2009;20(8):772–779. doi:10.1111/j.1399-3038.2009.00866.x
16. Griffiths RLM, El-Shanawany T, Jolles SRA, et al. Comparison of the performance of skin prick, ImmunoCAP, and ISAC tests in the diagnosis of patients with allergy. Int Arch Allergy Immunol. 2017;172(4):215–223. doi:10.1159/000464326
17. Soler R, de la Hoz B, Badia X, et al. Validación de la versión española del cuestionario de calidad de vida para pacientes con rinoconjuntivitis. Revista Clínica Española. 2004;204(3):131–138. doi:10.1016/s0014-2565(04)71417-4
18. Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310(3):170. doi:10.1136/bmj.310.6973.170
19. Rossi RE, Monasterolo G. Prevalence of serum IgE antibodies to the Staphylococcus aureus enterotoxins (SAE, SEB, SEC, SED, TSST-1) in patients with persistent allergic rhinitis. Int Arch Allergy Immunol. 2004;133:261–266. doi:10.1159/000076833
20. Shiomori T, Yoshida S, Miyamoto H, Makishima K. Asthma, rhinitis, other respiratory disorders relationship of nasal carriage of Staphylococcus aureus to pathogenesis of perennial allergic rhinitis. J Allergy Clin Immunol. 2000;105(3):449–454. doi:10.1067/mai.2000.104256
21. Bradley J. Immunoglobulins. J Med Genet. 1974;11:80–90. doi:10.1136/jmg.11.1.80
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
