Back to Archived Journals » Botanics: Targets and Therapy » Volume 6
Standardization of Prasaplai, a Thai traditional preparation for antidysmenorrhea
Authors Tangyuenyongwatana P, Gritsanapan W
Received 30 July 2015
Accepted for publication 15 October 2015
Published 23 December 2015 Volume 2016:6 Pages 1—9
DOI https://doi.org/10.2147/BTAT.S56492
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ayse Kuruuzum-Uz
Prasan Tangyuenyongwatana,1 Wandee Gritsanapan2,3
1College of Oriental Medicine, Rangsit University, Phathumthani, 2Department of Pharmacognosy, Faculty of Pharmacy, Mahidol University, 3Phyto Product Research, Bangsue, Bangkok, Thailand
Abstract: Prasaplai is a Thai traditional medicine preparation that has been used to treat dysmenorrhea for several decades. The preparation is composed of ten herbs and two compounds. Due to factors such as the inconsistency of active ingredients in raw materials, and the biological efficacy and reproducibility of therapeutic effects, traditional medicine preparations are required to be qualitatively and quantitatively standardized by modern methods so that they become more widely accepted. The prominent methods of choice are high-performance liquid chromatography and thin-layer densitometry. Each component of Prasaplai was collected from ten different locations in Thailand, and the amounts of active constituents in each of the component were analyzed using high-performance liquid chromatography. Prasaplai preparation also contains artifacts, which are formed during storage. These artifacts were evaluated periodically within 1 year of storage. Different extracting solvents for Prasaplai preparation were also analyzed to avoid the artifacts.
Keywords: Prasaplai, dysmenorrhea, standardization, HPLC, artifact
Introduction
Prasaplai is a Thai traditional medicine preparation used to treat dysmenorrhea. The word “Prasaplai” is derived from “Prasa”, which means 50% in amount, and “Plai”, which is a Thai name for Zingiber cassumunar Roxb. Altogether, Prasaplai refers to the preparation that contains 50% of Z. cassumunar. It has been commonly used for a long time by Thai traditional practitioners as an antiflatulent, to relieve dysmenorrhea, and to adjust the cycle of menstruation.1,2 Prasaplai has been included as a Thai traditional common household drug since the first list was established in 1968. Additionally, it has also been included in the Thai traditional common household drug list and the national essential drug list AD 1999 of Thailand (list of herbal medicinal products) for relieving dysmenorrhea, especially in women who have less menstruation or an irregular cycle of menstruation.3 Prasaplai formula consists of ten medicinal plants and two chemical compounds. It is a mixed powder of equal amounts of the pericarp of Citrus hystrix DC., the root of Acorus calamus L., the bulb of Allium sativum L., the bulb of Eleutherine americana (Aubl.) Merr. ex K. Heyne, the fruit of Piper nigrum L., the fruit of Piper retrofractum Vahl, the rhizomes of Zingiber officinale Roscoe and Curcuma zedoaria Roscoe, the seed of Nigella sativa L., and sodium chloride and camphor making a total of 50% of the formulation weight, and the remaining 50% comprises the rhizome powder of Z. cassumunar Roxb.2
Two clinical studies have investigated the efficiency and side effects of Prasaplai extract versus mefenamic acid in relieving primary dysmenorrhea.4,5 The results showed that Prasaplai resulted in less pain and less side effects compared with mefenamic acid (P<0.05),4 whereas Sriyakul et al reported no statistically significant difference between the effects of Prasaplai extract and mefenamic acid on the degree of pain relief. In addition, both Prasaplai extract and mefenamic acid showed no severe side effects.5 Based on such results, this preparation became a well-known commercial product and has been widely used in most of the hospitals and health care units in Thailand. However, standardization of the preparation is lacking, which is necessary to ensure efficiency and safety. The quality assessment of each herbal component is also important to control the quantity of its major active ingredients and the quality of the whole formulation.
Standardization of herbal components in Prasaplai preparation
Each herbal component of Prasaplai preparation was purchased during March–April 2007 from traditional drug stores in ten different locations in Thailand. The powder of each plant (100 mg) was sonicated three times in methanol (3 mL each) for 10 minutes. Each extract was decanted, filtered through a disk filter (0.45 μm), and adjusted to 10 mL in a volumetric flask to give a final concentration of 10 mg/mL. A 10 μL volume of the sample solution was injected to high-performance liquid chromatography (HPLC) system (Table 1).6
  | Table 1 HPLC system and common conditions |
Validation method of HPLC for quantitative analysis of each plant component of Prasaplai preparation
A. calamus L.
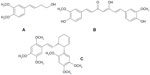
It is an herb of the Araceae family. The rhizome is traditionally used to soothe inflammation and treat skin infections. Calamus rhizome contains 2%–9% essential oil, which comprises sesquiterpenes and phenylpropanoids. cis-Isoasarone (β-asarone) is the main component (up to 80%) of the essential oil (Figure 1). Other compounds include cis-methyl isoeugenol, α- and γ-asarones, asarylaldehyde, acoroxide, acorin, calamine, linalool, calamol, calamenone, eugenol, methyl eugenol, azulene, pinene, cineole, camphor, and acorone, which is a sesquiterpene diketone with a bitter taste and spiral structure.7–9
  | Figure 1 Structure of marker compounds for Acorus calamus and Citrus hystrix. |
HPLC was performed using water and acetonitrile with gradient elution: 50:50, 43:57, 20:80, and 20:80 at 0, 10, 15, and 20 minutes, respectively. The linear regression equation is Y = 35.325X + 110.89 with r2=0.9998 for β-asarone. The concentrations range between 3.21 and 780.19 μg/mL. The marker for this plant is β-asarone, which is the major active compound in A. calamus. The content of β-asarone is in the range of 2.05%±0.02% to 2.65%±0.01% w/w. The average quantity of β-asarone in the dried powder is 2.17%±0.02%. Thus, A. calamus rhizome should contain β-asarone not <2.0% dry weight.6
C. hystrix DC
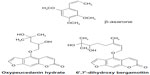
It is a medium tree of the Rutaceae family. Fresh peels and dried fruits are used to relieve nausea and flatulence and control menstruation. The fruit peel of C. hystrix contains an essential oil of which the major compounds are terpenes, such as β-pinene, limonene, β-phellandrene, citronellol, and citronellal. The other constituents are linalool, borneol, camphor, sabinene, germacrene D, aviprin, umbelliferone, and β-sitosterol.7,10 Coumarins are also isolated from the peel of C. hystrix. They include bergamottin, 6′,7′-dihydroxybergamottin (DHB), oxypeucedanin hydrate (OPH), psoralen, and auraptene.11 Flavonoids, including myricetin, quercetin, kaempferol, luteolin, and apigenin, are also found in the leaf of C. hystrix.12
HPLC was performed using water and acetonitrile with gradient elution: 85:15, 70:30, 50:50, 20:80, and 20:80 at 0, 10, 20, 30, and 35 minutes, respectively. DHB and OPH are the two markers for this plant (Figure 1). The linear regression equations for DHB and OPH are Y = 24.036X + 22.851 with r2=0.9999 and Y = 29.592X + 36.024 with r2=0.9999, respectively. The concentrations of DHB and OPH are 32.31–245.39 μg/mL and 31.37–238.23 μg/mL, respectively. The lower limits of dry weights of DHB and OPH are 0.300%±0.003% and 0.318%±0.003%, while the upper limits are 0.709%±0.002% and 0.543%±0.002%, respectively. The average amounts of DHB and OPH in the dried powder are 0.491%±0.124% and 0.446%±0.076% w/w, respectively. Thus, C. hystrix fruit peel should contain DHB and OPH not <0.40% dry weight.6
C. zedoaria Roscoe
It is an herb in the Zingiberaceae family. The rhizome is traditionally used as a decoction for flatulent colic. The essential oil from the rhizomes contains α-pinene, d-camphor, d-camphene, and cineole,13 while a great number of sesquiterpenes were also isolated. They are derived from germacrane-, elemane-, cadinane-, eudesmane-, and guainane-type skeletons. For example, curdione and germacrone-7,8-epoxide are germacrane type; zedoarone and curzerene are elemane type; pyrocurzerenone and curzeone are cadinane type; curcolone is eudesmane type; and procurcumenol and zedoarol are guainane type.14 In addition, curcumin (CCM) and its related compounds bis-(4-hydroxycinnamoyl)-methane and 4-hydroxycinnamoyl feruloyl methane are also detected in the rhizome of C. zedoaria.15
HPLC was performed using water and acetonitrile with gradient elution: 50:50, 50:50, 60:40, 60:40, 50:50, and 50:50 at 0, 8, 10, 15, 16, and 20 minutes, respectively. The linear regression equations and concentration ranges for three makers (Figure 2), namely, CCM, demethoxycurcumin (DMC), and bis-demethoxycurcumin (BMC), are shown in Table 2. The lower limits of CCM, DMC, and BMC are 0.320%±0.002%, 0.228%±0.002%, and 0.120%±0.001% w/w, respectively. The upper limits of CCM, DMC, and BMC are 0.732%±0.003%, 1.427%±0.055%, and 0.436%±0.021% w/w, respectively. The average quantities of CCM, DMC, and BMC in the dried powder are 0.616%±0.125%, 0.912%±0.352%, and 0.252%±0.104% w/w, respectively. Thus, C. zedoaria rhizome should contain CCM, DMC, and BMC not <0.5%, 0.6%, and 0.15% dry weight, respectively.
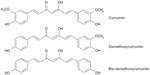  | Figure 2 Structures of markers for Curcuma zedoaria. |
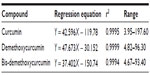  | Table 2 Linear regression equation and concentration ranges of markers for Curcuma zedoaria |
E. americana (Aubl.) Merr. ex K. Heyne
It is an herb of the Iridaceae family. The bulb of this plant is traditionally used as a carminative, and together with galangal, it can treat a cold in children. The ethanol extract from the bulb of E. americana contains three naphthalene derivatives, which are eleuterol, eleutherin, and isoeleutherin.16 Upon further investigation, hongconin, a new naphthalene derivative, was isolated. Its structure was elucidated based on chemical and spectroscopic methods.17 Anthraquinone and methyl ester of 3,4,8-trihydroxy-1-methyl-anthra-9,10-quinone-2-carboxylic acid were also found in the rhizome of E. americana.18 Recently, Paramapojn et al19 isolated a new compound, eleutherioside A, from the bulb of this plant (Figure 3). Its structure was also elucidated based on spectroscopic methods.
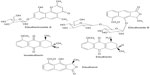  | Figure 3 Structures of markers for Eleutherine americana. |
HPLC was performed using water and acetonitrile with gradient elution: 85:15, 70:30, 55:45, 50:50, 40:60, 20:80, and 20:80 at 0, 10, 15, 20, 23, 25, and 30 minutes, respectively. The linear regression equations and concentration ranges for five makers, namely eleutherinoside A (A), eleuthoside B (B), isoeleutherin (C), eleutherin (D), and eleutherol (E), are shown in Table 3. The amounts of A, B, C, D, and E in the dried powder of E. americana bulbs collected from ten locations are found in the ranges of 0.011%±0.001% to 0.158%±0.001%, 0.001%±0.001% to 0.260%±0.006%, 0.089%±0.001% to 0.161%±0.002%, 0.138%±0.003% to 0.267%±0.004%, and 0.099%±0.002% to 0.196%±0.003% w/w, respectively. The average contents of A, B, C, D, and E in the dried powder are 0.091%±0.045%, 0.169%±0.083%, 0.112%±0.021%, 0.210%±0.039%, and 0.135%±0.028% w/w, respectively. Therefore, E. americana bulb should contain A, B, C, D, and E not <0.05%, 0.1%, 0.1%, 0.2%, and 0.1% dry weight, respectively.6
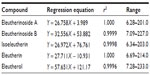  | Table 3 Linear regression equation and concentration ranges of makers for Eleutherine americana |
N. sativa L.
It is an herb of the Ranunculaceae family. The seed is used as a carminative, diuretic, and anthelmintic. The essential seed oil contains thymoquinone, nigellone, and 2-methyl-4-isopropyl-p-quinone.20 Seeds contain fatty acids, including palmitic, myristic, oleic, linoleic, and linolenic acids. β-Sitosterol and flavonol triglycosides are also found in the seeds of N. sativa.21
HPLC was performed using water and acetonitrile with gradient elution: 85:15, 80:20, 60:40, 50:50, 20:80, and 20:80 at 0, 5, 10, 15, 20, and 25 minutes, respectively. The linear regression equation is Y=95.279X+81.601 with r2=0.9998 for thymoquinone (Figure 4). The concentrations range between 1.47 and 357.06 μg/mL. The content of thymoquinone in the dried powder of N. sativa seeds from ten locations is found in the range of 0.112%±0.004% to 0.369%±0.004% w/w. The average amount of thymoquinone is 0.220%±0.106% w/w. Thus, N. sativa seeds should contain thymoquinone not <0.1% dry weight.6
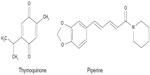  | Figure 4 Structures of markers for Nigella sativa and Piper retrofractum. |
P. retrofractum Vahl
This plant is a vine of the Piperaceae family. The fruit is traditionally used as a carminative and for preparing a decoction for the treatment of diarrhea. The major active constituents of the fruit are alkaloid piperine (Figure 4) and volatile oil. The other compounds are phenolic amides, such as guineensine, pellitorine, and N-isobutyl-2E,4E,8Z-eicosatrienamide. The neutral compounds are methyl piperate and β-sitosterol, while the acidic compound is piperic acid.22 Techadamrongsin et al reported many alkaloids in the fruits, including 2,4-decadienoylpiperidine, (2E,4E)-N-eicosadienoylpiperidine, (2E,14E)-N-eicosadienoylpiperidine, (2E,4E,14E)-N-isobutyleicosatrienamide, 1-(2E,4E)-octadecadienoylpiperidine, 1-(2E,4E)-octadecatrienoylpiperidine, piperamine, (2E,4E,14E)-N-isobutyloctadecatrienamide, piplartine, piperlonguminine, piperundecalidine, piperonaline, sylvetin, retrofractamide A, C, D, piperoctadecalidine, dihydropiperlonguminine, pipercide, octadecadienamide, eicosatrienamide, and n-isobutyldeca-trans-4-dienamide.23
HPLC was carried out using water and acetonitrile with gradient elution: 60:40, 55:45, 40:60, 30:70, 10:90, and 10:90 at 0, 10, 15, 25, 30, and 35 minutes, respectively. The linear regression equation is Y=22.541X−55.953 with r2=1.000 for piperine. The concentrations range between 2.06 and 501.29 μg/mL. The amount of piperine in the dried powder of P. retrofractum fruits from ten locations are found in the range of 2.84%±0.03% to 3.61%±0.03% w/w. The average content of piperine in the dried fruit powder is 3.24%±0.26% w/w. Therefore, P. retrofractum fruits should contain piperine not <3% dry weight.6
P. nigrum L.
It is a vine of the family Piperaceae. The fruit is traditionally used for cough, rhinitis, and anemia. The fruit consists of piperine, piperetine, amide-peperylin, piperoleins A and B, N-isobutyl-cicosa-trans-2-trans-4-diamide, guineensine, and sarmentine. Other alkaloids include chavicine, piperidine, piperettine, methyl caffeic acid, piperidide, β-methyl pyrroline, and a series of vinyl homologs of piperine and their stereoisomers. Essential oil from the fruit contains mainly sabinene (15%–25%), caryophyllene, α- and β-pinene, citronellol, α-thujene, myrcene, limonene, terpinene, p-humulene, selinene, camphene, linalool, terpineol, and nerolidol in varying amounts.24–27
The HPLC conditions, linearity, and range of concentration are the same as those for P. retrofractum. The content of piperine in the dried powder of P. nigrum fruits from ten locations is in the range of 2.54%±0.01% to 3.97%±0.01% w/w. The average amount of piperine in the dried powder is 3.37%±0.48% w/w. Thus, P. nigrum fruits should contain piperine not <3% dry weight.6
Z. cassumunar Roxb.
It is an herb of the family Zingiberaceae. The rhizome is used as a carminative and antispasmodic and for treatment of digestive problems. Amatayakul et al isolated six aromatic compounds from the rhizome of Z. cassumunar, which are cis-3-(2′,4′,5′-trimethoxyphenyl)-4-[(E)-2″′,4″′,5″′-trimethoxystyryl]cyclohex-1-ene; cis-3-(3′,4′-dimethoxyphenyl)-4-[(E)-3″′,4″′-dimethoxystyryl]cyclohex-1-ene; cis-3-(3′,4′-dimethoxyphenyl)-4-[(E)-2″′,4″′,5″′-trimethoxystyryl]cyclohex-1-ene; (E)-4-(3′,4′-dimethoxyphenyl)but-3-en-1-ol (compound D); (E)-4-(3′,4′-dimethoxyphenyl)but-3-en-1-yl acetate; and 8-(3,4-dimethoxyphenyl)-2-methoxynaphtho-1, 4-quinone.28 Kuronayaki et al reported three compounds with the same core structure.29 In 1981, Tuntiwachwuttikul et al reported phenylbutanoids from the rhizome of Z. cassumunar, which are (E)-4-(3′,4′-dimethoxyphenyl)but-1,3-diene; (E)-4-(3′,4′-dimethoxyphenyl)but-3-ene; (E)-4-(2′,4′,5′-trimethoxyphenyl)but-1,3-diene; (E)-4-(2′,4′,5′-trimethoxyphenyl)but-3-ene; (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-yl palmitate; 3,4-dimethoxybenzaldehyde; and 2,4,5-trimethoxybenzaldehyde.30
The HPLC conditions comprise BDS Hypersil C18 column as a stationary phase. The mobile phase comprises water and acetonitrile with gradient elution: 30:70, 45:65, 60:40, 45:55, 45:55, 70:30, 70:30, 55:45, and 30:70 at 0, 8, 10, 11.5, 30, 35, 40, 45, and 50 minutes, respectively. The linear regression equation and concentration ranges for the three markers (Figure 5), namely E-4-(3,4-dimthoxyphenyl)but-3-en-1-ol (A); CCM (B); and cis-3-(2′,4′,5′-trimethoxyphenyl)-4-[(E)-2′′′,4′′′,5′′′-trimethoxystyryl] cyclohex-1-ene (C), are shown in Table 4. The dried rhizomes of Z. cassumunar were collected from 14 locations. The contents of A, B, and C in the powdered rhizomes are in the range of 0.400%±0.001% to 1.642%±0.005%, 0.066%±0.001% to 0.265%±0.002%, and 0.027%±0.001% to 0.287%±0.004% w/w, respectively. The average contents of A, B, and C are 0.895%±0.374%, 0.131%±0.073%, and 0.126%±0.076% w/w, respectively. Therefore, Z. cassumunar rhizomes should contain A, B, and C not <0.8%, 0.13%, and 0.12% dry weight, respectively.6
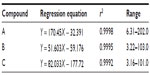  | Table 4 Linear regression equation and concentration ranges of makers for Zingiber cassumunar |
Z. officinale Roscoe (ginger)
It is an herb of the Zingiberaceae family. The rhizome is used as stomachic, antiemetic, and anti-inflammatory agent and as an expectorant. Ginger rhizome oleoresin contains mainly pungent principles such as homologous gingerol, shogoal, and zingerone. Zingerone and shogoal are found in small amounts in fresh ginger but in large amounts in stored products. The series of gingerol and related compounds are composed of [3]-, [4]-, [5]-, [6]-, [8]-, [10]-, and [12]-gingerols; [3]-, [4]-, [5]-, [6]-, [8]-, and [10]-shogoals; [4]-, [6]-, [8]-, and [10]-gingerdiols; [6]-methylgingerdiol; and [4]- and [6]-gingerdiacetate.31,32
HPLC was performed using water and acetonitrile with gradient elution: 50:50, 50:50, 30:70, 20:80, 10:90, and 10:90 at 0, 7, 10, 15, 20, and 30 minutes, respectively. The linear regression equation is Y=10.938X+17.08 with r2=1.000 for [6]-gingerol (Figure 6). The concentrations range between 1.61 and 390.63 μg/mL. The quantity of [6]-gingerol in the dried powder of Z. officinale rhizomes collected from ten locations is found in the range of 0.263%±0.018% to 1.120%±0.054% w/w. The average amount of [6]-gingerol in the dried powder is 0.641%±0.245% w/w. Thus, Z. officinale rhizomes should contain [6]-gingerol not <0.4% dry weight.6
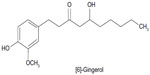  | Figure 6 Structure of a marker for Zingiber officinale. |
Formation of artifacts in Prasaplai preparation
During the investigation on Prasaplai formulation, three artifacts (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-yl linoleate (1); (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-yl oleate (2); and (E)-4-(3,4-dimethoxyphenyl)but-3-en-1-yl palmitate (3) are originated during storage of the preparation2 (Figure 7). These three compounds are formed by the interaction of components in Prasaplai preparation on the first day after mixing all ingredients together. After investigating the origin of the artifacts by a systematic preparation of two-component mixtures and analysis with HPLC, it is found that the artifacts are formed from the mixture of the rhizomes of Z. cassumunar and the seeds of N. sativa. A possible explanation for this phenomenon is that the formation of artifacts may occur from an esterification reaction between alcohol ((E)-1-(3,4-dimethoxyphenyl)but-3-en-1-ol or compound D) and carboxylic acids (fatty acids) in a special condition.33 These artifacts continue to be formed and saturated after 73 days of storage and are stable for 1 year.34
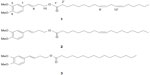  | Figure 7 Structures of artifacts 1–3. |
Standardization of artifacts in Prasaplai preparation
According to the Thai traditional way, Prasaplai can be administered by two approaches. First, it is recommended to take one teaspoon of the Prasaplai powder directly with warm water. The other method is to take it with spirit (40% ethanol). The preparation is macerated with 40% ethanol for a certain period of time and the decoction extract is taken.1 In order to produce Prasaplai preparation in a more modern pharmaceutical form, its extract is used instead of the powdered drug.
Preparation of Prasaplai extract
Tangyuenyongwatana and Gritsanapan studied the extraction method of traditional preparation of Prasaplai.35 Hexane, 40% ethanol, and distilled water were used as extracting solvents. The target markers used in this study were compound D ((E)-1-(3′,4′-dimethoxyphenyl)but-3-en-1-ol), (E)-4-(3′,4′-dimethoxyphenyl)butadiene (DMPBD),36 and compounds 1, 2, and 3. All of these compounds were synthesized with purity >95%.
For hexane extract, 1 g of Prasaplai preparation, which is equal to one teaspoon dose according to the medical indication, was transferred into a cotton bag and then the bag was sealed. The Prasaplai bag was placed in a 250 mL stopper flask and hexane (50 mL) was added. Then, the flask was sonicated for 30 minutes and the extract was decanted. The process was continued until exhausted as monitored by thin layer chromatography. The extracts were combined, filtered, and evaporated to dryness using a rotary evaporator. The weight of the crude extract was recorded.
For 40% ethanol and water extracts, the same procedure was carried out for each solvent except for freeze drying, which was used in the drying process of the water extract instead of using a rotary evaporator.
Determination of appropriate solvent for Prasaplai extraction
For each Prasaplai extract, four major standard compounds, which are compound D, DMPBD, and the three artifacts, were used to prepare the calibration curves for HPLC analysis.35 The HPLC system, a Knauer pump K-1001, and a Knauer Photometer K-2600 detector with detection at 254 nm were used for this experiment. The separation was performed on Kromasil 5 μm 100A C18, 250×4 mm column, flow rate was 0.8 mL/min, and the solvent system was a gradient elution of 1% acetic acid in water and CH3CN starting from 85:15, 70:30, 55:45, 50:50, 30:70, 15:85, 0:100, and 0:100 at 0, 8, 25, 30, 55, 65, 80, and 100 minutes, respectively.
Linearity of each compound was determined by using five concentrations in certain ranges. Linear regression equations, correlation coefficients (r2), and limit of quantification are shown in Table 5. The results of regression analysis of each compound had a correlation coefficient >0.9992.
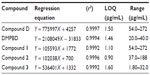  | Table 5 Calibration equation, correlation coefficient, and limit of quantification of standard compounds for standardization of Prasaplai preparation |
The hexane extract was prepared by sonicating Prasaplai preparation with hexane in an ultrasonic bath. After evaporation to dryness, 26.70±0.11 mg (2.7% w/w) of crude hexane extract was obtained as a brown residue. HPLC chromatogram showed that the hexane crude extract comprised the peaks of active anti-inflammatory ingredients, compound D, DMPBD, and three artifacts, which are compounds 1–3.
For the 40% ethanol extraction of Prasaplai preparation, the same procedure was carried out as mentioned in the previous experiment. After evaporation to dryness, 33.96±0.05 mg (3.40% w/w) of brown crude 40% ethanol extract was obtained. HPLC chromatogram showed that the 40% ethanol crude comprised peaks less than those of the hexane extract and lacked the three artifact peaks. Compound D and DMPBD still appeared in the crude 40% ethanol extract.
For the water extraction of Prasaplai preparation, after freeze drying, 49.83±0.30 (4.98% w/w) of crude water extract was obtained as a yellow residue. HPLC chromatogram showed that the water crude extract comprised mainly compound D peak and lacked the three artifact peaks.
Among the Prasaplai extracts, 40% ethanol extract seemed to be the appropriate extract for the development of modern dosage forms of Prasaplai because it contained the highest amount of major anti-inflammatory agents, compound D and DMPBD.
Conclusion
In modern times, standardization of herbal preparation is essential for the quality of drugs because of the variation in the concentration of their active principles. Quality assessment of an herbal formulation is important to justify their acceptability in modern medicine system. Prasaplai, a popular Thai traditional medicine preparation that has been used to treat dysmenorrhea, is composed of ten herbs that need to be standardized to avoid the inconsistency of active ingredients in the raw materials. Prasaplai preparation is standardized by a modern method using HPLC. The amounts of active compounds in each raw material collected from ten different places in Thailand were evaluated and the information was recorded as a guidance. Another interesting aspect in this preparation is the artifacts, which are originated during storage of the preparation. These artifacts can be avoided by using 40% ethanol extract of Prasaplai powder, which thus changes the way of administration of Prasaplai. The standardization of Prasaplai preparation is essential for assessing the quality of the product and promoting the interest of people who suffer from dysmenorrhea.
Acknowledgment
We thank Mr Panupon Khumsupan for his kind help in proofreading the manuscript.
Disclosure
The authors declare no conflicts of interest in this work.
References
Poomchusri NT. Ayurvedic Study. 2nd ed. Bangkok: Promjakkanpim; 1973:194. | |
Nualkaew S, Gritsanapan W, Petereit F, Nahrstedt A. New fatty acid esters originate during storage by the interaction of components in Prasaplai, a Thai traditional medicine. Planta Med. 2004;70:1243–1246. | |
National List of Essential Drugs Committee. National List of Essential Drug AD. 1999;1999:2. | |
Kamalashiran C, Lekskulchai O. The comparative study of the efficiency and side effect of Prasaplai extract versus mefenamic acid on relieving primary dysmenorrheal: a clinical phase II trials. Thammasart Med J. 2012;12(4):749–756. | |
Sriyakul K, Kietinun S, Pattaraarchachai J, Ruangrungsi N. A comparative double-blinded randomized study: the efficacy of Prasaplai herbal extract versus mefenamic acid in relieving pain among primary dysmenorrheal patients. Open Complement Med J. 2012;4:16–21. | |
Paramapojn S. Standardization and Biological Activities of Prasaplai, a Thai Traditional Medicine [Ph.D. thesis]. Salaya: Mahidol University; 2008. | |
Department of Medical Sciences, Ministry of Public Health. Thai Herbal Pharmacopoeia (Volumn II). Bangkok: Prachachon; 2000. | |
Department of Medical Sciences, Ministry of Public Health. Thai Herbal Pharmacopoeia. Bangkok: Prachachon; 1995. | |
Satyal P, Paudel P, Poudel A, et al. Chemical composition, biological activities of Acorus calamus essential oils from Nepal. Nat Prod Commun. 2013;8(8):1179–1181. | |
ASEAN Countries. Standard of ASEAN Herbal Medicine. Vol 1. Jakata: Aksara Berana Printing; 1993. | |
Murakami A, Gao G, Kim OK, et al. Identification of coumarins from the fruit of Citrus hystrix DC as inhibitors of nitric oxide generation in mouse macrophage RAW 264.7 cell. J Agric Food Chem. 1999;47:333–339. | |
Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaemferol, luteolin and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–3112. | |
Khare CP. Indian Herbal Remedies. Berlin, Germany: Springer-Verlag; 2004:176–177. | |
Tang W, Eisenbrand G. Chinese Drugs of Plant Origin. Berlin: Springer-Valag; 1992:79–86. | |
Syu WJ, Shen CC, Don MJ, Ou JC, Lee GH, Sun CM. Cytotoxicity of curcuminoids and some novel compounds from Curcuma zedoaria. J Nat Prod. 1998;61:1531–1534. | |
Chen ZX, Huang HZ, Wang CR, Li YH, Ding JM. Studies on the active constituents of Hong Cong (rhizomes of Eleutherine americana). Chin Trad Herb Drugs. 1981;12:484. | |
Chen ZX, Huang HZ, Wang CR, et al. Hongconin, a new naphthalene derivative from the rhizome of Eleutherine americana (Hong-Cong). Heterocycle. 1984;22:691–694. | |
Komura H, Mizukawa K, Minakata H, Huang H, Qin G, Xu R. New anthraquinones from Eleutherine americana. Chem Pharm Bull. 1983;31(11):4206–4208. | |
Paramapojn S, Ganzera M, Gritsanapan W, Stuppner H. Analysis of naphthoquinone derivatives in the Asian medicinal plant Eleutherine americana by RP-HPLC and LC-MS. J Pharm Biomed Anal. 2008;47:990–993. | |
Parrotta JA. Healing Plants of Penisular Indica. Oxon: CABT Publishing; 2001:601. | |
Merfort I, Wray V, Barakat HH, Hussein SAM, Nawwar MAM, Willuhn G. Flavonol triglycosides from seeds of Nigella sativa. Phytochemistry. 1997;46(2):359–363. | |
Nakatani N, Inatani R, Ohta H, Nishioka A. Chemical constituents of peppers (Piper spp.) and application to food preservation: natural occuring antioxidative compounds. Environ Health Perspect. 1986;67:135–142. | |
Techadamrongsin Y, Dechatiwongse Na Ayudhya T. Chemical and physical studies of commercial Java long pepper. Mahidol Univ J Pharm Sci. 1996;23(1):20–28. | |
Dorman HJ, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88(2):308. | |
Jain SR, Kar A. Antibacterial activity of some essential oils and their combinations. Planta Med. 1971;20:118. | |
Rao CSS, Nigam SS. Antimicrobial activity of some Indian essential oils. Indian Drugs. 1976;14:62. | |
Jain SR, Jain MR. Antifungal studies on some indigenous volatile oils and their combinations. Planta Med. 1972;22:136. | |
Amatayakul T, Cannon JR, Dampawan P, et al. Chemistry and crystal structures of some constituents of Zingiber cassumuar. Aust J Chem. 1979;32:71–88. | |
Kuronayaki M, Fukushima S, Yoshihira K, et al. Further characterization of the constituents of a Thai medicinal plant, Zingiber cassumuar Roxb. Chem Pharm Bull. 1980;28(10):2948–2959. | |
Tuntiwachwuttikul P, Pancharoen O, Jaipetch T, Reutrakul V. Phenylbutanoids from Zingiber cassumuar. Phytochemistry. 1981; 20(5):1164–1165. | |
Thomson M, Al-Qattan KK, Al-Sawan SM, Alnaqeeb MA, Khan I, Ali M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandin Leukot Essent Fatty Acids. 2002;67(6):475–478. | |
Kiuchi F, Iwakami S, Shibuya M, Hanako F, Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem Pharm Bull. 1992;40(2):387–391. | |
Tangyuenyongwatana P, Gritsanapan W. Mechanism evaluation of artifacts formation in Prasaplai preparation, a Thai traditional medicine. Planta Med. 2008;74:1403–1405. | |
Tangyuenyongwatana P, Gritsanapan W. Quantitative analysis and toxicities determination of artifacts originated in a Thai traditional medicine Prasaplai. Pharm Biol. 2010;48(5):584–588. | |
Tangyuenyongwatana P, Gritsanapan W. Determination of appropriate solvent for preparation of Thai traditional medicine Prasaplai extract. Songklanakarin J Sci Technol. 2009;31(5):527–531. | |
Jeenapongsa R, Yoorathaworn K, Pongprayoon U, Sriwatanakul K. Anti-inflammatory activity of (E)-4-(3′,4′-dimethoxyphenyl)butadiene from Zingiber cassumuar Roxb. J Ethnopharmacol. 2003;87:143–148. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.