Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
Standardisation of Clinical Assessment, Management and Follow-Up of Acute Hospitalised Exacerbation of COPD: A Europe-Wide Consensus
Authors Ramakrishnan S , Janssens W , Burgel PR , Contoli M , Franssen FME , Greening NJ, Greulich T, Gyselinck I, Halner A, Huerta A , Morgan RL , Quint JK , Vanfleteren LEGW , Vermeersch K, Watz H, Bafadhel M
Received 20 October 2020
Accepted for publication 11 December 2020
Published 16 February 2021 Volume 2021:16 Pages 321—332
DOI https://doi.org/10.2147/COPD.S287705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Sanjay Ramakrishnan,1– 3 Wim Janssens,4 Pierre-Regis Burgel,5 Marco Contoli,6 Frits ME Franssen,7 Neil J Greening,8 Timm Greulich,9 Iwein Gyselinck,4 Andreas Halner,1 Arturo Huerta,10 Rebecca L Morgan,11 Jennifer K Quint,12 Lowie EGW Vanfleteren,13 Kristina Vermeersch,4 Henrik Watz,14 Mona Bafadhel1
1Respiratory Medicine Unit, Nuffield Department of Medicine - Experimental Medicine, University of Oxford, Oxford, UK; 2 National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), University of Oxford, Oxford, UK; 3School of Medical and Health Sciences, Edith Cowan University, Perth, Australia; 4Department of Respiratory Diseases, UZ Leuven, Research Group BREATHE, KU Leuven, Leuven, Belgium; 5Faculty of Medicine, University of Paris and INSERM 1016 Institut Cochin, Cochin Hospital, Assistance Publique Hôpitaux de Paris, Paris, France; 6Department of Morphology, Surgery and Experimental Medicine, University of Ferrara, Ferrara, Italy; 7Department of Respiratory Medicine, Maastricht University Medical Center, Maastricht, Netherlands; 8Department of Respiratory Sciences, NIHR Leicester Biomedical Research Centre (Respiratory), Glenfield Hospital, Leicester, UK; 9Department of Medicine, Pulmonary and Critical Care Medicine, University Medical Centre Giessen and Marburg, Philipps University, Member of the German Centre for Lung Research (DZL), Marburg, Germany; 10Pulmonary and Critical Care Division, Clinica Sagrada Familia, IDIBAPS August Pi i Sunyer Biomedical Research Institute, Barcelona, Spain; 11Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, Canada; 12National Heart & Lung Institute, Imperial College, London, UK; 13COPD Center, Department of Respiratory Medicine and Allergology, Sahlgrenska University Hospital, Department of Internal Medicine and Clinical Nutrition at Institute of Medicine, SU Sahlgrenska, Göteborg, Sweden; 14Pulmonary Research Institute at LungenClinic Grosshansdorf, Airway Research Center North (ARCN), German Center for Lung Research (DZL), Grosshansdorf, Germany
Correspondence: Mona Bafadhel
Respiratory Medicine Unit, Nuffield Department of Medicine Experimental Medicine, University of Oxford, Oxford, UK
Tel +44 1865 612898
Email [email protected]
Background: Despite hospitalization for exacerbation being a high-risk event for morbidity and mortality, there is little consensus globally regarding the assessment and management of hospitalised exacerbations of COPD. We aimed to establish a consensus list of symptoms, physiological measures, clinical scores, patient questionnaires and investigations to be obtained at time of hospitalised COPD exacerbation and follow-up.
Methods: A modified Delphi online survey with pre-defined consensus of importance, feasibility and frequency of measures at hospitalisation and follow-up of a COPD exacerbation was undertaken.
Findings: A total of 25 COPD experts from 18 countries contributed to all 3 rounds of the survey. Experts agreed that a detailed history and examination were needed. Experts also agreed on which treatments are needed and how soon these should be delivered. Experts recommended that a full blood count, renal function, C-reactive protein and cardiac blood biomarkers (BNP and troponin) should be measured within 4 hours of admission and that the modified Medical Research Council dyspnoea scale (mMRC) and COPD assessment test (CAT) should be performed at time of exacerbation and follow-up. Experts encouraged COPD clinicians to strongly consider discussing palliative care, if indicated, at time of hospitalisation.
Interpretation: This Europe-wide consensus document is the first attempt to standardise the assessment and care of patients hospitalised for COPD exacerbations. This should be regarded as the starting point to build knowledge and evidence on patients hospitalised for COPD exacerbations.
Keywords: COPD, disease exacerbation, hospitalisation, patient care, consensus development, expert opinion
Introduction
Hospitalised exacerbations of chronic obstructive pulmonary disease (COPD) account for a significant proportion of bed pressures and hospital costs throughout the world, including Europe1,2 and North America.3 These exacerbations also confer a high risk of in-hospital mortality of approximately 5–8%4,5 and carry up to 58% risk of re-admissions within 1 month6,7 and up to a 20–25%5 risk of mortality in the 12 months following discharge.
Major international COPD guidelines8,9 provide clinicians with very little guidance for standardisation of clinical assessment, examination, laboratory and radiological tests and treatment in hospitalised exacerbations of COPD (HECOPD). There is also no consensus on patient follow-up frequency and the details on what should be measured during the post hospitalisation follow-up phase. For example, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) suggests measuring spirometry in everyone hospitalised for an exacerbation of COPD.8 A 2016 European audit4 showed that more than half of patients admitted to hospital for an exacerbation of COPD had never had a spirometry recorded. Similarly, the GOLD report8 recommends the measurement of an arterial blood gas, yet uptake of this is incomplete.4 Even in long-term treatment decisions, such as the use of long-term oral corticosteroids, physician practices do not match the guidelines.10
Our colleagues in other fields of acute hospital care, such as cardiology and rheumatology, have enviable evidence-based guidelines.11,12 These often define what, when, how and the frequency a patient should have assessment of symptoms, tests, outcomes and treatments. This has led to standardisation of treatment protocols and clinical trial endpoints. Without a doubt, this has played a major contributory role in the improvements in patient outcomes in rheumatoid arthritis13 and myocardial disease.14 There are also clear lessons that COPD specialists can take on board from other clinical areas. In 2004,15 the Outcome Measures in Rheumatology (OMERACT) collaborative set out to achieve an expert consensus statement on different outcome measures and treatment goals in caring for patients with psoriatic arthritis. Like for HECOPD, they set out from a place of limited evidence and aimed to achieve a standardised starting point to then build their evidence base on. Within a few years, the collaborative achieved global expert consensus on outcomes in psoriatic arthritis care,16 which was then taken up by major international professional bodies.17
With this context, we have investigated consensus as well as the areas of disagreement in the evaluation of the expert view on demographic, clinical characteristics, comorbidities, investigations and clinical outcomes for patients who are hospitalised for acute exacerbations of COPD as part of the CICERO collaboration.18
Methods
This study used a modified, 3-round online survey based on the Delphi method19 to establish a defined list of variables that should be measured at the time of a HECOPD. The survey was conducted via a secure online survey platform (surveymonkey.com). The variables were divided into symptoms, examination findings, co-morbidities, clinical scores, laboratory tests, point of care test (eg, ECG, spirometry), other tests (eg, radiology, detailed lung function tests), treatments and clinical outcomes of importance. All the items were assessed for use at time of hospitalisation and at the post hospitalisation follow-up phase. Excepting the history-taking sections, the feasibility of undertaking each of these assessments and treatments was also assessed. Ethics or institutional review board approval was not required. This survey was exempt from approval as it was non-invasive, undertaken voluntarily by medical professionals and did not involve any patients.
Expert Selection
To understand current practice in Europe and to derive a consensus list of variables, we set out to invite a diverse panel of COPD experts from as many countries in Europe as possible. Policy Delphi20 methodologists recommend a panel size of between 15–40 experts to achieve an appropriate balance between points of view. Experts were contacted by the European Respiratory Society if they met 2 or more of the following criteria:
- Board-certified pulmonologists who currently spend at least 20% of their time caring for patients hospitalised for acute exacerbation of COPD
- Evidence of publication of important COPD research relevant to assessment or management of patients hospitalised for exacerbations of COPD
- A history of participation in the development of local or national guidelines for the management of COPD
Delphi Process
The modified Delphi process consisted of 3 iterative rounds (subsequently called rounds 1, 2 and 3). Each expert was provided a unique secure link to an online questionnaire platform. The variables were listed in groups, and experts were asked to rate the importance, feasibility and a suggested frequency on a Likert scale. Experts were reminded that the survey sought to obtain their opinion on clinical care for HECOPD. Free text capability for expert comments were sought for each section. Any new item suggested was added to the following round of the electronic survey. Members then returned the completed online surveys anonymously. Experts were asked to return surveys within a 3-week period. Reminder e-mails were sent to encourage completion, and extensions were given when necessary. This is summarised in Figure 1.
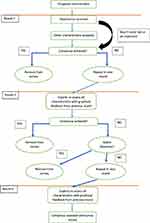 |
Figure 1 Schematic illustrating the Delphi survey process. |
Consensus, dissensus and stability criteria for the Delphi process were pre-determined prior to Delphi process commencement. Consensus21,22 was defined as an interquartile range (IQR) of ≤1 for a 4- or 5-point Likert scale item. For 3-point Likert scales and for Yes/No items, an IQR of 0 was needed to achieve consensus. A Wilcoxon signed rank test was performed on paired results of expert’s responses to assess stability of responses between rounds. If the responses were not statistically significantly different (p value ≥ 0.05), responses were considered stable. At the completion, any item that reached a score of “important“, “important to very important“ or “very important“ was included in the final consensus list. The corresponding feasibility and frequency were also reported if consensus was achieved. If items were rated “neutral“ or “neutral to important“, they were assigned as “to be considered” in any future evaluation. Items where consensus was not achieved are also reported.
Variable Selection
A detailed literature review was undertaken by SR and MB to assess the current evidence basis for symptoms, examination findings, co-morbidities, clinical scores, laboratory tests, point of care test (eg, electrocardiogram, spirometry), other test (radiology, detailed lung function tests), treatments and clinical outcomes of importance for use in hospitalised patients with exacerbation of COPD (search criteria used are available in supplementary Table 1), prior to design of the Delphi survey. A final decision for survey input was made at a face to face meeting by SR, MB, WJ and AH.
Round 1
As we were aiming to establish experts’ views on many variables and many aspects of the variable (importance, frequency, feasibility etc), a skip logic was programmed to help reduce the survey burden. If an expert marked a variable as “Not at all important” on the importance Likert scale, the item was removed from the survey for that expert for all subsequent lower order items (eg, feasibility). In other words, if experts rated something as not important, then aspects of that item, eg, feasibility and frequency, were deemed irrelevant for the remainder of round 1.
Round 2
All items that achieved consensus in round 1 were removed from round 2. If consensus was only achieved on one aspect of the item, for example the importance of a particular clinical test, but not the feasibility or frequency, the importance section was not re-evaluated in round 2, but the other aspects were re-evaluated. Any variable that was re-evaluated was accompanied by a histogram of the previous round’s expert responses and a median of the responses. Any items suggested by experts in round 1 were also included in round 2. No skip logic was programmed for round 2.
Round 3
Again, variables achieving consensus were removed. For items that did not achieve consensus, stability was assessed. If a variable remained in dissensus and remained stable, the item was removed and marked as “stable disagreement”. If a variable remained in dissensus but had changed significantly, the item was marked for re-evaluation in round 3. Like round 2, any new suggestions from experts in round 2 were included. Questions were modified for clarity and/or specificity in response to experts’ suggestions. There was no skip logic. Any variable that was re-evaluated was accompanied by a histogram of the previous round’s expert responses and a median of the responses for both rounds.
Results
A total of 25 COPD experts from 19 European countries completed all 3 rounds of the Delphi survey. There were 3 experts from the UK; 2 each from the Netherlands, France, Germany and Italy; and 1 each from Belgium, Spain, Switzerland, Portugal, Croatia, Estonia, Serbia, Latvia, Sweden, Turkey, Slovenia, Finland, Greece and Poland. There were 8 (32%) female experts, and the majority were aged between 41–50 years (56%). All experts worked in the field of COPD in secondary or tertiary/academic institutions. On average, experts spent 22% of their time caring for respiratory inpatients. All but one of the experts were actively involved with research into COPD care. After round 2, no further new items were recommended to gain consensus. The survey was sent and completed prior to the coronavirus disease 2019 (COVID-19) pandemic.
Expert Consensus Opinion During an Acute Hospitalised Exacerbation of COPD
Symptoms
There were 29 symptoms that were assessed for importance, method of symptom data capture (binary vs severity scale) and frequency of symptom capture. After 3 rounds, no consensus was achieved on 3 items (low mood, sneezing and poor sleep). A further 2 symptoms, namely runny eyes and itchiness, were excluded by experts. Of the remaining 24 symptoms, experts recommend that 12 symptoms must be recorded at time of exacerbations and 12 that ought to be considered (see Table 1). The experts endorse that most symptoms could be recorded in a binary form (ie, present or absent). For the symptoms of cough and sputum purulence respectively, experts could not agree whether this should be reported quantitively (on a scale of severity) or qualitatively (absent or present) after 3 rounds.
 |
Table 1 Recommended Symptom Data Capture at Time of Hospitalised Exacerbation |
Co-Morbidities
Respondents recommended that a complete and detailed medical history was necessary at time of exacerbation. Some medical history items, namely, history of atopy, osteoporosis, chronic kidney disease, human immunodeficiency virus (HIV) status and non-lung primary malignancy were rated as being less important for routine recording but should be considered.
Clinical Signs
Consensus was achieved on the importance of recording respiratory clinical signs. However, expert opinion was that treating physicians need only consider recording some cardiac signs, such as heart murmurs, pulsus paradoxus, jugular venous pressure (JVP), in addition to body weight at time of hospitalised exacerbations (see Table 2). Experts agreed that menstrual cycle, forearm and quadriceps strength, abdominal distension, abdominal tenderness and pulsatile liver edge did not need to be actively recorded unless relevant to the patient history. The consensus list of clinical signs to elicit at the post hospitalisation follow-up phase are listed in supplementary Table 2.
 |
Table 2 Recommended Clinical Signs Data Capture at Time of Hospitalised Exacerbation |
Clinical Tests
An expert consensus was reached on which tests should be performed at the time of a severe hospitalised exacerbation of COPD (see Table 3). Experts recommend that a full blood count, renal function, C-reactive protein (CRP), cardiac troponin and BNP are essential tests and should be completed within 4 hours of hospitalisation. Similar to this, the experts recommended that a chest radiograph and ECG should be performed as soon as possible. The optimum timing of when other essential tests should be performed (namely arterial blood gas, sputum cultures and viral swabs) could not be decided. Other routinely available tests were recommended by experts to be investigations to consider, reflecting on occasion the healthcare system available to experts. This included common tests such as lactate dehydrogenase (LDH) and nasopharyngeal swab for non-influenza respiratory viruses. Tests not included in the consensus recommendation are listed in supplementary Table 3. Experts could not agree on the importance of performing any point of care assessment of lung function or inflammation at time of acute exacerbation (including peak flow, spirometry and exhaled nitric oxide). All tests at the post hospitalisation follow-up phase that were recommended for consideration and excluded by expert consensus are listed in supplementary Table 3.
 |
Table 3 Recommended Tests to Perform at Time of Hospitalised Exacerbation of COPD |
Clinical Scores and Questionnaires
A wide range of related clinical severity scores and questionnaires were assessed for their utility and feasibility (see supplementary Table 4). Experts felt that only 3 should be done at time of hospitalised exacerbation; these were the modified Medical Research Council (mMRC) dyspnoea scale, the COPD assessment test (CAT) and asking about frequency of exacerbations (frequent exacerbator phenotype) (see Table 4). The majority of clinical scores/questionnaires were excluded by consensus (see supplementary Table 5), including the SGRQ, APACHE and EXACT-Pro. These recommendations also extended to the post hospitalisation follow-up phase (see supplementary Table 5).
 |
Table 4 Recommended Clinical Scores and Questionnaires to Be Taken at Time of Hospitalised Exacerbation |
Pharmacological Treatments (When Indicated)
Expert opinions related to treatments, when clinically indicated, achieved the greatest amount of consensus early in the Delphi, including the treatments that need to be given and when they should be commenced (see Table 5). When clinically indicated, experts recommended that patients hospitalised with an exacerbation of COPD be treated with systemic corticosteroids and antibiotics, for 5 to 7 days in total, with systemic corticosteroids dosing equivalent to 30–50 mg of prednisolone daily. Experts recommended that nebulised therapy duration should be given for a maximum of 5 days, although there was a greater variation of opinion on duration. Experts strongly recommended that long-term inhaler optimisation should be performed prior to discharge.
 |
Table 5 Recommendations Regarding Treatment Allocation, if Indicated, at the Time of Hospitalised Exacerbation of COPD |
Non-Pharmacological Treatments
Experts expected smoking cessation advice to be provided at every hospitalisation, in addition to seeing a respiratory physiotherapist. A referral to pulmonary rehab was considered a routine requirement. Although ideal, seeing a COPD specialist nurse at every hospitalisation was not deemed feasible. Experts also recommend that it was very important and very feasible to discuss palliative care, goals of care, symptom control and resuscitation status at every hospitalised exacerbation.
Outcomes at the Post Hospitalisation Follow-Up Phase
The consensus opinion among experts was that patients should only be considered stable at 6 weeks (median, IQR 6 to 12 weeks) post hospitalisation for COPD exacerbation. Experts were also able to make recommendations on the list of outcomes that should be used to define “treatment failure”, both in clinical practice and in research (see Table 6).
 |
Table 6 Recommended Treatment Failure Assessments at 30 Days After Hospitalised Exacerbation of COPD |
Potential Controversies
Experts recommended that an echocardiogram should be part of clinical care for patients with a hospitalised exacerbation of COPD following round 3; however, no consensus was reached as to when this should be performed (ie, in hospital or after discharge) or whether this should be performed routinely or only if clinically indicated. Computed tomography (CT) scans of the thorax and peak flow measurements led to strongly conflicting opinions of experts saying it was either “not at all important” or “very important”, and these polarised opinions were not resolved after 3 rounds.
Discussion
We report here the expert consensus recommendations from a detailed Delphi study in standardisation of measurements in the management of patients with HECOPD. These included symptoms, examination findings, co-morbidities, clinical scores, laboratory tests, point of care tests and treatments.
This is the first proposal of standardised data collection in clinical practice for severe hospitalised exacerbations of COPD (see https://www.cicero-copd.net/ for hospital exacerbation standardisation tool). This Delphi survey is a robust method to obtain consensus on standardisation of many aspects of hospitalised COPD management and provides a real-life perspective on the components prioritised by COPD physicians. The experts selected represent COPD physicians from across Europe with diverse health systems. We believe our high retention rate of experts throughout the three survey rounds is indicative of the importance of gaining standardisation for hospitalised exacerbation management, in addition to certain features of programming such as skip logics which ultimately reduced participant “click” burden. We found that, overall, there was a great deal of consensus amongst COPD experts. We specifically assessed importance and feasibility separately to establish an ideal set of measures; this assessment of a clinician's opinion on importance and how feasible a measure is has not been made before.8 We also pre-defined consensus and stability criteria prior to study commencement to prevent post hoc adjustments to affect inclusion threshold.22 We found very few occasions where an item (eg, a question in the survey) was found to be rated important and regarded as not feasible simultaneously.
As expected, all experts agreed that a detailed medical history and physical exam were important at time of hospitalisation, as per current recommendations.8 However, the inability of experts to agree on how to measure two very common symptoms, such as cough and sputum purulence, clearly exemplifies the need to urgently standardise recording of these symptoms. Our effort will almost certainly aid clinical practice, research practice and consequently patient outcomes. Furthermore, it is recognised that cardiovascular disease is a substantial cause of morbidity and mortality in patients with COPD;23 however, the expert opinion in our survey predominantly graded respiratory physical signs of higher importance than cardiac signs. This may reflect bias in asking respiratory experts or that simply assessing cardiac signs alone is insufficient to address cardiovascular risk. Moreover, it is worth noting that our experts recommend that cardiac biomarkers such as BNP, troponin, ECG and echocardiogram are essential tests in the management of a patient hospitalised with an exacerbation, and in the case of BNP, troponin and an ECG these should be performed within 4 hours (or as early as possible) of the admission. This recommendation highlights the importance of assessing cardiovascular risk in patients with COPD, where mortality is high.23 It is recognised that there is an increased risk of cardiovascular events within 30 days of hospitalisation for an exacerbation of COPD24 and that it is highly likely that clinical or sub-clinical cardiovascular disease may worsen during a severe exacerbation.
The experts’ views on blood, radiology and assessments of lung function at the time of hospitalised COPD exacerbation were interesting. Unlike asthma guidelines, current COPD guidelines do not recommend point of care lung function testing during an exacerbation.8,9 This was also reflected by our experts not agreeing on any point of care test (including peak flow) and thus not recommending this as an investigation at time of hospitalisation. This could reflect the limitations with spirometry or peak flow as an available tool25 and the lack of evidence to suggest it alters clinical management at the time of an exacerbation. This contrasts with the expert consensus regarding the value of spirometry at time of follow-up, as a diagnostic requirement. It is conceivable, however, that other more sensitive tools assessing airway obstruction, such as impulse oscillometry testing,26 need to be considered at time of hospitalisation or follow-up.
In contrast to this, there was expert consensus and recommendation that full blood count, renal function and CRP should be performed within 4 hours of an admission. The use of the peripheral blood eosinophil count27 and serum C-reactive protein28 during a HECOPD is still being evaluated. Meanwhile, although other tests were considered to be important, there was no consensus as to when these should be performed. We believe that the variability in the severity of HECOPD could impact on timeline decision-making and is thus reflected as dissensus in this survey.
The experts recommended that a chest radiograph should be performed as soon as possible within the admission. This is in line with current recommendations. Chest X-ray is used frequently29–31 despite evidence showing that it rarely alters30 clinical management. Research on the value of a CT of the chest during HECOPD is ongoing;32 whilst the relative infeasibility of CT scans likely contributed to strongly polarised views on its importance at time of HECOPD.
Finally, experts agreed that only the mMRC33 and the CAT34 must be assessed at time of hospitalisation. Despite there being a wide variety of risk tools to assess COPD exacerbation and mortality risk, external validation of these risk tools at the time of a hospital admission is limited35 and is likely to have attributed to expert opinion on which risk tools/scores are required at the time of a hospitalisation of COPD.
Following this expert consensus, we have defined a patient to be stable following a hospitalisation of COPD at 6 weeks, with a range of timepoints from 6 to 12 weeks to define stability, after hospitalisation. We believe it is of great importance that our experts were able to provide opinion on how to define a treatment failure. Our experts recommended that a treatment failure outcome should be measured at 30 days; in addition to currently approved definitions (of death or re-treatment for example), we should also capture outcomes related to cumulative systemic corticosteroid use, use of short-acting inhaler use and new or worsening of concomitant co-morbidities. These additional components seek to address harm of treatment, where the evidence is now increasing.36
There are several limitations to discuss. Firstly, it is important to note that the consensus decisions derived from this survey reflect expert opinion, and there is little evidence supporting the practice of measuring these outcomes at time of hospitalisation for an exacerbation of COPD. However, due to the paucity of evidence or best practice, this is where the Delphi method works best. In particular, this Delphi approach in management of hospitalised COPD exacerbations can serve to act as a springboard to start standardising, building evidence and importantly to improve care. We feel that COPD physicians should not accept the status quo simply because there is no evidence to guide change. Secondly, as expected,19 an expert-led Delphi process would favour more detail and intervention than that which is potentially plausible in day to day practice. To resolve this, we specifically asked about the feasibility of all items, especially considering local costs, and practice limitations. All the items proposed reached a rating of “feasible to very feasible“. We believe that our success at bringing together a broad expert panel from across Europe makes this document workable in clinical practice across Europe. This has led to recommendations of symptoms, signs, tests and outcomes which are all eminently feasible. A further limitation is that our experts were pre-selected pulmonologists practising in Europe. This may potentially make our results difficult to generalise to low- and middle-income countries; however, we feel this limitation will only apply to the selection of investigations at the time of a hospitalised exacerbation. The exclusion of allied health professionals from the expert panel could also limit the generalisability/acceptability of the proposed standardisation consensus. As part of the CICERO clinical research collaborative, we are now seeking patients' views on our expert consensus through a multi-national, multi-lingual survey, run in collaboration with the European Lung Foundation. This standardisation is also being piloted in a Europe-wide cohort study of 1000 hospitalised COPD exacerbations, another pre-defined goal of the CICERO collaboration. These novel, patient and end-user driven validation attempts are unique in clinical care standardisation. Finally, we limited the survey to three rounds a priori, where further rounds may yield further consensus,19 although in current practice a minimum of 3 rounds are commonly recommended.22 The statistical approaches used to define consensus and dissensus, and the use of the Wilcoxon signed rank test could have falsely shown stability. However, very few elements failed to achieve stability of either consensus or dissensus after 3 rounds.
In conclusion, we have developed an expert consensus tool through a pre-defined Delphi process, that recommends which measures should be undertaken as part of standardisation of routine clinical care. To improve COPD clinical care, the respiratory field should move beyond the status quo from a position of limited standardisation. Adoption of this expert consensus will provide the first starting point to do this for patients hospitalised for exacerbations of COPD.
Acknowledgments
We are very grateful to all the experts who contributed generously to this project, including Alan Altraja; Giovanna Elisiana Carpagnano; Joanna Chorostowska-Wynimko; Christian Clarenbach; Aikaterini Dimakou; Marta Drummond; Aleksandra Dudvarski Ilic; Matjaz Flezar; Ana Hećimović; Hannu Kankaanranta; Huib Kerstjens; Nurdan Köktürk; Jose Luis Lopez-Campos Bodineau; Eric Van Ganse. We also thank Ms Alessandra Marguerat and Dr Elise Heuvelin at the European Respiratory Society for their technical and programming support.
Sanjay Ramakrishnan was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Disclosure
Dr. Ramakrishnan reports non-financial support from AstraZeneca, PhD scholarship from Australian Government Research Training Program (RTP) and junior researcher salary support from National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC), during the conduct of the study and outside the submitted work.
Dr. Janssens reports grants from Chiesi, AstraZeneca and GSK, advisory board membership for Boerhinger Ingelheim, AstraZeneca, Chiesi, GSK outside the submitted work; and W. Janssens is cofounder of ARTIQ, a spinoff company of KULEUVEN.
Dr. Burgel reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Novartis, Pfizer, Insmed and Zambon, and grants and personal fees from GSK and Vertex, outside the submitted work.
Dr. Contoli reports grants and personal fees from Chiesi, AstraZeneca and GlaxoSmithKline, personal fees from Boehringer Ingelheim, Alk-Abello, Novartis and Zambon and grants from University of Ferrara, Italy, outside the submitted work.
Dr. Franssen reports grants and personal fees from AstraZeneca and Novartis, and personal fees from Boehringer Ingelheim, Chiesi, GlaxoSmithKline and TEVA, outside the submitted work.
Dr. Greening reports grants and personal fees from GSK, personal fees and non-financial support from Chiesi, and Boehringer Ingelheim outside the submitted work.
Dr. Greulich reports personal fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GSK, Novartis, grants from German Centre for Lung Research (DZL), Marburg, Germany (Deutsches Zentrum für Lungenforschung), grants and personal fees from Grifols and CSL-Behring and lectures and advisory boards for GSK, Novartis and Roche, outside the submitted work.
Dr. Gyselinck reports other non financial support from KU-Leuven, outside the submitted work.
Dr. Quint reports grants from MRC, during the conduct of the study; personal fees from GSK, grants from Asthma UK, Chiesi, MRC and The Health Foundation, grants and personal fees from AZ and BI, Bayer outside the submitted work.
Dr. Vanfleteren reports grants and personal fees from AstraZeneca, personal fees from Novartis, GSK, Chiesi, Menarini, Pulmonx, Resmed, Boehringer, Verona Pharma and AGA Linde outside the submitted work.
Dr. Bafadhel reports grants and personal fees from AstraZeneca, personal fees from Chiesi and GSK, grants and non-financial support from AZ, non-financial support from Chiesi and GSK and advisory board membership for Albus Health and ProAxsis, outside the submitted work.
The authors report no other potential conflicts of interest for this work.
References
1. Nielsen R, Johannessen A, Benediktsdottir B, et al. Present and future costs of COPD in Iceland and Norway: results from the BOLD study. Eur Respir J. 2009;34(4):850–857. doi:10.1183/09031936.00166108
2. Ornek T, Tor M, Altın R, et al. Clinical factors affecting the direct cost of patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. Int J Med Sci. 2012;9(4):285–290. doi:10.7150/ijms.4039
3. Pasquale MK, Sun SX, Song F, Hartnett HJ, Stemkowski SA. Impact of exacerbations on health care cost and resource utilization in chronic obstructive pulmonary disease patients with chronic bronchitis from a predominantly medicare population. Int J Chron Obstruct Pulmon Dis. 2012;7:757–764. doi:10.2147/COPD.S36997
4. Hartl S, Lopez-Campos JL, Pozo-Rodriguez F, et al. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD audit. Eur Respir J. 2016;47(1):113. doi:10.1183/13993003.01391-2014
5. Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459–467. doi:10.1378/chest.124.2.459
6. Jacobs DM, Noyes K, Zhao J, et al. Early hospital readmissions after an acute exacerbation of chronic obstructive pulmonary disease in the nationwide readmissions database. Ann Am Thorac Soc. 2018;15(7):837–845. doi:10.1513/AnnalsATS.201712-913OC
7. Royal College of Physicians & British Thoracic Society. National COPD Audit Programme: Outcomes from the Clinical Audit of COPD Exacerbations Admitted to Acute Units in England 2014. London; 2017.
8. GOLD. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2020 Report. 2019.
9. Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European respiratory society/American thoracic society guideline. Eur Respir J. 2017;49(3):1600791. doi:10.1183/13993003.00791-2016
10. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
11. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS): the task force for the diagnosis and management of acute pulmonary embolism of the European society of cardiology (ESC). Eur Heart J. 2019;41(4):543–603.
12. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;61(4):e78–e140.
13. Sokka T. Long-term outcomes of rheumatoid arthritis. Curr Opin Rheumatol. 2009;21(3):284–290. doi:10.1097/BOR.0b013e32832a2f02
14. Nauta ST, Deckers JW, Akkerhuis KM, van Domburg RT. Short- and long-term mortality after myocardial infarction in patients with and without diabetes: changes from 1985 to 2008. Diabetes Care. 2012;35(10):2043–2047. doi:10.2337/dc11-2462
15. Gladman DD, Strand V, Mease PJ, Antoni C, Nash P, Kavanaugh A. OMERACT 7 psoriatic arthritis workshop: synopsis. Ann Rheum Dis. 2005;64(suppl 2):ii115. doi:10.1136/ard.2004.032615
16. Ogdie A, de Wit M, Callis Duffin K, et al. Defining outcome measures for psoriatic arthritis: a report from the GRAPPA-OMERACT working group. J Rheumatol. 2017;44(5):697–700. doi:10.3899/jrheum.170150
17. Singh JA, Guyatt G, Ogdie A, et al. 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken). 2019;71(1):2–29. doi:10.1002/acr.23789
18. Janssens W, Bafadhel M. The CICERO (collaboration in COPD ExaceRbatiOns) clinical research collaboration. Eur Respir J. 2020;55(3):2000079. doi:10.1183/13993003.00079-2020
19. Linstone HA, Turoff M. The Delphi Method. MA: Addison-Wesley Reading; 1975.
20. Turoff M. The policy delphi. In: Linstone HATM, editor. The Delphi Method: Techniques and Applications.
21. Rayens MK, Hahn EJ. Building consensus using the policy Delphi method. Policy Polit Nurs Pract. 2000;1(4):308–315. doi:10.1177/152715440000100409
22. von der Gracht HA. Consensus measurement in Delphi studies. Technol Forecast Soc Change. 2012;79(8):1525–1536. doi:10.1016/j.techfore.2012.04.013
23. Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32(4):962–969. doi:10.1183/09031936.00012408
24. Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. a post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):51–57. doi:10.1164/rccm.201711-2239OC
25. Rea H, Kenealy T, Adair J, Robinson E, Sheridan N. Spirometry for patients in hospital and one month after admission with an acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:527–532. doi:10.2147/COPD.S24133
26. Jetmalani K, Timmins S, Brown NJ, et al. Expiratory flow limitation relates to symptoms during COPD exacerbations requiring hospital admission. Int J Chron Obstruct Pulmon Dis. 2015;10:939–945. doi:10.2147/COPD.S78332
27. Sivapalan P, Lapperre TS, Janner J, et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicentre, randomised, controlled, open-label, non-inferiority trial. Lancet Respir Med. 2019;7(8):699–709. doi:10.1016/S2213-2600(19)30176-6
28. Prins HJ, Duijkers R, van der Valk P, et al. CRP-guided antibiotic treatment in acute exacerbations of COPD admitted to hospital. Eur Respir J. 2019.
29. Sha J, Worsnop CJ, Leaver BA, et al. Hospitalised exacerbations of chronic obstructive pulmonary disease: adherence to guideline recommendations in an Australian teaching hospital. Intern Med J. 2020;50(4):453–459. doi:10.1111/imj.14378
30. Sherman S, Skoney JA, Ravikrishnan KP. Routine chest radiographs in exacerbations of chronic obstructive pulmonary disease. Diagnostic value. Arch Intern Med. 1989;149(11):2493–2496. doi:10.1001/archinte.1989.00390110077016
31. Zimmermann SC, Tonga KO, Thamrin C. Dismantling airway disease with the use of new pulmonary function indices. Eur Respir Rev. 2019;28(151):180122. doi:10.1183/16000617.0122-2018
32. Rangelov BA, Young AL, Jacob J, et al. Thoracic imaging at exacerbation of chronic obstructive pulmonary disease: a systematic review. Int J Chron Obstruct Pulmon Dis. 2020;15:1751–1787. doi:10.2147/COPD.S250746
33. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581. doi:10.1136/thx.54.7.581
34. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648. doi:10.1183/09031936.00102509
35. Bellou V, Belbasis L, Konstantinidis AK, Tzoulaki I, Evangelou E. Prognostic models for outcome prediction in patients with chronic obstructive pulmonary disease: systematic review and critical appraisal. BMJ. 2019;367:l5358. doi:10.1136/bmj.l5358
36. Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. doi:10.1136/bmj.j1415
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
