Back to Journals » Cancer Management and Research » Volume 9
Standard of care and direct medical costs of the treatment of chronic lymphocytic leukemia among the adult population in Ukraine, Russia, and Kazakhstan: data from the LEUKOSPECT study
Authors Vasylyev A, Molostvova V, Rebrov BA , Makarova J, Zaritskey A, Ptushkin V, Ramazanova R, Popovych Y, Tsyapka O, Pashanov E
Received 19 April 2017
Accepted for publication 23 June 2017
Published 7 September 2017 Volume 2017:9 Pages 387—395
DOI https://doi.org/10.2147/CMAR.S139915
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Averyan Vasylyev,1 Valentina Molostvova,2 Boris A Rebrov,3 Janina Makarova,4 Andrey Zaritskey,5 Vadim Ptushkin,6 Raigul Ramazanova,7 Yuriy Popovych,8 Orest Tsyapka,9 Evgeny Pashanov10
1CIS Medical, GlaxoSmithKline, Kiev, Ukraine; 2Regional Clinical Hospital No. 1, Khabarovsk, Russia; 3Lugansk Regional Clinical Hospital, Lugansk, Ukraine; 4Russia Medical Department, GlaxoSmithKline, Moscow, Russia; 5Oncohaematology Department, Almazov Federal Heart, Blood and Endocrinology Centre, Saint Petersburg, Russia; 6S.P. Botkin Hospital, Moscow, Russia; 7Kazakh Scientific Research Institute of Oncology and Radiology, Almaty, Kazakhstan; 8Zakarpatskaya Regional Clinical Hospital, Zakarpatskaya, Ukraine; 9Institute of Blood Pathology and Transfusion Medicine, Lviv, Ukraine; 10Novartis Pharma LLC, Moscow, Russia
Purpose: The LEUKOSPECT study aimed to describe health service utilization and to estimate the direct medical costs (DMCs) of chronic lymphocytic leukemia (CLL) in 2013 in the adult population of three post-Soviet countries – Russia, Ukraine, and Kazakhstan. As oncologic medical care is provided by federal state-owned, specialized medical institutions, the cost estimation in this study primarily informs from a state budget perspective. Patients’ contributions to medical costs were not included in the cost evaluation.
Patients and methods: This was a multinational, multicenter, retrospective study conducted in eight specialized centers (four in Russia, three in Ukraine, and one in Kazakhstan). The investigators captured data from the medical documents of all adult patients with an established CLL diagnosis before December 31, 2013, and who made at least one visit to their respective center between January 1 and December 31, 2013.
Results: A total of 319 adult CLL patients were enrolled (124 in Kazakhstan, 106 in Russia, and 89 in Ukraine). In 2013, the DMCs of CLL management (without CLL therapy) were €215.40 in Kazakhstan, €1,342.20 in Russia, and €13,260.70 in Ukraine. Hospitalizations formed the largest proportion of total cost: 18.1%, 23.1%, and 40.4%, respectively. The mean cost of CLL medical treatment was €13,580.60 (Russia), €399.40 (Kazakhstan), and €7,453.00 (Ukraine).
Conclusion: CLL treatment standards varied across the selected countries; higher usage of biologic therapy was noted in Russia. Future research is needed to assess DMCs which include CLL treatment, which is another essential factor contributing to CLL DMCs.
Keywords: chronic lymphocytic leukemia, standard of care, direct medical cost, Russia, Ukraine, Kazakhstan
Introduction
Chronic lymphocytic leukemia (CLL) often occurs during or after middle age and is the most common type of leukemia in Western countries, accounting for approximately 30% of all leukemias in the USA.1 CLL is more common in men, with a male to female ratio of approximately 1.7:1.2
The majority of epidemiology studies in CLL have been conducted in Western Europe and the USA, but aspects such as standard of care (SoC) and direct medical costs (DMCs) are often left unexplored. The majority of conventional first-line therapies are non-curative and are only used to treat symptomatic or progressive CLL.3,4 Modern treatment options such as monoclonal antibodies have made it possible to advance CLL treatment, and the SoC is frequently changing. However, there is little knowledge of the SoC, treatment standards, and burden of hematologic malignancies in post-Soviet countries.5 Such studies would be helpful in these regions for health authorities to further improve the management of hematologic diseases, including CLL.
This LEUKOSPECT study (GlaxoSmithKline protocol no. 117047) was the first epidemiologic study of CLL in these post-Soviet countries. The first part of the study was aimed at assessing the prevalence (in 2013) and cumulative 5-year (2009–2013) incidence of CLL; these data were published in a separate manuscript6 and will not be discussed further here. This paper focusses on the investigation of SoC, health care resource utilization, and DMCs in 2013 in the adult populations of cities across three post-Soviet countries – the Russian Federation (Russia), Ukraine, and Kazakhstan.
Material and methods
Study design
LEUKOSPECT was a retrospective, multinational, multicenter study using data from patient medical charts. Eight centers (four in Russia [Moscow, Yekaterinburg, Saint Petersburg, Khabarovsk], three in Ukraine [Lugansk, Lviv, Uzhhorod], and one in Kazakhstan [Almaty]) participated in the SoC part of the study. The Lugansk site was closed prematurely due to the political situation in the region; however, the majority of data at this site were captured, and therefore this site was included in the final analysis.
Data were captured from the medical records of all adult patients with an established CLL diagnosis before December 31, 2013, and who made at least one visit to the center (specialized hematologic and/or oncologic center) between January 1 and December 31, 2013. Investigators (qualified hematologists and oncologists) assessed data for this period through medical documentation both from the center and from other clinics or hospitals (eg, copies of discharge summaries).
Health care resource use was expressed as rates of services (ie, per year) and the mean number of services used in 2013. Services included clinic visits (hospitalizations, emergency room [ER] visits, outpatient visits), drugs for CLL treatment (not including comorbidities), visits to specialists, imaging, and laboratory tests. The study was reviewed and approved by independent ethics committees in Russia and Kazakhstan, and by local ethics committees of all participating sites in Ukraine, according to the specific local legal requirements. The approving ethics committees were The Independent Interdisciplinary Ethics Committee of Ethical Review for Clinical Trials (Russia), Central Ethics Committee of the Ministry of Health of Kazakhstan (Kazakhstan), and local ethics committees of Lugansk Regional Clinical Hospital, The Institute of Blood Pathology and Transfusion Medicine, Lviv, and Zakarpatskaya Regional Clinical Hospital in the Ukraine. Informed consent was obtained from all patients in compliance with the consent process reviewed by relevant ethics committees.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics software version 21.0 (IBM Corporation, Armonk, NY, USA) and R software version 3.1.2 (R Core Team, Vienna, Austria). Descriptive analyses (proportion, mean, SD) and statistical analyses of cost evaluations were performed for each country.
Cost evaluation
Routine sources for cost estimation were used. In each country, the investigators were asked to provide the cost of interventions, medications, etc, according to compulsory health insurance tariffs established in their region in 2013. In the countries investigated in this study, oncologic medical care (including management of hematologic malignancies) is provided by federal state-owned specialized medical institutions. Specialized medical care is provided by oncologists and radiotherapists in oncologic dispensaries, or health care organizations which have the necessary materials and resources, including certified specialists, inpatient, and day hospitals. Therefore, the cost estimation in this study is from a state budget perspective. Patients’ contributions to medical costs were not included in the cost evaluation.
The investigators provided data in the local currency, and at statistical analysis costs were converted to euros based on the currency rate as of November 29, 2013 (1 Russian ruble = €44.99; 1 Kazakhstan tenge = €209.71; 1 Ukrainian hryvnia = €1.09). DMCs were calculated for each patient on a yearly basis. The following direct cost components during 2013 were added to the DMC evaluation: laboratory tests, bone marrow biopsies, cytogenetic and visualization tests, outpatient visits, hospitalizations, ER visits, and visits to specialists. Costs of medication (for CLL treatment only, not comorbidities) were not available for all patients in each country; therefore, the cost of CLL treatment was evaluated in a subset of patients with available data.
Results
Study population and baseline characteristics in each country
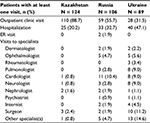
A total of 319 patients (124 in Kazakhstan, 106 in Russia, and 89 in Ukraine) were enrolled. The ratio of men to women was almost 1:1 in the study population: 63 (50.8%) male patients were included in Kazakhstan, 54 (50.9%) in Russia, and 48 (53.9%) in Ukraine. All patients in Russia and Ukraine were Caucasian, whereas in Kazakhstan, 30 (24.2%) patients were Asian and the remainder were Caucasian. About half of the patients were older than 65 years at CLL diagnosis: 66 (53.2%) in Kazakhstan, 48 (45.3%) in Russia, and 39 (43.8%) in Ukraine. The median time between diagnosis and final data capture was 5.9 years in Kazakhstan, 1.9 years in Russia, and 2.7 years in Ukraine.
The proportion of patients with comorbidities of interest varied from 29.8% in Kazakhstan to 62.8% in Ukraine. Among these comorbidities, cardiovascular disorders predominated: coronary heart disease (seen in 36.4% of patients in Ukraine, 14.5% in Kazakhstan, and 11.9% in Russia) and arterial hypertension (22.7% in Ukraine, 17.7% in Kazakhstan, and 17.8% in Russia) were the most common comorbidities. Most of the patients had CLL Rai stage I–III, and Binet stage A disease slightly predominated over Binet stage B and C. Participants’ baseline demographics and clinical characteristics are presented in Table 1.
  | Table 1 Demographics and clinical characteristics of CLL patients Note: aComorbidities with a frequency of more than 5% are presented. Abbreviation: CLL, chronic lymphocytic leukemia. |
Proportion of patients by treatment regimen
CLL treatment regimens were evaluated in 303 patients with valid data and included first-line therapy and other (second-line and subsequent regimens) CLL therapy (Table 2). A total of 135 patients (46 in Kazakhstan, 40 in Russia, and 49 in Ukraine) were prescribed CLL therapy at their first visit in 2013. Among them, 121 patients (44 in Kazakhstan, 41 in Russia, 36 in Ukraine) received a first-line treatment regimen; other lines of CLL therapy were prescribed to 37 patients (15, 2, and 20, respectively). In Kazakhstan and Ukraine, the most frequently prescribed first-line treatment regimens were chlorambucil monotherapy (34.1% of patients in Kazakhstan and 44.4% in Ukraine) and fludarabine + cyclophosphamide (34.1% and 22.2% of patients, respectively). In Russia, however, a fludarabine + cyclophosphamide + rituximab regimen was prescribed most frequently (48.8% of patients; 34.1% of patients received bendamustine + rituximab). In later lines of CLL therapy, the most frequent regimens in Kazakhstan were cyclophosphamide + doxorubicin + vincristine + prednisone and cyclophosphamide + vincristine + prednisone (COP), while in Ukraine, rituximab + COP (R-COP) was the most frequent regimen. In Russia, of the two patients who received later lines of therapy, one received rituximab + bendamustine and the other received R-COP. Other treatment regimens were prescribed less frequently (Table 2).
Health care resource utilization in 2013
Laboratory tests and procedures in 2013
Almost all patients had hematology tests during 2013; the greatest number of hematology tests received per patient was 26 in Kazakhstan, 23 in Russia, and 22 in Ukraine. The most frequently investigated parameters of blood chemistry were total protein (48%–72% of patients), aspartate aminotransferase (48%–68%), alanine aminotransferase (47%–76%), total bilirubin (47%–81%), creatinine (48%–73%), and glucose (46%–67%). The greatest number of blood chemistry tests per patient was 26 in Kazakhstan, 23 in Russia, and 22 in Ukraine.
Among immunology tests, immunoglobulins were investigated in 28.3% of patients in Russia, 6.7% of patients in Ukraine, and none of the patients in Kazakhstan; beta-2 microglobulin was tested in 15.1%, 7.9%, and 0% of patients, respectively; and the Coombs test was performed in 30.2% of patients in Russia, 10.5% of patients in Kazakhstan, and no patients in Ukraine. Blood immunophenotyping and cytogenetic tests were generally performed less frequently. Blood immunophenotyping was performed in approximately 20%–30% of patients in Kazakhstan and Ukraine, and in more than 60% of patients in Russia. The greatest number of blood immunophenotyping tests per patient was two in Kazakhstan, six in Russia, and two in Ukraine.
The rate of bone marrow aspiration in 2013 reached 60%–61% (Kazakhstan and Russia), whereas bone marrow biopsies were performed quite rarely. The greatest number of bone marrow aspirations per patient was three in Kazakhstan, five in Russia, and three in Ukraine.
Among visual investigations, about 60% of patients underwent an abdominal ultrasound in 2013 and 25%–33% of patients had an X-ray, whereas computed tomography was conducted less frequently. The greatest number of visual investigations per patient was three in Kazakhstan, four in Russia, and five in Ukraine. Table 3 shows the frequency of laboratory, bone marrow, and visual investigations in the study countries in 2013.
  | Table 3 Laboratory, bone marrow, and visual investigations by country (number of patients with at least one test/procedure performed in 2013) |
Visits to clinics and specialists, ER visits, and hospitalizations related to CLL in 2013
The rate of outpatient clinic visits during 2013 varied from 31.5% in Ukraine to 88.7% in Kazakhstan (Table 4). The mean ± SD number of such visits per patient in 2013 was 2.5 ± 2.9 (maximum 16) in Kazakhstan, 4.3 ± 8.2 (maximum 62) in Russia, and 2.1 ± 6.4 (maximum 53) in Ukraine.
The proportion of patients with at least one CLL-related hospitalization was 47.1% in Ukraine, 20.2% in Kazakhstan, and 32.7% in Russia. The mean ± SD number of hospitalizations per patient in 2013 was 0.4 ± 1.1 (maximum 8) in Kazakhstan, 0.9 ± 1.7 (maximum 8) in Russia, and 1.1 ± 1.6 (maximum 7) in Ukraine. The mean ± SD duration of hospitalization was 5.4 ± 14.1 days in Kazakhstan, 11.2 ± 22.9 days in Russia, and 13.5 ± 18.4 days in Ukraine. ER visits were not widespread: there were two patients with ER visits in 2013, both from Russia. One patient had one visit with a duration of 3 days, while the other patient had two ER visits with a total duration of 8 days.
The rate of visits to specialists did not exceed 15%. The greatest number of visits to specialists per patient in 2013 was four in Kazakhstan, seven in Russia, and eleven in Ukraine. Table 4 shows the frequency of visits, including visits to various specialists.
Total DMCs for adult CLL patients in 2013
The highest mean total DMCs of CLL management (without therapy) in 2013 was recorded in Ukraine (€13,260.70 [median €1,706.60]) and the lowest in Kazakhstan (€215.40 [median €26.70]). In Russia, the mean DMC was €1,342.20 (median €463.90). Data on the cost evaluation of different categories of CLL management (hospitalizations and ER visits, laboratory and visual investigations, outpatient visits, visits to specialists) are presented in Table 5.
Hospitalizations made up the largest proportion of the total cost of CLL management in 2013: the proportion of hospitalization costs in relation to the total cost (mean value) per patient was 18.1% in Kazakhstan, 23.1% in Russia, and 40.4% in Ukraine. The proportions of all categories in relation to the total cost per patient are detailed in Figure 1.
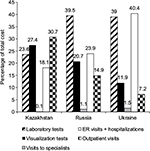  | Figure 1 Percentages of different categories in relation to total CLL cost per patient in 2013. Abbreviations: CLL, chronic lymphocytic leukemia; ER, emergency room. |
Data on CLL medical treatment (drugs and dosage regimens) were available for 115 patients (32 in Kazakhstan, 50 in Russia, and 33 in Ukraine). The mean cost of CLL medical treatment in 2013 was €13,580.60 (median €6,683.00) in Russia, €399.40 (median €48.79) in Kazakhstan, and €7,453.00 (median €1,092.30) in Ukraine.
Discussion and conclusion
Study findings and fit with current knowledge
As expected, CLL patients were elderly (about half of the patients were older than 65 years), and ethnic demographics differed between the study countries (all patients in Russia and Ukraine were Caucasian, while in Kazakhstan the proportion of Asians was 24.2%). On average, patients in Kazakhstan had been followed up for longer (5.9 years) than those in Russia (1.9 years) or Ukraine (2.7 years); however, the length of follow-up is unlikely to influence the DMC for a single year as has been explored in this study. The CLL treatment standards in 2013 varied across the study countries: thus, monoclonal antibodies (rituximab) as a component of first-line CLL therapy were administered predominantly in Russia; in Ukraine, rituximab was used mostly in second-line and later treatment regimens, and in Kazakhstan it was rarely used.
The health care resource utilization results of our study show wide usage of various routine clinical and diagnostic methods (eg, hematology, blood chemistry tests, ultrasound, and X-ray) and less frequent utilization of more specific and expensive methods such as computed tomography scanning, blood immunophenotyping, cytogenetic tests, and biopsy. It is known that the use of expensive methods depends not only on their necessity (as they are quite specific) but also on their availability. The availability of expensive investigations depends on state financial programs, eg, “high-tech medical care”.
The mean total direct cost of CLL management (without therapy) in 2013 in Kazakhstan was only €215.40, while in Russia it was €1,342.20, and in Ukraine it was €13,260.70. In comparison, in Germany, Blankart et al7 evaluated the direct and indirect costs of CLL for 4,198 CLL patients identified in 2007 and 2008. The cost attributable to CLL for each prevalent case amounted to €4,946.00 from the payer’s perspective and €7,910.00 from a societal perspective (eg, including productivity losses). Inpatient hospital stays and pharmaceuticals were the main cost drivers.7 Another study conducted in Russia showed that the main component of direct costs was medication charges.8 However, it should be noted that our study did not include in its cost calculation categories such as nursing care at home or rehabilitation, which could change the overall costs.
The main cost drivers identified for CLL are chemotherapy, bone marrow transplantation, and inpatient stays.7,9 The results of the LEUKOSPECT study support these facts: the proportion of hospitalization costs in relation to the DMCs was 18%–40%. A local CLL treatment cost study conducted in Ukraine by Mandrik et al5 found that only hospital choice had a significant impact on the cost of drug treatment, and the authors gave risk-patient selection or differences in treatment practice between hospitals as possible explanations: the average annual cost (2010) of a patient’s drug treatment was €2,047.00 and the average cost of a hospital stay was €542.00 per person, resulting in total expenditures of €2,589.00. The authors concluded that because many patients pay out of pocket for in-hospital drugs, these costs represent a high economic burden for patients with CLL.5
Several other studies have evaluated the cost of CLL treatment. Lafeuille et al10 evaluated the average lifetime costs of patients with CLL compared with similar patients without cancer during 1999–2007 in Montreal, Canada. The average lifetime costs were $87,151.00 for patients with CLL compared with $47,642.00 for matched controls without cancer. Among common CLL treatments, the average costs per patient were $5,140.00 for rituximab and $953.00 for radiation therapy.10
An observational study in the Netherlands performed by Holtzer-Goor et al11 assessed data from 19 patients with CLL diagnosed between 1999 and 2003 (Group 1); an additional group of patients (diagnosed between 2003 and 2008; Group 2) was included who were treated with fludarabine + cyclophosphamide, fludarabine + cyclophosphamide + alemtuzumab, fludarabine + cyclophosphamide + rituximab, or alemtuzumab as first- or second-line therapy. The mean total costs per patient per year were €5,898.00 in Group 1 and €13,996.00 in Group 2.11
Varker et al12 identified data from patients with newly diagnosed CLL in Seattle, USA, in 2004–2013. Among unfit (for chemotherapy)/relapsed patients, per-patient per-month total costs by first and second lines of therapy were US$15,907.00 (SD US$19,893.00) and US$18,506.00 (SD US$36,977.00), respectively. The most commonly used regimens for unfit/relapsed CLL patients included rituximab.12
In the LEUKOSPECT study, the mean cost of CLL medical treatment in 2013 was €399.40 in Kazakhstan, €13,580.60 in Russia, and €7,453.00 in Ukraine. We suggest that the higher cost of CLL treatment in Russia is related to a greater use of biologic drugs, taking into account the studies described previously. The higher economic burden of DMC and CLL treatment in Ukraine compared with Kazakhstan is likely to be primarily driven by the higher number of hospitalizations (40.4% of the total cost) and visits to specialists, as the study population had higher prevalence of comorbidities (Table 1). This might explain in part the higher proportion of patients with progressive disease in Ukraine compared with Kazakhstan. Another factor influencing the differing response to treatment in the Ukraine could be compliance to the treatment regimen. Since the majority of patients were required to cover the costs of the medication in Ukraine (no reimbursement or co-payment schemes available), there may have been inconsistency with regard to initiation and continuation of treatment for CLL leading to a lower rate of complete response (CR) + complete response with incomplete blood count recovery (iCR) in Ukraine than in countries with reimbursed access to treatment (Kazakhstan, Russia).
Study limitations
As with other studies, the LEUKOSPECT study has some limitations. First, some data were missing due to the retrospective design of the study. Even though the investigators used medical documentation from both the study centers and other clinics and hospitals (eg, copies of discharge summaries), it cannot be guaranteed that the collected data are complete.
The study aimed to collect data of the majority of CLL patients in the selected cities, but this cannot be guaranteed. The inclusion criteria meant that the study center had to be the “patient’s primary place for CLL care”. In some cities (Lugansk, Lviv, and Uzhgorod in Ukraine; Yekaterinburg and Khabarovsk in Russia), the study centers were unique institutions specializing in CLL, and in other cities the study centers were non-specialized, but still leading CLL centers. These facts allow us to presume that the study population included the majority of CLL patients in the selected cities.
Because this study was conducted in selected cities, the data should be extrapolated to national populations with caution. Furthermore, health care resources might vary between large and small cities. In Kazakhstan, only one center participated (in Almaty), whereas four centers participated in Russia and three in Ukraine, including centers in large cities with well-developed health care systems.
This study had a retrospective, non-interventional design rather than a randomized or cohort study design; therefore, the apparent differences between the study countries cannot be fully interpreted, as they may be related to real differences or to various data registration and management differences among the hospitals.
Generalizability
Study limitations need to be considered when assessing the relative generalizability of the results. This study considers only the direct costs and these were evaluated from a state budget perspective. Indirect costs from a societal perspective, eg, productivity losses, were not taken into account. As cost estimation in this study is from a state budget perspective, patients’ out-of-pocket contributions to medical costs (although considered a DMC) were not included in the cost evaluation of this study.
Furthermore, the cost evaluation did not include CLL treatment, which is known to be a major part of DMCs for CLL patients. However, the study did assess other main categories of CLL management, including hospitalizations, specialist visits, tests, and procedures.
Conclusion
CLL treatment standards varied across the selected countries, with higher usage of biologic therapy noted in Russia. The mean cost of CLL management was lower than has previously been reported in Western countries, and hospitalizations formed the major component of CLL costs in all three countries. Future research is needed to assess DMCs including CLL treatment, which is an essential factor contributing to DMCs in CLL.
Acknowledgments
This work was supported by GlaxoSmithKline; Arzerra is an asset of Novartis AG as of March 2, 2015. The sponsor was involved in the study design, collection, analysis, and interpretation of the data, in the writing of the report, and in the decision to submit the article for publication.
Marcelo Horacio S Pereira (Rio de Janeiro State University [UERJ], Brazil), Irada Nurasheva (GlaxoSmithKline, Kazakhstan), Zaira Smailova (GlaxoSmithKline, Kazakhstan), Zvenislava Maslyak (Institute of Blood Pathology and Transfusion Medicine, Ukraine), and all the investigators are thanked for their contribution to the study.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
A Vasylyev and J Makarova are employed by GlaxoSmithKline. E Pashanov is employed by Novartis. V Molostvova, B Rebrov, A Zaritskey, V Ptushkin, R Ramazanova, Y Popovych, and O Tsyapka were supported by GlaxoSmithKline, during the conduct of the study.
References
Wierda WG, Keating MJ, O’Brien S. Chronic lymphocytic leukemia. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2278–2292. | ||
Harris L, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–3849. | ||
Byrd JC, Jones JJ, Woyach JA, Johnson AJ, Flynn JM. Entering the era of targeted therapy for chronic lymphocytic leukemia: impact on the practicing clinician. J Clin Oncol. 2014;32(27):3039–3047. | ||
Seung AH. Standard of care and novel treatments for chronic lymphocytic leukemia. Am J Health Syst Pharm. 2010;67(21):1813–1824. | ||
Mandrik O, Corro Ramos I, Zalis’ka O, Gaisenko A, Severens JL. Cost for treatment of chronic lymphocytic leukemia in specialized institutions of Ukraine. Value in Health Regional Issues. 2013;2(2):205–209. | ||
Vasylyev A, Loginov A, Molostvova V, et al. Prevalence and cumulative 5-year incidence of chronic lymphocytic leukemia in the adult population in the Russian Federation and Ukraine: data from the LEUKOSPECT study. Hematology. 2017;22(1):16–24. | ||
Blankart CR, Koch T, Linder R, Verheyen F, Schreyögg J, Stargardt T. Cost of illness and economic burden of chronic lymphocytic leukemia. Orphanet J Rare Dis. 2013;8:32. | ||
Kolbin AS, Vilum IA, Balykina Yu E, Proskurin MA. Фармакоэкономический анализ применения ибрутиниба в первой линии терапии хронического лимфолейкоза у пациентов с делецией 17р. [Pharmacoeconomic analysis of the use of ibrutinib in therapy of the first line of a chronic lymphocytic leukaemia for patients with 17p deletion]. Good Clinical Practice. 2015;1:32–43. Russian. | ||
Stephens JM, Gramegna P, Laskin B, Botteman MF, Pashos CL. Chronic lymphocytic leukemia: economic burden and quality of life: literature review. Am J Ther. 2005;12(5):460–466. | ||
Lafeuille MH, Vekeman F, Wang ST, Kerrigan M, Menditto L, Duh MS. Lifetime costs to Medicare of providing care to patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(6):1146–1154. | ||
Holtzer-Goor KM, Bouwmans-Frijters CA, Schaafsma MR, Uyl-de Groot CA. A Cost of Illness and Quality of Life Study in Patients with B-cell Chronic Lymphocytic Leukemia (CLL) in the Netherlands. Rotterdam, the Netherlands: Erasmus University Rotterdam; 2011. | ||
Varker H, Song X, Meyer N, Gregory SA, Pinilla-Ibarz J, Ramsey SD. Treatment patterns, mortality, and costs of care in unfit patients (pts) with relapsed chronic lymphocytic leukemia (CLL). J Clin Oncol. 2014;32(5 Suppl):7105. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.