Back to Journals » Pathology and Laboratory Medicine International » Volume 11
Squamous-cell carcinoma arising in a pericoccygeal–rectal epithelial inclusion cyst with adjacent benign notochordal cell tumor: first case report and review of the literature
Authors Lopez L , Schoeniger L, Zhou Z
Received 11 May 2018
Accepted for publication 13 September 2018
Published 6 May 2019 Volume 2019:11 Pages 1—5
DOI https://doi.org/10.2147/PLMI.S173802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Paul Zhang
Lorraine Lopez,1 Luke Schoeniger,2 Zhongren Zhou1
1Department of Pathology and Laboratory Medicine, University of Rochester, Rochester, NY, USA; 2Department of Surgery, University of Rochester, Rochester, NY, USA
Background: Malignant transformation of epidermal inclusion cysts (EICs) is rare. Only one case has been reported in the pericoccygeal–rectal region. Benign notochordal cell tumor (BNCT) is also rare. There have been no reports of squamous-cell carcinoma (SCC) arising in EICs with adjacent BNCT in the English-language literature.
Case presentation: An 83-year-old male patient with a history of malignant fibrous histiocytoma of the thigh after resection and irradiation presented clinically with a pericoccygeal–rectal, flocculent, painful mass 7 cm in diameter. No osseous destruction was associated on magnetic resonance imaging. Partial sacrectomy and coccygectomy with en bloc resection of retrorectal cystic tumor were performed. Histology showed SCC arising from an EIC. SCC cells were positive for p53, EGFR, p16, and cytokeratin 5/6 on immunostaining and negative for human papillomavirus. Adjacent to the EIC, one tumor showed bland cells with numerous intracytoplasmic vacuoles. These cells were positive for epithelial membrane antigen, pancytokeratin, S100, and negative for CD68, which confirmed the diagnosis of BNCT. The diagnosis was well moderately differentiated invasive SCC arising in an EIC with an adjacent BNCT abutting to bone.
Conclusion: We report the first case of SCC arising from an EIC with an adjacent BNCT in a patient with a history of malignant fibrous histiocytoma. Partial sacrectomy and coccygectomy with en bloc resection of retrorectal cystic tumor and wide margins taken prevented recurrence of both tumors.
Keywords: squamous cell carcinoma, epidermal inclusion cyst, benign notochordal cell tumor, malignant transformation, pericoccygeal–rectal region
Background
Epidermal inclusion cysts (EICs) are common benign skin lesions that can occur anywhere in the body. Malignant transformation, however, is exceptionally rare.1 There are some reports of malignant and premalignant lesions arising in EICs, including squamous-cell carcinoma (SCC), Merkel-cell carcinoma, basal-cell carcinoma, Bowen disease, Paget disease, and mycosis fungoides.1 SCC is considered the most common malignant transformation tumor from EICs. There have been 25 cases reported in the English-language literature since 1976, one in the pericoccygeal region.1–12 Of 25 cases, 14 occurred in the head and neck area,1–3,9,11 four in the buttock region,1,4,7,10 five in the trunk and extremities,1,2,5,6 one in perineum,11 and only one occured in the perirectal region.12 SCC in these cysts has been reported in up to 0.045% of cases.3 Males and females are affected equally, regardless of age.8
Benign notochordal cell tumors (BNCTs) are rare vertebral lesions composed of bland physaliphorous cells.13,14 These tumors are usually found incidentally, and are thought to be possible precursors to chordoma.13,14 In a review of the literature, many case reports used multiple terms. “Notochordal rest”, “notochord remnant”, “ecchordosis physaliphora”, and “notochord vestige” were used interchangeably, and are synonymous designations for any persistent postnatal notochordal tissue.13–17 Here, we report the first case of SCC arising from a pericoccygeal–rectal EIC with an adjacent BNCT in a patient with a history of malignant fibrous histiocytoma.
Case presentation
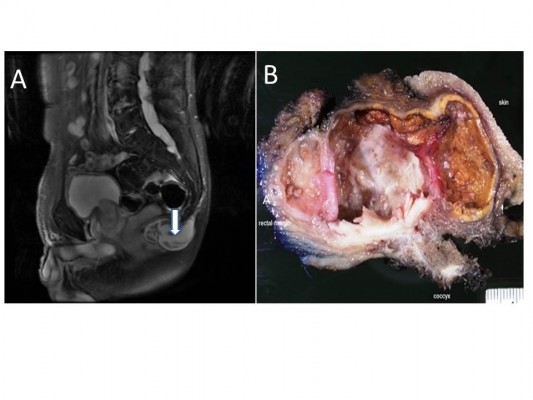
An 83-year-old Caucasian gentleman with a medical history of malignant fibrous histiocytoma at the right posterior thigh status after resection and irradiation presented with a pericoccygeal–rectal cystic mass. The mass was considered related to trauma, presenting and growing for 10 months after a fall. Clinically, the mass was 7 cm in diameter, flocculent, and very painful. Magnetic resonance imaging showed a T2 heterogeneous, multilocular, peripherally enhancing 6.1×3.8×4.2 cm lesion inferior to the tip of the coccyx (Figure 1A). No osseous destruction was associated with this lesion. Although there was mass effect on the closely associated lower rectum and anus, it did not appear continuous with the thecal sac. The lesion had previously measured 3.6×2.8 cm.
Fine-needle aspiration of the mass at 7 months after the fall was performed, since it is a cheap and safe procedure. The sample showed atypical cells with abundant keratinous-type debris, suggestive of a dermoid IC/EIC. Some atypical squamous cells and cells of uncertain origin, probably cartilaginous cells, were noted, with mild nuclear atypism. Although these may represent degenerative changes, a more serious process could not be entirely excluded.
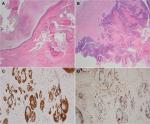
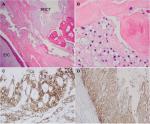
Partial sacrectomy and coccygectomy with en bloc resection of retrorectal cystic tumor were performed, with wide margins taken. Intraoperatively, the surgeon observed no direct evidence of malignancy. There was no clinical evidence of recurrence of malignant fibrous histiocytoma postoperatively. Grossly, the cystic lesion contained soft yellow material, consistent with keratin-like material (Figure 1B). Histologically, the cyst lining showed a focal area of well moderately differentiated invasive SCC arising from the EIC (Figure 2A and B). Immunostaining of the tumor cells showed positivity for p53, EGFR, p16, and cytokeratin 5/6 (Figure 2C and D). Tumor cells were negative for human papillomavirus (HPV). In addition, adjacent to this EIC and abutting the coccygeal bone, there was a grouping of large foamy cells with abundant microvesicular cytoplasm and small bland nuclei (Figure 3A and B) with immunostaining positive for S100, EMA, cytokeratin 5/6 (Figure 3C and D) and negative for CD68, which confirmed physaliphorous cells. The diagnosis was a BNCT.
The patient had 7-year follow-up. No recurrent SCC, chordoma, or malignant fibrous histiocytoma was identified.
Ethical approval
The Research Subjects Review Board of the University of Rochester waived the need for ethical approval for this case report.
Discussion
To the best of our knowledge, this is the first report of SCC arising from an EIC with an adjacent BNCT in the pericoccygeal–rectal region in a patient with a history of malignant fibrous histiocytoma. While epidermal cysts of the skin are very common, malignant transformation of these lesions is very unusual, especially in the pericoccygeal region. In a review of the English-language literature, we found 25 cases of SCC arising in an EIC.1–12 Of 25 cases, 14 occurred in the head and neck area,1–3,9,11 four in the buttock region,1,4,7,10 five in the trunk and extremities,1,2,5,6 one in perineum,11 and only one occured in the perirectal region.12
Many of these were cured with complete excision. Only three presented with very aggressive disease, with metastases and death within 5–10 months.8 It is generally recommended that EICs, especially enlarged ones that are symptomatic, be entirely resected and examined thoroughly for any invasive or carcinoma in situ within the squamous epithelium. Our case had partial sacrectomy and coccygectomy with en bloc resection of retrorectal cystic tumor with wide negative margins, which completely removed the SCC. No recurrent SCC had been identified after 5-year follow-up.
Based on the immunohistochemical studies, the SCC cells were positive for EGFR, p53, and p16, but negative for HPV. EGFR, p53, and p16 immunostaining were used to study the transition from normal cystic squamous epithelium to SCC. We did see squamous cells with hyperkeratinization between normal squamous epithelium and SCC, but no definite squamous dysplasia was identified. Anton-Badiola et al used cyclin D1, p53, and Ki67 immunostaining to highlight the transition zone between the wall of the cyst and the tumor. They found the transition was very abrupt, without demonstrable dysplastic changes between normal and cancer tissues. In addition, HPV was negative in our case, which has been reported in other studies.2,8 The radiation therapy took place 14 years ago. There is no clear data for the radiation field location; however, the location of the current squamous cell carcinoma is far away from original malignant fibrous histiocytoma tumor of the thigh. Therefore, we consider that the radiation effect should not be considered in regard to the current SCC.
During normal development, the notochordal cells, composed of physaliphorous cells, become the nucleus pulposus within the intervertebral disks by 10 weeks of gestation, the remaining notochordal cells degenerate and are not normally found within the disks. However, this regression can arrest at any point during development, thus yielding persistent ectopic notochord resets that may later undergo hyperplasia.14 Embryological studies have shown ectopic remnants may persist anywhere along the axial path of evolution of the notochord, most commonly at its cephalic (clivus – ecchordosis physaliphora) and caudal (coccyx) ends.14 In our case, the BNCT presented adjacent to the coccyx and rectum, with SCC arising from the EIC.
In 1996, at an International Skeletal Society meeting, an idea was put forth that there existed symptomatic lesions of the axial skeleton morphologically different from chordoma.18 BNCTs are well demarcated, but unencapsulated with sheets of bland adipocyte-like vacuolated or eosinophilic cells, with fewer vacuoles and mild pleomorphism. Bone trabeculae are often sclerotic, but without bony destruction. There are no intercellular myxoid matrices, necrosis, or mitotic figures. However, the chordoma is osteolytic and consists of multiple lobules separated by thin fibrous septa. The lobules contain cords, strands, or sheets of physaliphorous cells with myxoid matrices, and cells have mild-marked nuclear atypism.
In a review of the literature, the terminology of BNC remnants was confused. Many terms, including “notochord rest”, “notochord remnant”, “ecchordosis physaliphora”, “notochord vestige”, “giant notochord rest”, “benign notochordal cell tumor”, and “benign notochordal cell lesion” referred to the same morphological entity.13–17 Recently, the terminology BNCT has become gradually accepted.13 However, whether BNCT represents benign or premalignant lesions of chordomas are still controversial.13–17,19 Most case reports indicate the possible malignant transformation from BNCT to malignant chordoma. In the current study, we found that the BNCT was adjacent pericoccygeal–rectal region without damage to the bone. In addition, at the clinical 4-year follow-up, no chordoma was identified in this patient.
Conclusion
We have reported the first case of SCC arising from an EIC and an adjacent BNCT (remnant) in a patient with a history of malignant fibrous histiocytoma. Treatment was partial sacrectomy and coccygectomy with en bloc resection of retrorectal cystic tumor and wide margins taken, which prevented recurrence of both tumors and helped us to find other adjacent BNCTs. It is recommended that entire lesions be resected with wide margins if EICs or BNCTs are present.
Consent for publication
As the patient was lost to follow-up, his written informed consent for publication of this case was not possible. The details have been sufficiently anonymized to protect his identity.
Acknowledgments
We want to thank the histological and immunohistochemistry laboratory at the University of Rochester for help with staining. An abstract of this paper was presented at the College of American Pathologists Annual Meeting 9–12 September, 2012; San Diego, CA, USA; as a poster presentation with interim findings, and has been published.20
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. ZZ diagnosed this case and wrote and edited the manuscript, LS is a gastrointestinal surgeon who worked on the operation and provided all clinical information, and LL collected clinical and pathological information, took pictures, and wrote the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
López-Ríos F, Rodríguez-Peralto JL, Castaño E, Benito A. Squamous cell carcinoma arising in a cutaneous epidermal cyst: case report and literature review. Am J Dermatopathol. 1999;21(2):174–177. | ||
Morgan MB, Stevens GL, Somach S, Tannenbaum M. Carcinoma arising in epidermoid cyst: a case series and aetiological investigation of human papillomavirus. Br J Dermatol. 2001;145(3):505–506. | ||
Cameron DS, Hilsinger RL Jr. Squamous cell carcinoma in an epidermal inclusion cyst: case report. Otolaryngol Head Neck Surg. 2003;129(1):141–143. | ||
Debaize S, Gebhart M, Fourrez T, Rahier I, Baillon JM. Squamous cell carcinoma arising in a giant epidermal cyst: a case report. Acta Chir Belg. 2002;102(3):196–198. | ||
Nemoto I, Shibaki A, Aoyagi S, Tsuji-Abe Y, Shimizu H. Aggressive squamous cell carcinoma developing in a giant epidermal cyst of the abdomen. Int J Dermatol. 2006;45(12):1444–1446. | ||
Chiu MY, Ho ST, St H. Squamous cell carcinoma arising from an epidermal cyst. Hong Kong Med J. 2007;13(6):482–484. | ||
Jehle KS, Shakir AJ, Sayegh ME. Squamous cell carcinoma arising in an epidermoid cyst. Br J Hosp Med. 2007;68(8):446. | ||
Antón-Badiola I, San Miguel-Fraile P, Peteiro-Cancelo A, Ortiz-Rey JA. Squamous cell carcinoma arising on an epidermal inclusion cyst: a case presentation and review of the literature. Actas Dermosifiliogr. 2010;101(4):349–353. | ||
Shabbir A, Loss L, Bogner P, Zeitouni NC. Squamous cell carcinoma developing from an epidermoid cyst of the ear. Dermatol Surg. 2011;37(5):700–703. | ||
Kshirsagar AY, Sulhyan SR, Deshpande S, Jagtap S. Malignant change in an epidermal cyst over gluteal region. J Cutan Aesthet Surg. 2011;4(1):48–50. | ||
Pusiol T, Zorzi MG, Piscioli F. Squamous cell carcinoma arising in epidermal and human papillomavirus associated cysts: report of three cases. Pathologica. 2010;102(3):88–92. | ||
Blatchford GJ, Oliverius SJ, Karrer FW, Schenken JR, Moor BJ, Rose SG. Malignant degeneration in a presacral squamous cyst. Nebr Med J. 1982;67(12):336–339. | ||
Kreshak J, Larousserie F, Picci P, et al. Difficulty distinguishing benign notochordal cell tumor from chordoma further suggests a link between them. Cancer Imaging. 2014;14:4. | ||
Lagman C, Varshneya K, Sarmiento JM, Turtz AR, Chitale RV, Criteria PD. Proposed Diagnostic Criteria, Classification Schema, and Review of Literature of Notochord-Derived Ecchordosis Physaliphora. Cureus. 2016;8(3):e547. | ||
Kikuchi Y, Yamaguchi T, Kishi H, et al. Pulmonary tumor with notochordal differentiation: report of 2 cases suggestive of benign notochordal cell tumor of extraosseous origin. Am J Surg Pathol. 2011;35(8):1158–1164. | ||
Yamaguchi T, Iwata J, Sugihara S, et al. Distinguishing benign notochordal cell tumors from vertebral chordoma. Skeletal Radiol. 2008;37(4):291–299. | ||
Yamaguchi T, Suzuki S, Ishiiwa H, Shimizu K, Ueda Y. Benign notochordal cell tumors: A comparative histological study of benign notochordal cell tumors, classic chordomas, and notochordal vestiges of fetal intervertebral discs. Am J Surg Pathol. 2004;28(6):756–761. | ||
Kyriakos M. Benign notochordal lesions of the axial skeleton: a review and current appraisal. Skeletal Radiol. 2011;40(9):1141–1152. | ||
Nishiguchi T, Mochizuki K, Ohsawa M, et al. Differentiating benign notochordal cell tumors from chordomas: radiographic features on MRI, CT, and tomography. AJR Am J Roentgenol. 2011;196(3):644–650. | ||
Lopez L, Zhou Z. Squamous Cell Carcinoma Arising In a Pericoccygeal Epithelial Inclusion Cyst with Adjacent Benign Notochordal Cell Tumor: First Case Report. Arch of Pathol & Lab Med. 2012;136(9):95. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.