Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Spotlight on Tocilizumab in the Treatment of CAR-T-Cell-Induced Cytokine Release Syndrome: Clinical Evidence to Date
Authors Si S , Teachey DT
Received 28 May 2020
Accepted for publication 24 July 2020
Published 4 August 2020 Volume 2020:16 Pages 705—714
DOI https://doi.org/10.2147/TCRM.S223468
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Stephanie Si,1,2 David T Teachey1,2
1Children’s Hospital of Philadelphia, Division of Oncology, Philadelphia, PA, USA; 2Department of Pediatrics, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA
Correspondence: Stephanie Si
University of Hawai’i Cancer Center, 701 Ilalo Street, Honolulu, Hawaii 96813, USA
Tel +1 310 266 8480
Email [email protected]
Abstract: Immune-based therapies such as chimeric antigen receptor (CAR)-T-cell therapy have revolutionized the landscape of cancer treatment in recent years. Although this class of therapy has demonstrated impressive clinical efficacy against cancers that were once thought to be incurable, its success is in part limited by unique toxicities which can be severe or even fatal. Cytokine release syndrome (CRS) is the most commonly observed toxicity and occurs as a result of non-antigen specific immune activation. Similar to macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH), CRS is associated with elevated levels of several cytokines including interleukin-6 (IL-6) that serve as a driver for host immune dysregulation. As a direct anti-cytokine drug, tocilizumab has been a cornerstone in the treatment of CAR-T-associated CRS through its ability to dampen CRS without compromising CAR-T-cell function. However, optimal timing of administration is yet unknown. Here, we review the use of tocilizumab in the management of CAR-T-associated CRS, emphasizing on the clinical efficacy across various CAR constructs and its role in current CRS management algorithms. We also discuss alternative therapies that may be considered for refractory CRS therapy and the use of tocilizumab in the current COVID-19 global pandemic.
Keywords: chimeric antigen receptor-T-cell therapy, cytokine release syndrome, tocilizumab, pediatric
Introduction
The landscape of cancer treatment has changed drastically over the past few decades.1 Unlike classic cytotoxic chemotherapies, adoptive cellular therapies such as chimeric antigen receptor (CAR)-T-cell therapy allow us to harness the power of the immune system to fight cancer cells by redirecting cytolytic T-cell activity towards tumor cells.2 Immunotherapies have demonstrated impressive clinical efficacy in treatment of a number of cancers that were once thought to be incurable.2,4 T-cell engaging immunotherapies, including CAR-T and Bispecific T-cell engagers (BiTEs), also elicit unique toxicities. Two of these toxicities, cytokine releases syndrome (CRS) and neurotoxicity, can occur early after treatment with CARs or BiTES and be life-threatening. CRS occurs as a result of non-antigen specific immune activation that clinically and biologically mimics macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH).5,6 Therapies are needed that treat these unwanted side-effects without impacting the efficacy of the immunotherapies.
Of particular interest is tocilizumab, a humanized, immunoglobin G1κ (IgG1κ) anti-human interleukin-6 receptor (anti-IL-6R) monoclonal antibody (mAb) that originally received US Food and Drug Administration (FDA) approval in the late 2000s for treatment of various rheumatologic diseases such as rheumatoid arthritis, systemic and polyarticular juvenile idiopathic arthritis, and giant cell arteritis.7,11 In 2012, our institution treated the first pediatric patient with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL) with CD19 antigen directed CAR-T-cell therapy.12 Several days after infusion of engineered T-cells, she became critically ill with unrelenting high fevers, requiring invasive mechanical ventilation and multiple vasopressors. Etanercept was tried empirically without benefit. A cytokine panel was subsequently sent which revealed significantly elevated levels of a number of cytokines including IL-6, therefore tocilizumab was given. Within several hours of receiving this drug, her condition radically improved, and she has since remained leukemia free. This led to adoption of tocilizumab for CRS, eventually leading to its FDA approval for the treatment of CAR-T-associated CRS in patients 2 years of age and older in 2017.13 Almost a decade after first being used for CRS, the experience with the use of tocilizumab for this indication has increased significantly. The goal of this review will be to highlight updated clinical evidence for the use of tocilizumab in the management of CRS and address current challenges and limitations of this drug.
Basics of Drug
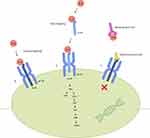
IL-6 is a soluble mediator with a pleiotropic effect on inflammation, immune response, and hematopoiesis.8 During inflammation, it has been shown that IL-6 can up-regulate Th17/Treg balance, promote T-follicular helper-cell differentiation, induce differentiation of CD8+ T-cells into cytotoxic T-cells, and activate B-cells into antibody-producing plasma cells.14,16 IL-6R exist as two forms, either membrane bound or soluble. Binding of IL-6 to IL-6R alone does not lead to signaling, but instead requires the IL-6/IL-6R complex to interact with gp130, a protein that is expressed on all cells. This will subsequently induce homodimerization of gp130 and initiate intracellular signaling via the Jak/Stat pathway.17 In classical IL-6 signaling, IL-6 binds to membrane bound IL-6R and gp130. However, as membrane-bound IL-6R is only expressed on hepatocytes, some epithelial cells and leukocytes, most cells are not responsive to classical IL-6 signaling. In trans-IL-6 signaling, IL-6 binds to soluble IL-6R, and this complex then interacts with gp130 expressing cells (Figure 1).17,18 The composite effect of these changes is thought to serve as the driver for host immune dysregulation, including autoimmune diseases, and acute inflammatory responses such as cytokine release syndrome.
Because of this, targeting of IL-6 became an attractive treatment strategy for various immune-mediated diseases where elevated IL-6 or activated Jak-Stat signaling are involved in the pathogenesis of the disease. Tocilizumab is a humanized anti-IL-6R monoclonal Ab of the IgG1 class that was generated by grafting the complementarity determining regions of a mouse antihuman IL-6R Ab onto human IgG1.7 This drug works by blocking IL-6-mediated signal transduction by inhibiting IL-6 binding to IL-6R (Figure 1). Specifically, data suggest tocilizumab blocks both cis- and trans-IL-6 signaling pathways.18 Pharmacokinetic (PK) and pharmacodynamic (PD) relationships have been studied extensively in both healthy subjects and patients with rheumatologic diseases.19,20 As c-reactive protein (CRP) is induced primarily by IL-6 under inflammatory conditions, it is often used as a PD parameter of tocilizumab.21 Phase I studies have shown that adult patients with rheumatoid arthritis treated with 2 mg/kg, 4 mg/kg, and 8 mg/kg tocilizumab intravenously every 2 weeks had significantly decreased dose-dependent levels of CRP, as well as decreased erythrocyte sedimentation rate (ESR), with the greatest change observed with 8 mg/kg. CRP levels normalized as early as week 2 in the 8 mg/kg cohort. The serum level of CRP remained undetectable as long as the concentration of free tocilizumab was > 1 μg/mL, suggesting that tocilizumab inhibits IL-6 induced increases in CRP levels directly. Fixed dosed subcutaneous formulation of tocilizumab (162 mg given weekly or every 2 weeks) has similar efficacy as compared to intravenous formulation of tocilizumab (8 mg/kg given every 4 weeks).20 Tocilizumab also has a non-linear PK profile in the dose range of 2–8 mg/kg and undergoes biphasic elimination from the circulation.21 Bioavailability of tocilizumab is rapid, and maximal serum concentration is reached after 2 hours (with infusion of 10 mg/kg intravenously). In patients with underlying rheumatologic diseases, the half-life of this drug at steady state is ≤11 days for 4 mg/kg given every 4 weeks intravenously, and 13 days for 8 mg/kg given every 4 weeks.21 Specifically, in patients with adoptive cellular therapy associated CRS, using a dosing parameter established in previous non-oncologic studies (<30 kg received 12 mg/kg, and ≥30kg received 8 mg/kg), PK and PD of tocilizumab in pediatric B-ALL patients who received CTL019 CAR-T therapy (former name of tisagenlecleucel) was found to have increased clearance over other disorders, and did not impair the function of CAR-T-cells.13,22
Clinical Efficacy of Tocilizumab
Tisagenlecleucel
In the Phase 1/2a CTL019 study conducted in 2014 of patients receiving tisagenlecleucel (a second-generation CAR-T-cell product using CD137 (4–1BB) as co-stimulatory domain) for children and young adults with r/r B-ALL, CRS occurred in all 30 patients. Although assessment and grading of CRS have varied considerably across clinical trials, generally, severe CRS is defined as the presence of life-threatening organ dysfunction, often requiring significant cardiovascular and respiratory support.23,25 Eight of the 30 patients developed severe CRS and required intensive care management and vasopressor support for hypotension with varying degrees of respiratory support. All patients with severe CRS received tocilizumab for persistent hypotension unresponsive to fluid boluses, which resulted in rapid defervescence and stabilization of blood pressure, with improvement over a period of 1–3 days. Six patients also received short courses of glucocorticoids, and four patients received a second dose of tocilizumab for recrudescence of CRS.2 Interim analysis showed 14 of 39 (36%) patients developed cardiovascular dysfunction, of which 13 had profound fluid-refractory vasoplegic shock treated with α-agonist infusions and one had cardiomyopathy with diminished left ventricular systolic function supported with milrinone. Within this cohort, all 13 patients with fluid-refractory shock were considered to have severe CRS and were treated with tocilizumab. Nine of thirteen patients received one dose, and four received two or three doses due to partial response or recurrence of symptoms. The first dose of tocilizumab was administered after a median of 5 days after CTL019 infusion in this cohort of patients, and catecholamine-dependent shock demonstrated complete resolution over a median of 4 days after tocilizumab administration. Of note, eight subjects were also treated with short courses of corticosteroids, including the four subjects who received multiple doses of tocilizumab secondary to clinical deterioration at that time. All patients requiring tocilizumab and/or steroids recovered from severe CRS and achieved disease remission.26 Most recent updated analysis demonstrates CRS occurring in 50 of 65 patients (77%), and 39% of patients received tocilizumab with or without other anti-cytokine therapies. Thirty-one patients required intensive care unit level care, but all cases of CRS were reversible.27
Incidence of CRS in the ELIANA trial, a Phase 2, single cohort, multicenter global study occurred in 58 of 75 patients, and the median time to onset was 3 days (range=1–22 days). Thirty-five of 75 patients were admitted to the intensive care unit for management of CRS; 19 patients were treated with high dose vasopressors, 33 received oxygen supplementation, 10 received mechanical ventilation, and seven underwent dialysis. Twenty-eight patients received tocilizumab for management of severe CRS.28 In an updated analysis, CRS occurred in 75 of 97 (77%) patients, of which 48% had severe CRS. Thirty-nine percent of patients with severe CRS received tocilizumab with or without other anti-cytokine therapies. Similar to the CTL019 trial, all cases of CRS were reversible.27
In both the CTL019 and ELIANA trial, no significant adverse events were associated with the administration of tocilizumab. Use to tocilizumab did not seem to affect clinical efficacy of CAR-T-cells. All patients who received tocilizumab were able to achieve complete remission, had robust proliferation, and continued persistence of CAR-T-cells.2,28 However, the optimal timing to intervene with tocilizumab remains unknown, and use of tocilizumab as a prophylactic treatment strategy is being explored. There is an ongoing trial (NCT02906371) evaluating the efficacy of early tocilizumab in pediatric patients with high disease burden in order to mitigate tisagenlecleucel-associated severe CRS, with the hypothesis that preemptive immunomodulation could decrease rates of severe CRS without affecting engraftment and persistence of CAR-T-cells.29,30
Axicabtagene Ciloleucel Trial
In the phase 2 multicenter ZUMA-1 trial of patients receiving KTE-C19 (a second-generation CAR-T-cell product using CD28 as co-stimulatory domain) for adults with refractory aggressive non-Hodgkin lymphoma (NHL), CRS developed in 94 of 101 (93%) of patients. Twelve of the 94 patients (13%) had severe CRS, and vasopressors were used in 17% of these patients. The median time after infusion until the onset of CRS was 2 days (range=1–12 days). Forty-three percent of all patients, regardless of CRS severity, received tocilizumab and 27% received glucocorticoids. All CRS events were reversible and use of immunosuppression in treatment of CRS did not appear to affect the overall response among patients in this study.31 In a retrospective analysis of pooled data from studies involving KTE-C19, it was reported that the median time from the start of CRS to the first dose of tocilizumab was 3 days (range=0–14 days). Five of 15 (33%) patients received two or three doses of tocilizumab per day, and the median total number of tocilizumab was two (range 1–13). Using the response criteria of being afebrile and off vasopressors for at least 24 hours within 14 days of the first dose of tocilizumab, eight of 15 (53%) patients demonstrated a response after a single dose.13
Other CAR Constructs and T-Cell-Engaging Therapies
In addition to tisagenlecleucel and axicabtagene, other CAR constructs have also demonstrated encouraging clinical efficacy. However, different CAR constructs confer different rates and severity of CRS. As the list of novel T-cell engaging therapies is exhaustive, this section will highlight several key trials that will serve to exemplify the effectiveness of tocilizumab in ameliorating unwanted toxicities. In the phase 1 CTL119 study using CD19-humanized CAR-T-cells, CRS was observed in 28 of 30 (93%) patients. Three patients had severe CRS, and all received tocilizumab with complete reversal of CRS symptoms.32 In an updated analysis of a phase 1 trial using CD22-CAR-T-cells with a 4–1BB co-stimulatory domain, CRS occurred in 50 of 58 (86%) of patients, of which 12 (24%) had severe CRS. Twenty-three patients received tocilizumab, and 18 patients received corticosteroids. These interventions resulted in reversal of CRS symptoms. Interestingly, authors in this trial observed an increase in HLH-like features in those with CRS after incorporation of CD4/8 T-cell selection of starting apheresis products. In this subset of patients with HLH-like features, corticosteroids and/or anakinra, an anti-IL-1R antagonist was used. All patients had resolution of HLH-like symptoms with preserved CAR-T-cell expansion.33,34 Patients who receive blinatumomab infusion, a CD19/CD3 BiTE antibody, are also at risk for CRS syndrome. In the phase 2 multicenter, single-group study that treated 189 patients with r/r Philadelphia chromosome negative B-ALL, 2% of all patients developed severe CRS.35 Though incidence of CRS is less common with blinatumomab compared to other CAR-T products, the clinical signs and symptoms are similar, and timing of CRS is generally limited to the first cycle of drug infusion.36,37 A retrospective review conducted using the Amgen global safety database reported a CRS incidence of 3.9% (39 of approximately 1000 cases) and, of these patients, six received tocilizumab. Tocilizumab was administered concurrently with corticosteroids in three cases, after corticosteroids in two cases, and both before and after corticosteroids in one case. Other interventions include stopping blinatumomab infusion, and all six patients had complete resolution of CRS symptoms.38 Flotetuzumab, a novel CD123/CD3 bispecific dual-affinity re-targeting (DART) antibody, similarly confers a high risk of CRS. In the ongoing phase 1/2 multi-center, a single-arm study (NCT02152956) evaluating 30 adult patients with r/r acute myeloid leukemia (AML), almost all patients developed CRS. Severe CRS occurred in four patients, and most commonly occurred within the first week of treatment. Twenty (67%) patients received at least one dose of tocilizumab (median=2 doses, range=1–12 doses), and all patients recovered from CRS toxicity without an impact on flotetuzumab anti-leukemic activity.39
Advantages and Limitations of Using Tocilizumab for Management of CAR-T-Cell Therapy-Associated Toxicities
As stated above, tocilizumab demonstrates impressive efficacy in the management of CAR-T-associated CRS. Unlike corticosteroids, it does not appear to suppress T-cell function and/or induce T-cell apoptosis.40,41 This is important as the challenge in toxicity management lies in controlling CRS symptoms without compromising clinical efficacy. Reassuringly, studies to date indicate that use of tocilizumab does not seem to affect the function of CAR-T-cells. In both the phase 1/2a CTL019 and ELIANA trials, all patients who received tocilizumab had robust proliferation and continued persistence of CAR-T-cells.2,28 Similarly, in both phase 1 and 2 of the ZUMA-1 trial, use of tocilizumab did not appear to ablate CAR-T-cell expansion nor alter CAR-T-cell related elevation of cytokines, chemokines, and immune effector molecules. Durable responses were also observed in patients who received tocilizumab and/or corticosteroids for toxicity management as well as those who did not.31,42 Finally, Davila et al43 reported use of tocilizumab as monotherapy did not dampen expansion of CAR-T-cells in both peripheral blood and bone marrow.
Aside from CRS, another significant CAR-T-cell therapy associated toxicity is immune effector cell-associated neurotoxicity syndrome (ICANS).26 Incidence of ICANS is noteworthy, as 30 of 75 (40%) patients in the ELIANA trial, and 65 of 111 (64%) patients in the ZUMA-2 trial (phase 2 KTE-C19 for patients with r/r mantle cell lymphoma) developed symptoms of neurotoxicity.28,31 Unlike CRS, the underlying pathophysiology of ICANS remains somewhat unclear. Recent studies are in support of a model whereby CAR-associated T-cell stimulation induces central nervous system (CNS) endothelial cell (EC) activation as an early event, initiating a cascade of coagulopathy and enhanced endothelial permeability, resulting in a breakdown of blood–brain barrier. High levels of inflammatory cytokines such as monocyte-derived IL-1 and IL-6 and CAR-T-cells then enter the CNS and initiate a feed-forward loop of continued EC and pericyte activation.44,47 As a result, common ICANS symptoms include headache, delirium, language disturbance, seizures, focal deficits, and diminished consciousness including coma. In severe cases, patients can develop cerebral edema and necrosis associated with intravascular microthrombi.25 Despite such grave symptoms, limited therapeutic interventions are available, and management is primarily supportive. Unfortunately, targeting of IL-6R using tocilizumab has not been shown to be effective for either prevention or treatment of neurotoxicity.48 This is likely secondary to the inefficient distribution into the CNS,49 upregulation of IL-6, or involvement of other cytokines.45,50,51 Therefore, tocilizumab is not an approved treatment for neurotoxicity associated with CAR-T-cell therapy.13 However, owing to the increased level of IL-1 that is also seen in CRS and severe neurotoxicity, prophylactic treatment with anakarina, an IL-1R antagonist, has been promising. Mice that were treated prophylactically with anakinra compared to tocilizumab or placebo at the time of CAR-T-cell infusion were found to have an absence of meningeal thickening on postmortem examination, a finding that is supportive of neurotoxicity prevention.46
Safety/Adverse Events
In general, tocilizumab is a very well tolerated drug with minimal adverse events (AEs). Despite its potent immunosuppressive effects, the risk of serious infections attributed to tocilizumab monotherapy is comparable to other biologic disease-modifying antirheumatic drugs (DMARDs).52,53 In a study with 48 healthy subjects, tocilizumab was not associated with increased serious AEs. However, it is important to note that a black box warning in tocilizumab’s label describing the increased risk of serious infections is present. This is based upon the ACTERMRA-IV data that included five double-blind, controlled, multicenter studies where patients received either 8 mg/kg tocilizumab monotherapy, 8 mg/kg tocilizumab in combination with disease modifying anti-rheumatic drugs including methotrexate, or 4 mg/kg tocilizumab in combination with methotrexate. Results indicate that the rate of serious infections in the tocilizumab monotherapy group was 3.6 per 100 patients-years compared to 1.5 per 100 patient-years in the methotrexate group. Aside from this, common AEs include headache (17% of patients), diarrhea (8% of patients), and neutropenia (60%) as defined by an absolute value below 1.5x109/l and a decrease of 20% below baseline. No difference in AEs were found between intravenous or subcutaneous route of administration.19 Similarly, in patients with various rheumatologic diseases, tocilizumab was also found to be well tolerated. Most common AEs in this population cohort include infection, gastrointestinal complaints, rash, headache, and rates of withdrawal of tocilizumab due to AEs were low.20,54 Finally, based on the FDA approval summary for tocilizumab for treatment of CAR-T-cell-associated CRS, no reports of AEs were attributable to tocilizumab. Although five patients in the CTL019 series and five patients in the KTE-C19 series died within 30 days of the first dose of tocilizumab, none were thought to be attributed to tocilizumab.13
Management of Severe CRS with Tocilizumab
As CAR-T therapy-associated CRS can potentially be life-threatening, a major focus of research has been placed on identification of risk factors in developing severe CRS in order to determine optimal timing of tocilizumab administration. One of the most commonly identified risk factors is disease burden before CAR-T infusion, as higher tumor burden can presumably lead to higher levels of T-cell activation.2,55,56 There are also clear data that demonstrate a higher infusion dose of CAR-T-cell products is associated with more severe CRS.29,55,57 Although varying CAR-T-cell therapy constructs may have different in vivo expansion kinetics, different dosing strategies have been attempted. Interventions such as delivering an initial lower dose of CAR-T-cells to patients with higher disease burden, or increasing infusion time over 3 days with doses held on days 2 and/or 3 for early signs of CRS have all seemed to reduce rates of severe CRS at the respective institutions.56,57 Early elevation of inflammatory markers or cytokine profiles such as interferon gamma (IFN-γ), soluble gp130, soluble IL-6R, and IL-6 have also been found to be predictive of CRS severity and may potentially be used in guiding pre-emptive anti-cytokine directed treatment.18 However, interpretation of the risk factors for severe CRS remains challenging, as different studies includes different diseases, disease stages, trial design, pre-CAR-T lymphodepleting regimen, and CRS grading scales.
Regardless, there is general consensus that tocilizumab should be administered at the time of moderate-to-severe signs of CRS in order to minimize rates of life-threatening CRS without altering CAR-T-cell efficacy. Both Tisagenlecleucel and Axicabtagene have published recommended guidelines for management of CRS with tocilizumab.13 For Tisagenlecleucel, tocilizumab should be considered for patients who developed CRS symptoms requiring moderate-to-aggressive intervention (ie, hemodynamic instability despite intravenous fluids and vasopressor support, worsening respiratory distress including pulmonary infiltrates, increased oxygen requirement including high-flow oxygen and/or need for mechanical ventilation, or signs of rapid clinical deterioration). Tocilizumab may be repeated every 8 hours if there is no clinical improvement, but doses of tocilizumab should be limited to a maximum of four doses. Concurrent administration of methylprednisolone should also be considered if no improvement after first dose of tocilizumab.58 For Axicabtagene, the recommended threshold for tocilizumab administration is somewhat lower than tisagenlecleucel. Patients who endorse moderate CRS symptoms (ie, oxygen requirement less than 40% FiO2, hypotension responsive to fluids or low-dose vasopressor) may be given tocilizumab. Similarly, doses can be repeated every 8 hours for a maximum of four total doses, and alterative CRS treatment such as corticosteroids should be considered if no clinical improvement is seen within 24 hours of Tocilizumab administration.59 It is important to note that such proposed algorithms only serve as guidelines, and the decision for the optimal timing of tocilizumab administration will inevitably rely heavily on the clinical subjectivity on the treating institution. When managing a complex syndrome such as CRS, a multi-disciplinary approach cannot be overstated, and the critical care team should be made aware of all CAR-T therapy-treated patients in the hospital in order to facilitate urgent transfers to the intensive care unit when needed.
Unfortunately in rare circumstances, CRS may be refractory to tocilizumab and additional immunosuppression is needed.60 Mechanisms of failure are unclear, but may be due to inadequate tocilizumab dosing to suppress a rise in IL-6, alternative cytokines driving the hypercytokinemia, or a compensatory feedback in IL-6 signaling as described earlier. Investigations for alternative therapies are underway, especially for patients with tocilizumab and corticosteroids refractory CRS.61,62 Siltuximab, an IL-6 antagonist, is an attractive candidate, especially as serum IL-6 levels have been shown to increase after administration of tocilizumab, presumably by preventing IL-6R mediated uptake of IL-6 into peripheral tissue.50 Regardless, experience with siltuximab in CRS management is limited, and there has not been a head to head comparison between the two drugs to determine superiority.63 Recent pre-clinical studies have also demonstrated encouraging results on dasatinib, a multityrosine kinase inhibitor, for patients refractory to standard CRS treatment by potently and reversibly inhibiting CAR-T-cell function.64,65 Other potential therapies include ruxolitinib, a Janus kinase inhibitor, anakinra, an IL-1 receptor antagonist, and Cytosorb cartridge columns; all of these have been used with anecdotal success, but prospective clinical trials will be needed to confirm their clinical efficacy as either first-line or refractory CRS therapy.66,68
Use of Tocilizumab in COVID-19-Associated CRS-Like Syndrome
The current coronavirus disease 2019 (COVID-19) pandemic has inflicted a devastatingly high morbidity and mortality rate across the globe.69,70 Although we are only beginning to understand this disease’s underlying pathophysiology, a unique sequelae of COVID-19 is the presence of a CRS-like response. A pathology report from the first patient in China that died from severe COVID-19 revealed a high concentration of proinflammatory cytokines,71 a finding that was later confirmed in other published studies.72,73 Patients who develop this so called “hyperinflammatory state” will often rapidly progress into multisystem organ failure, therefore much urgency is needed to find an appropriate prophylactic or treatment strategy. In particular, elevated levels of interferon gamma (IFN-γ), IL-6, IL-8, IL-10, and IL-12 are associated with severe lung injury and adverse outcomes.74 Based on these findings and the vast experience with this drug, tocilizumab has since been used to treat patients with severe COVID-19 and has demonstrated encouraging results.72,73,75
In China, a single center reported use of tocilizumab (dosing ranged from 80 mg to 600 mg) in 15 adult patients, of which 13 had severe disease. Five received two or more doses of tocilizumab, and eight patients received corticosteroids concurrently. The mortality rate was 20% (three of 15 patients) and, interestingly, deaths occurred in patients who only received one dose of Tocilizumab.73 In another single center experience from Italy, two doses of tocilizumab 8 mg/kg were administered to 100 adult patients with severe disease. Thirteen received a third dose of tocilizumab, and none received other anti-cytokine or immune-modulating therapies. The mortality rate was 20% (20 of 100 patients).72 Although these results provide encouraging evidence on tocilizumab’s clinical efficacy in the treatment of severe COVID-19, larger randomized trials are needed to confirm this. Moreover, further studies will determine if tocilizumab may play a role in the treatment or prevention of the post-inflammatory Kawasaki-like disease observed in children with COVID-19.76,77
Conclusion
CAR-T-cell therapy has revolutionized the cancer therapy landscape over the past decade. However, despite such impressive clinical efficacy in the treatment of r/r neoplasms, its success is in part limited by potentially life-threatening CRS. Using targeted anti-cytokine therapy, tocilizumab remains a cornerstone in the treatment of CAR-T-cell therapy associated CRS as evidenced by its ability to dampen CRS without compromising CAR-T-cell efficacy along with its minimal toxicity profile. However, further studies are needed to elucidate the optimal timing of intervention, especially in patients who are at risk of developing severe CRS or if alternative agents are more efficacious in either first-line or refractory CRS therapy.
Disclosure
The authors have no relevant affiliations or financial involvement with the subject matter or materials discussed in the manuscript. David Teachey reports grants from NIH (NCI R01CA193776), Leukemia and Lymphoma Society TRP and SCOR, Cookies for Kids Cancer, Alex’s Lemonade Stand Foundation, and Children’s Oncology Group Foundation, during the conduct of the study; and serves on advisory boards for Amgen, La Roche, Sobi, and Janssen, other from Amgen, outside the submitted work.
References
1. Teachey DT, Hunger SP. Acute lymphoblastic leukaemia in 2017: immunotherapy for ALL takes the world by storm. Nat Rev Clin Oncol. 2018;15(2):69–70. doi:10.1038/nrclinonc.2017.176
2. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi:10.1056/NEJMoa1407222
3. von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/Phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381–4389. doi:10.1200/JCO.2016.67.3301
4. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–753. doi:10.1056/NEJMoa1509277
5. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi:10.1182/blood-2014-05-552729
6. Billiau AD, Roskams T, Van Damme-lombaerts R, Matthys P, Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-gamma-producing lymphocytes and IL-6- and TNF-alpha-producing macrophages. Blood. 2005;105(4):1648–1651. doi:10.1182/blood-2004-08-2997
7. Sato K, Tsuchiya M, Saldanha J, et al. Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res. 1993;53(4):851–856.
8. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi:10.1101/cshperspect.a016295
9. Navarro G, Taroumian S, Barroso N, Duan L, Furst D. Tocilizumab in rheumatoid arthritis: a meta-analysis of efficacy and selected clinical conundrums. Semin Arthritis Rheum. 2014;43(4):458–469. doi:10.1016/j.semarthrit.2013.08.001
10. Yokota S, Miyamae T, Imagawa T, et al. Therapeutic efficacy of humanized recombinant anti-interleukin-6 receptor antibody in children with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2005;52(3):818–825. doi:10.1002/art.20944
11. Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627–2632. doi:10.1182/blood-2004-12-4602
12. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi:10.1056/NEJMoa1215134
13. Le RQ, Li L, Yuan W, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23(8):943–947. doi:10.1634/theoncologist.2018-0028
14. Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. doi:10.1002/eji.201040391
15. Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209(7):1241–1253. doi:10.1084/jem.20120994
16. Okada M, Kitahara M, Kishimoto S, Matsuda T, Hirano T, Kishimoto T. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol. 1988;141(5):1543–1549.
17. Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. Int J Biol Sci. 2012;8(9):1237–1247. doi:10.7150/ijbs.4989
18. Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6(6):664–679. doi:10.1158/2159-8290.CD-16-0040
19. Zhang X, Georgy A, Rowell L. Pharmacokinetics and pharmacodynamics of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, following single-dose administration by subcutaneous and intravenous routes to healthy subjects. Int J Clin Pharmacol Ther. 2013;51(6):443–455. doi:10.5414/CP201819
20. Song SN, Yoshizaki K. Tocilizumab for treating rheumatoid arthritis: an evaluation of pharmacokinetics/pharmacodynamics and clinical efficacy. Expert Opin Drug Metab Toxicol. 2015;11(2):307–316. doi:10.1517/17425255.2015.992779
21. Nishimoto N, Yoshizaki K, Maeda K, et al. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J Rheumatol. 2003;30(7):1426–1435.
22. Lee C, Bittencourt H, Rives S, et al. Pharmacokinetics and Pharmacodynamics of Tocilizumab for the Management of Cytokine Release Syndrome (CRS) in Pediatric and Young Adult Patients with Relapsed/Refractory (R/R) B-Cell Acute Lymphoblastic Leukemia (B-ALL) Treated with CAR T-Cell Therapy, CTL019. Atlanta, Georgia: The American Society of Hematology (ASH); 2017.
23. Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11(1):35. doi:10.1186/s13045-018-0571-y
24. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi:10.1038/nrclinonc.2017.148
25. Lee DW, Santomasso BD, Locke FL, et al. ASBMT consensus grading for cytokine release syndrome and neurological toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2018.
26. Fitzgerald JC, Weiss SL, Maude SL, et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45(2):e124–e131. doi:10.1097/CCM.0000000000002053
27. Grupp SA, Maude S, Rives S, et al. Updated Analysis of the Efficacy and Safety of Tisagenlecleucel in Pediatric and Young Adult Patients with Relapsed/Refractory (r/r) Acute Lymphoblastic Leukemia. The American Society of Hematology; 2018.
28. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi:10.1056/NEJMoa1709866
29. Gardner R, Leger KJ, Annesley CE, et al. Decreased rates of severe CRS seen with early intervention strategies for CD19 CAR-T cell toxicity management. Blood. 2016;128(22):586. doi:10.1182/blood.V128.22.586.586
30. Myers R, Kadauke S, Li Y, et al. Risk-Adapted Preemptive Tocilizumab Decreases Severe Cytokine Release Syndrome (CRS) after CTL019 CD19-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for Pediatric B-Cell Acute Lymphoblastic Leukemia (B-ALL). Biol Blood Marrow Transplant. 2020;26(3):S39. doi:10.1016/j.bbmt.2019.12.105
31. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi:10.1056/NEJMoa1707447
32. Maude S, Barrett D, Rheingold S, et al. Efficacy of humanized CD19-targeted chimeric antigen receptor (CAR)-modified T cells in children with relapsed ALL. Abstract presented at The American Society of Hematology; December 2, 2016; San Diego, CA.
33. Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–28. doi:10.1038/nm.4441
34. Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-cell selection affects Chimeric Antigen Receptor (CAR) T-cell potency and toxicity: updated results from a Phase I Anti-CD22 CAR T-cell trial. J Clin Oncol. 2020;JCO1903279.
35. Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi:10.1016/S1470-2045(14)71170-2
36. Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):567–572. doi:10.1182/asheducation-2016.1.567
37. Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood. 2013;121(26):5154–5157. doi:10.1182/blood-2013-02-485623
38. Choudhry J, Parson M, Wright J. A Retrospective Review of Tocilizumab for the Management of Blinatumomab (a Bispecific T Cell Engager)-Induced Cytokine Release Syndrome (CRS). San Diego, CA: The American Society of Hemotology; 2018 November 29.
39. Jacobs K, Viero C, Godwin J, et al. Management of Cytokine Release Syndrome in AML Patients Treated with Flotetuzumab, a CD123xCD3 Bispecfiic Dart Molecule for T-Cell Redirected Therapy. San Diego, CA: The American Society of Hematology; 2018.
40. Paliogianni F, Ahuja SS, Balow JP, Balow JE, Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through IL-2 receptor. J Immunol. 1993;151(8):4081–4089.
41. Lanza L, Scudeletti M, Puppo F, et al. Prednisone increases apoptosis in in vitro activated human peripheral blood T lymphocytes. Clin Exp Immunol. 1996;103(3):482–490. doi:10.1111/j.1365-2249.1996.tb08306.x
42. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: A multicenter study of KTE-C19 Anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther. 2017;25(1):285–295. doi:10.1016/j.ymthe.2016.10.020
43. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra225. doi:10.1126/scitranslmed.3008226
44. Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7(12):1404–1419. doi:10.1158/2159-8290.CD-17-0698
45. Mackall CL, Miklos DB. CNS endothelial cell activation emerges as a driver of CAR T cell-associated neurotoxicity. Cancer Discov. 2017;7(12):1371–1373. doi:10.1158/2159-8290.CD-17-1084
46. Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24(6):739–748. doi:10.1038/s41591-018-0036-4
47. Gallenga CE, Pandolfi F, Caraffa A, et al. Interleukin-1 family cytokines and mast cells: activation and inhibition. J Biol Regul Homeost Agents. 2019;33(1):1–6.
48. Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019;111(7):646–654. doi:10.1093/jnci/djz017
49. Nellan A, McCully CML, Cruz Garcia R, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018;132(6):662–666. doi:10.1182/blood-2018-05-846428
50. Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112(10):3959–3964. doi:10.1182/blood-2008-05-155846
51. Mueller KT, Maude SL, Porter DL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130(21):2317–2325. doi:10.1182/blood-2017-06-786129
52. Pawar A, Desai RJ, Solomon DH, et al. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: a multidatabase cohort study. Ann Rheum Dis. 2019;78(4):456–464. doi:10.1136/annrheumdis-2018-214367
53. Yamamoto K, Goto H, Hirao K, et al. Longterm safety of tocilizumab: results from 3 years of followup postmarketing surveillance of 5573 patients with rheumatoid arthritis in Japan. J Rheumatol. 2015;42(8):1368–1375. doi:10.3899/jrheum.141210
54. Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199–219. doi:10.1146/annurev-pharmtox-010611-134715
55. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi:10.1016/S0140-6736(14)61403-3
56. Park JH, Riviere I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi:10.1056/NEJMoa1709919
57. Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+: CD8+composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–2138. doi:10.1172/JCI85309
58. Novartis. Cytokine Release Syndrome Treatment Algorithm. 2018.
59. Gilead. CRS Grading and Management Guidelines. 2019.
60. Ishii K, Shalabi H, Yates B, et al. Tocilizumab-Refractory Cytokine Release Syndrome (CRS) Triggered by Chimeric Antigen Receptor (CAR)-Transduced T Cells May Have Distinct Cytokine Profiles Compared to Typical CRS. San Diego, CA: The American Society of Hematology; 2016 December 2.
61. Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi:10.1200/JCO.2014.56.2025
62. Frey N, Levine BL, Lacey S, et al. Refractory Cytokine Release Syndrome in Recipients of Chimeric Antigen Receptor (CAR) T Cells. San Francisco, CA: The American Society of Hematology; 2014 December 6.
63. Chen F, Teachey DT, Pequignot E, et al. Measuring IL-6 and sIL-6R in serum from patients treated with tocilizumab and/or siltuximab following CAR T cell therapy. J Immunol Methods. 2016;434:1–8. doi:10.1016/j.jim.2016.03.005
64. Weber EW, Lynn RC, Sotillo E, Lattin J, Xu P, Mackall CL. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 2019;3(5):711–717. doi:10.1182/bloodadvances.2018028720
65. Mestermann K, Giavridis T, Weber J, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med. 2019;11:499. doi:10.1126/scitranslmed.aau5907
66. Haile WB, Gavegnano C, Tao S, Jiang Y, Schinazi RF, Tyor WR. The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol Dis. 2016;92(Pt B):137–143. doi:10.1016/j.nbd.2016.02.007
67. Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014;20(2):119–122. doi:10.1097/PPO.0000000000000035
68. Bottari G, Merli P, Guzzo I, et al. Multimodal therapeutic approach of cytokine release syndrome developing in a child given chimeric antigen receptor-modified T cell infusion. Crit Care Explor. 2020;2(1):e0071. doi:10.1097/CCE.0000000000000071
69. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMoa2002032
70. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574. doi:10.1001/jama.2020.5394
71. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi:10.1016/S2213-2600(20)30076-X
72. Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020:102568.
73. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92(7):814–818. doi:10.1002/jmv.25801
74. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi:10.1016/j.ebiom.2020.102763
75. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi:10.1073/pnas.2005615117
76. Viner R, Whittaker E. Kawasaki-like disease: emergency complication during the COVID-19 pandemic. The Lancet. 2020.
77. Verdoni L, Mazza A, Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.