Back to Journals » Psoriasis: Targets and Therapy » Volume 9
Spotlight on itolizumab in the treatment of psoriasis – current perspectives from India
Authors Budamakuntla L, Shree-Lakshmi HV, Bansal A, Venkatarayaraju SK
Received 13 October 2017
Accepted for publication 11 January 2019
Published 3 May 2019 Volume 2019:9 Pages 19—27
DOI https://doi.org/10.2147/PTT.S154073
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Uwe Wollina
Leelavathy Budamakuntla,1 HV Shree-Lakshmi,1 Akshi Bansal,1 Shankar Kumar Venkatarayaraju2
1Department of Dermatology, Venerology and Leprosy, Bangalore Medical College and Research Institute, Bengaluru, Karnataka 560002, India; 2Department of Endocrinology, MS Diabetes Centre, Indiranagar, Bangalore 560038, India
Abstract: Psoriasis is a chronic, debilitating, immune-mediated, systemic inflammatory disease affecting mainly skin, nails, and joints. Several therapeutic modalities are available depending on the severity of the disease. Long-term use of these drugs results in unwanted effects and toxicities. Recently, itolizumab, a humanized monoclonal immunoglobulin G1 antibody to CD6, has shown appreciable clinical effects and safety profile in patients with moderate-to-severe chronic plaque psoriasis. A literature search was conducted using the keywords “anti-CD6”, “psoriasis”, “phase trials”, “case series”, and “case reports”. The data from all studies conducted in India on efficacy of itolizumab in psoriasis and published before September 2017 were collected. This article provides an overview of the clinical data obtained in these published articles. Itolizumab has immunomodulatory and anti-inflammatory effects. It is efficacious and provides a good duration of remission, and hence represents a new biological agent that could be added to the therapeutic armamentarium of psoriasis.
Keywords: psoriasis, biologic, monoclonal antibody, anti-CD6, Humanised IgG1 monoclonal antibody, anti CD6
Introduction
Psoriasis is a chronic relapsing inflammatory disease affecting ~1%–3% of the world’s population.1,2 Recently, there has been a growing consensus that psoriasis is a systemic disorder rather than a papulosquamous disorder affecting the skin. Increased co-incidence of inflammatory arthropathy (in about 25% of the cases) and metabolic syndrome with psoriasis has supported this opinion.3 Frequent episodes of exacerbations and remissions of the disease not only lead to physical impairment but also cause psychological, social, and even financial difficulties.3 Several drugs are available for the management of psoriasis depending upon the severity and type of the disease. Conventional drugs including corticosteroids, retinoids, methotrexate, and anthralin are prescribed in moderate-to-severe degree of psoriasis with some success. However, these drugs on long-term use can cause toxicity. Biological agents like etanercept, adalimumab, and infliximab are considered to be superior to the conventional drugs both in terms of safety and efficacy, as they are highly specific in their action (target-specific molecules important in the pathogenesis of psoriasis).4
Pathogenesis of psoriasis: immunological aspects
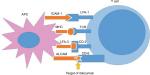
In a genetically predisposed individual, different immune cells like dendritic cells, histiocytes, keratinocytes, monocytes, etc, under specified stimuli release several cytokines like tumor necrosis factor (TNF-α) and IL-1.1,5 These cytokines then activate the antigen-presenting cells (APCs) which along with the processed antigens then activate the naïve T-cells following migration to the regional lymph nodes. Next, differentiation of naïve T-cells into different helper T-cells like Th1, Th2, Th17, and T regulatory cells occur.2 APC-mediated activation of the T-cells requires two signals. The first one depends on the antigen (antigen-specific) and requires interaction between the T-cell receptor and the major histocompatibility complex molecules located on the membranes of the APCs, whereas the second one is not antigen specific and requires the presence of different co-stimulatory signals.1,5 Activation of T-cells fails in the absence of co-stimulation, leading to apoptosis of T-cells.
Role of CD6 in the pathogenesis of psoriasis
CD6 has been identified as an important immune component in the pathogenesis of psoriasis. CD6 is a surface glycoprotein found on the outer surface of mature T-cells and immature B-cells; its molecular weight ranges between 105 and 130 kDa.1,6–8 There are three scavenger receptor cysteine-rich (SRCR) domains (D) present in the extracellular region of CD6. There is a CD6 ligand, also known as CD166 or activated leukocyte-cell adhesion molecule (ALCAM), that is expressed on different cells like the T- and B-lymphocytes, APCs, and thymocytes.1,6–8 ALCAM binds with the SRCR domain 3 (D3) of CD6 and plays an important role in interaction between T-cells and APCs.1,6–8 Again, CD6–ALCAM interaction contributes to the formation of immunological interactions like facilitation of stable adhesion between the T-cells and the APCs and also plays important role in differentiation, proliferation, and maturation of T-cells.1,6–8 CD6 can also activate the T-cells when there is a decrease in the levels of intracellular phosphoproteins (Figure 1).7 It can also stimulate the proliferation of CD3 and increase the number of CD25 molecules via activating a number of co-stimulatory pathways. In addition to all these functions, CD6 can also increase the release of TNF-α, IFN-γ, and IL-6.1 Through all these actions, CD6 can finally lead to dermal inflammation which in turn leads to activation of keratinocytes and consequent psoriatic changes like acanthosis, hyperkeratosis, and parakeratosis.1
Itolizumab
Itolizumab, a humanized recombinant monoclonal antibody of immunoglobulin G1 type with a molecular weight of 148 kDa, is a selective T-cell co-stimulation modulator, targeting the SRCR-D1 of CD6 on T-cells.1 It has two heavy chains with 449 amino acids and two light chains with 214 amino acids linked with a disulfide bond.1
The murinemonoclonal anti-CD6 (ior-T1) has therapeutic effects in diseases like rheumatoid arthritis and psoriasis.9 Itolizumab, the humanized version of ior-T1, shows less immunogenicity as well as a better safety profile but exhibits the same therapeutic benefits as ior-T1.1,8
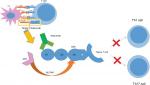
Mechanism of action
Several hypotheses regarding the mechanism of itolizumab have been proposed, although many of them require further evidences. The most popular theory is that itolizumab can modulate T-lymphocyte activation and proliferation through binding with CD6 (Figure 2).1,8 However, it does not have any effect on interaction of ALCAM with CD6-expressing HEK293 cells in experimental settings and does not cause T-cell depletion in rheumatoid arthritis patients.1,7 Also, the widespread presence of CD6 in different types of T-cells and B-cells should enable itolizumab to modulate immunological pathways in various diseases, although clinical evidence in this regard is lacking.1,6,7
Dosage and administration
In chronic plaque psoriasis, the recommended dosage schedule includes administration of itolizumab at 1.6 mg/kg body weight, once every 2 weeks for 12 weeks followed by once in 4 weeks for a total period of 24 weeks.1,8 It is mixed with 250 mL of sterile normal saline at room temperature and administered as slow intravenous infusion over 2 hours, 50 mL in the first hour and the remaining 200 mL in the next hour.1,8
Contraindications
The drug is contraindicated in active and latent infections and in patients with hypersensitivity to any of the components of the itolizumab injection or murine proteins.1 Hence, screening active as well as latent infections like tuberculosis is mandatory before initiating therapy. As safety of the drug is yet to be evaluated in patients with neutropenia and lymphopenia, AIDS, tuberculosis, and hepatitis B and C, itolizumab is avoided in psoriasis patients with these coexisting conditions.1 Again, safety of itolizumab is not established in pregnant (can cross placental barrier) and lactating mothers (secreted in milk), in children <18 years, and in patients with liver and kidney dysfunctions. Hence, it is better to avoid the use of itolizumab in these people.1
Safety and efficacy of itolizumab for psoriasis in Indian patients
In India, the efficacy and safety of itolizumab was assessed in two different randomized, multicentric studies involving patients with stable chronic plaque psoriasis, aged ≥18 years, with Psoriasis Area and Severity Index (PASI) score ≥10 (severe disease) and in patients who showed minimal or no benefit from other systemic drugs (treatment failures or resistant).
In the study conducted by Anand et al,10 a 32-week, randomized, single-blind, and parallel phase II study, itolizumab was administered at different doses at different intervals to 40 patients allotted to eight different groups, each group consisting of 5 patients for a total period of 8 weeks with follow-up period extended for 24 weeks.10 The study showed statistically significant improvement in mean PASI score, Physician’s Global Assessment (PGA) score, and Psoriasis Severity Scale (Table 1).10 Quality of life of the study participants also improved with itolizumab assessed by the Dermatology Quality Life Index (DLQI) and Short Form-6.10 At 12th week, 72.5%, 45%, 30%, and 7.5% of the patients achieved PASI scores of 50, 75, 90, and 100, respectively.10 The study observed that 62% of patients improved or maintained their PASI improvement measured at the 8th week till the 12th week even after stoppage of the drug.10
In a study by Krupashankar et al11 and in a letter to the editor by Dogra et al,12 a 52-week, randomized, double-blind, placebo-controlled, parallel-arm, one-way crossover, multicentric, randomized withdrawal, phase III study of 225 patients with moderate-to-severe plaque psoriasis (PASI ≥10), the patients were randomized (2:2:1) into three groups: A, B, and C. Groups A and B were assigned different dosing schedules (week 0–12: arm A patients received a loading dose of 0.4 mg/kg/week of itolizumab for 4 weeks, followed by 1.6 mg/kg every 2 weeks as induction regimen; arm B patients received 1.6 mg/kg every 2 weeks) and group C was on placebo during week 0–12.11After 12 weeks, placebo crossover was conducted and group C was administered the study drug (1.6 mg/kg of itolizumab every 2 weeks till 24 weeks).11 At 12 weeks, 27%, 36.4%, and 2.3% of patients were reported to achieve at least 75% improvement in PASI score in groups A, B, and C, respectively, thus meeting the primary end point, while 58.4%, 67.0%, and 23.3% of patients were reported to achieve PASI 50 in groups A, B, and C, respectively.11 The reduction in PASI score was statistically significant in the treatment groups. The proportion of patients who achieved at least 75% improvement in PASI was greater for patients with baseline PASI ≥20 than for those with PASI <20. The study also observed PASI 90, reported for 11.2% and 17% of patients in groups A and B, respectively.11 After crossover of the placebo group to receive itolizumab at week 12, the improvement in PASI scores was comparable to other treatment groups by week 20.11 At week 28, 46.1%, 45.5%, and 41.9% of patients were reported to achieve at least 75% improvement in PASI score in groups A, B, and C, respectively, while 78.7%, 80.7%, and 79.1% of patients were reported to achieve at least 50% improvement in PASI score in groups A, B, and C, respectively.11 At week 28, the study observed at least 90% improvement in PASI score in 19.1%, 21.6%, and 27.9% of patients in groups A, B, and C, respectively. Quality of life scores and PGA also demonstrated similar trend as PASI scores.11 At week 12, the proportion of patients with PGA scores of “clear” or “minimal” was greater than placebo (A: 20.0%; B: 16.0%; C, 4.9%), and at week 28, it was similar across the groups (21%–23%).11
Furthermore, out of the 199 patients in the above-mentioned trial, at week 28, 177 patients entered randomized withdrawal phase.12 At week 28, patients with PASI score ≥75 were re-randomized (1:1) into group 1M and group 1P to receive maintenance itolizumab therapy and placebo, respectively.12 Patients with PASI score <50 were withdrawn, while patients with PASI score ≥50 but <75 at week 28 were reinitiated with itolizumab induction therapy. The patients of the three subgroups can be categorized as: good responders (GR), achieving long-term remission after controlled drug cessation; good responders with maintenance therapy (GM), achieving long-term remission with maintenance therapy; and late responders (LR) achieving PASI ≥75 after itolizumab reinitiation with the induction regimen.12 At 1-year follow-up, 52.5% in group 1P maintained PASI score ≥75 (representing GR) and 70% maintained PASI score ≥50, which is a clinically meaningful response, comparable to other biologics.12 In group 1M, significant remission was observed with 66.7% patients who maintained PASI score at 75% and 84.6% patients who maintained PASI score at 50.12 In group 1M, 44.7% of patients maintained PGA scores of “clear” or “minimal” at week 52 vs 46.2% at week 28; in group 1P, these proportions were reported as 30% vs 50%.12 (Table 1)
The safety of itolizumab was also established in a case series of five patients reported by Nott et al,13 a case series of seven patients by Singh,14 a case series of 20 patients by Parthasaradhi,15 a case series of 155 patients by Parthasaradhi et al,16 a case series of five patients by Pai and Pai,17 a case report presented by Trasi et al,18 a case report by Gupta et al,19 and a case report by Budamakuntla et al.20 In all the clinical studies and individual case reports or case series, itolizumab was safe and well tolerated (Tables 2 and 3).
  | Table 2 Case series on efficacy and adverse events of itolizumab Abbreviations: DLQI, Dermatology Quality Life Index; PASI; Psoriasis Area and Severity Index; PGA, Physician’s Global Assessment. |
Discussion
Itolizumab was efficacious with a significant reduction in the mean PASI, PGA, and DLQI scores in both the above-mentioned phase II and III trials. The common side effects noted were diarrhea, infusion-related reactions, and anti-drug antibodies with no effect on safety and efficacy of the drug.11 In various case series and case reports, the results were comparable and itolizumab gave good remission. The comparative data of all the studies and case reports are summarized in Table 4.
Efficacy of itolizumab as compared to various biologics available in India for psoriasis is tabulated in Table 5.
  | Table 5 Comparison of efficacy of various biologics available in India for psoriasis Abbreviation: PASI; Psoriasis Area and Severity Index. |
In various randomized control trials conducted on Indian patients, PASI 75 response at week 12 was 58.5% in those who received 300 mg/kg of secukinumab,22 50% in those who received 150 mg/kg of secukinumab,22 36.4% in those who received 1.6 mg/kg two weekly who received 1.6mg/kg of Itolizumab once in 2 weeks,11 27% in those who received 0.4 mg/kg/week for 4 weeks and then 1.6 mg/kg every 2 weeks of itolizumab,11 and 19.4% in those who received etanercept.22
Conclusion
Psoriasis is a chronic inflammatory disease that can result in significant physical, psychological, and social morbidity with severe impairment of quality of life. Being a T-helper cell-mediated, type 1 immunological disease, it does not just affect the skin but goes more than “skin deep” and evokes a state of systemic inflammation.
Most of the conventional treatment modalities for psoriasis provide only temporary relief and are riddled with potential toxicities which result in high rates of treatment failures and dissatisfaction among these patients. Introduction of “biological” agent as a therapeutic option has revolutionized the treatment of psoriasis. The threshold for treatment success has now changed and achieving clear or almost clear skin with 90%–100% improvement in baseline PASI scores is most desirable and relevant. Until recently, biological treatment was limited to TNF-α inhibitors (infliximab, etanercept, and adalimumab), IL-12/IL-23 antagonist (ustekinumab), and IL-17 antagonist (secukinumab).
Itolizumab is a novel biological agent that targets CD6 receptors on T-cells. It has better side effect profile but lower efficacy than other biologicals. The clinical trials on its use in moderate-to-severe plaque psoriasis have shown that itolizumab has favorable clinical effects and a safety profile as monotherapy in patients who fail to respond to conventional systemic therapies. Few studies have also shown that itolizumab provides a longer remission even after treatment withdrawal. The drug was first approved in Cuba. Based on the multicentric clinical phase II and III trial results, the Drugs Controller General of India approved this drug in India in January 2013 for the management of plaque psoriasis.
Disclosure
The authors report no conflicts of interest in this work.
References
Menon R, David BG. Itolizumab – a humanized anti-CD6 monoclonal antibody with a better side effects profile for the treatment of psoriasis. Clin Cosmet Investig Dermatol. 2015;17:215–222. | ||
Handa S, Mahajan R. Pathophysiology of psoriasis. Indian J Dermatol Venereol Leprol. 2013;79(7):1–9. | ||
Oliveira MD, Rocha BD, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90(1):9–20. | ||
Raychaudhuri SP, Raychaudhuri SK. Biologics: target-specific treatment of systemic and cutaneous autoimmune diseases. Indian J Dermatol. 2009;54(2):100–109. | ||
Jariwala SP. The role of dendritic cells in the immunopathogenesis of psoriasis. Arch Dermatol Res. 2007;299(8):359–366. | ||
Nair P, Melarkode R, Rajkumar D, Montero E. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin Exp Immunol. 2010;162(1):116–130. | ||
Alonso-Ramirez R, Loisel S, Buors C, et al. Rationale for targeting CD6 as a treatment for autoimmune diseases. Arthritis. 2010;2010(6):1–9. | ||
Dogra S, Uprety S, Suresh SH. Itolizumab, a novel anti-CD6 monoclonal antibody: a safe and efficacious biologic agent for management of psoriasis. Expert Opin Biol Ther. 2017;17(3):395–402. | ||
Hernández P, Moreno E, Aira LE, Rodríguez PC. Therapeutic targeting of CD6 in autoimmune diseases: a review of Cuban clinical studies with the antibodies IOR-T1 and Itolizumab. Curr Drug Targets. 2016;17(6):666–677. | ||
Anand A, Assudani D, Nair P, et al. Safety, efficacy and pharmacokinetics of T1h, a humanized anti-CD6 monoclonal antibody, in moderate to severe chronic plaque psoriasis – results from a randomized phase II trial. J Immunol. 2010;184:96–103. | ||
Krupashankar DS, Dogra S, Kura M, et al. Efficacy and safety of itolizumab, a novel anti-CD6 monoclonal antibody, in patients with moderate to severe chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, phase-III study. J Am Acad Dermatol. 2014;71(3):484–492. | ||
Dogra S, Krupashankar DS, Budamakuntla L, et al. Long-term efficacy and safety of itolizumab in patients with moderate-to-severe chronic plaque psoriasis: a double-blind, randomized-withdrawal, placebo-controlled study. J Am Acad Dermatol. 2015;73(2):331–333. | ||
Nott A, Raghavan V, Sashidharan N. Use of Itolizumab in chronic plaque psoriasis patients: clinical experience. Ejpmr. 2016;3(3):413–416. | ||
Singh V. Clinical outcome of a novel anti-CD6 biologic Itolizumab in patients of psoriasis with comorbid conditions. Dermatol Res Pract. 2016;2016(5):1–4. | ||
Parthasaradhi A. Safety and efficacy of Itolizumab in the treatment of psoriasis: a case series of 20 patients. J Clin Diagn Res. 2016;10(11):WD01–WD03. | ||
Parthasaradhi A, Singh V, Parasramani SG, et al. A real-world study to assess the effectiveness of itolizumab in patients with chronic plaque psoriasis. Indian Dermatol Online J. 2017;8(4):246–249. | ||
Pai G, Pai AH. Itolizumab – a new biologic for management of psoriasis and psoriatic arthritis. Case Rep Dermatol. 2017;9(2):141–145. | ||
Trasi. S, Sashidharan N. Itolizumab as the first line of treatment in moderate to severe chronic plaque posriasis: a case report. Asian J Pharm Clin Research. 2016;9(2):12. | ||
Gupta A, Sharma Y, Deo K, Kothari P. Severe recalcitrant psoriasis treated with itolizumab, a novel anti-CD6 monoclonal antibody. Indian J Dermatol Venereol Leprol. 2016;82(4):459–461. | ||
Budamakuntla L, Madaiah M, Sarvajnamurthy S, Kapanigowda S. Itolizumab provides sustained remission in plaque psoriasis: a 5-year follow-up experience. Clin Exp Dermatol. 2015;40(2):152–155. | ||
Sridhar J, Desylva P, Singh YD. Chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72(2):133–135. | ||
Bhat RM, Leelavathy B, Aradhya SS, et al. Secukinumab efficacy and safety in Indian patients with moderate-to-severe plaque psoriasis: sub-analysis from fixture, a randomized, placebo-controlled, phase 3 study. Indian Dermatol Online J. 2017;8(1):16. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.