Back to Journals » International Journal of Women's Health » Volume 14
Spiral Suture of the Lower Uterine Segment with Temporary Aortic Balloon Occlusion in Morbidly Adherent Placenta Previa Cases
Authors Yin Y, Qu L, Jin B, Yang Z, Xia J, Sun L, Zhou X
Received 23 March 2022
Accepted for publication 3 August 2022
Published 25 August 2022 Volume 2022:14 Pages 1161—1171
DOI https://doi.org/10.2147/IJWH.S367654
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Supplementary video by Yin, Lin Qu, Bai Jin et al.
Views: 2564
Yin Yin,1 Lin Qu,1 Bai Jin,1 Zhengqiang Yang,2 Jinguo Xia,2 Lizhou Sun,1 Xin Zhou1
1Department of Obstetrics and Gynecology, The First Affiliated Hospital of Nanjing Medical University Hospital, Nanjing, People’s Republic of China; 2Department of Interventional Radiology, The First Affiliated Hospital of Nanjing Medical University Hospital, Nanjing, People’s Republic of China
Correspondence: Xin Zhou, Department of Obstetrics & Gynecology, The First Affiliated Hospital of Nanjing Medical University Hospital, Nanjing, People’s Republic of China, Tel +86 25 8620 0133, Fax +86-25-8371-6602, Email [email protected]
Purpose: We aimed to investigate the combined effect of spiral suture of the lower uterine segment with intraoperative aortic balloon occlusion in morbidly adherent placenta previa cases.
Patient and Methods: This retrospective, single-center study involved patients from 2017 to 2020. The study considered 68 cases of morbidly adherent placenta previa cases from medical records retrospectively with age ranging from 23 to 42 years. Bilateral uterine artery embolization was performed, to control excessive bleeding. Perioperative blood loss, hysterectomy rate, amount of blood transfusion, balloon occlusion time, fetal and maternal radiation dose, and postpartum complications were assessed.
Results: A total of 68 patients underwent surgery. Hysterectomy was performed in three patients and uterine artery embolization in 21 patients. Of 53 patients who required blood transfusions, the amount of packed red blood cells given was 800 mL and the amount of plasma given was 400 mL. Median abdominal aortic balloon occlusion time was 17 minutes. Fetal and maternal radiation doses were 5 mGy and 12 mGy, respectively. One patient experienced surgery-related complications, a bladder injury. No major catheterization-related and postpartum complications were observed.
Conclusion: Fertility-sparing surgery for women with morbidly adherent placenta could include abdominal aortic balloon occlusion and spiral suture of lower uterine segment.
Keywords: morbidly adherent placenta previa, spiral suture, lower uterus, aortic balloon occlusion
Introduction
Placenta accreta spectrum or morbidly adherent placenta occurs mostly on a previous cesarean scar when placenta fails to detach due to abnormal invasion of placenta in to the uterine wall.1,2 The incidence of morbidly adherent placenta increased in the past decade from 0.8 per 1000 deliveries to 3 per 1000 deliveries.3 The increase in the incidence is attributed to increase in cesarean deliveries from 1 in about 2500 births to 1 in 500 births.4 The number of previous cesarean deliveries is the most important antenatal risk factor. A recent retrospective study reported that the rate of accreta for previous 1, 2, 3, 4, and 5 cesarean deliveries was found to be 26.7%, 43.5%, 65.5%, 55.6%, and 66.7, respectively. The management of morbidly adherent placenta previa is challenging due to the high risk of massive hemorrhage following delivery due to invasion of the placenta into surrounding tissues in those with placenta percreta.5,6 The massive hemorrhage often leads to multiorgan failure and the need for morbid hysterectomy and blood transfusion.2,7 It is also one of the common causes of maternal mortality with a mortality incidence of 7% worldwide.8 Several predictive models were developed to assess the outcomes of placenta accreta spectrum and uterine sparing techniques.9,10
Peripartum emergency hysterectomy is the most effective and safe intervention to stop postpartum hemorrhage.10 In around 75% of cases with morbidly adherent placenta previa, peripartum hysterectomy is preferred.11 However, many women desire fertility-sparing surgery, if possible, for which several conservative methods have been adopted. Postpartum hemorrhage is primarily managed with the help of conservative approaches such as using uterotonic agents, bimanual uterine massage as well as laceration suturing. When these methods fail in effective management, other uterine sparing surgical procedures are used, which include uterine compression sutures, uterine arterial embolization, and ligation of the uterine or hypogastric artery.7,9,12 Internal iliac artery ligation can be an effective way to control hemorrhage and preserve the uterus, but may be technically challenging in urgent settings.13 The external compression suture techniques have a bleeding control success rate of around 76% to 100% for uterine atony or the simple placenta previa and decreased the need for cesarean hysterectomy.14,15 In placenta previa/accreta cases, an overall success rate of 86% in achieving hemostasis was reported by Li et al12 and a success rate of 98.2% by Mohamed et al16 while only 14.28% of success rate was reported in preserving the uterus of placenta increta cases.14 Ligation is often challenging due to anatomical changes and enlargement of the uterine vessels’ plexus during pregnancy.17 The ring suture is parallel to the cervical os, and the suture needs to be removed through the vagina afterwards.18
Tightening during previously used suturing techniques may induce lacerations of the posterior wall increasing the bleeding and even leading to infertility due to the restrictive compression and drainage of the uterine cavity.19 In modern society, due to the progress of surgical and interventional radiology, hemostasis is achieved by directly suturing the bleeding site instead of indirect compression sutures. However, in some cases all these methods can be insufficient in controlling bleeding from the lower uterine segment. Risk associated with the use of multiple square sutures and the circular isthmic-cervical sutures include uterine cavity occlusion due to the presence of bloods clot and debris entrapment. Even though cervical canal closure will be prevented by using the parallel vertical penetrating sutures, these sutures do not include the whole length and thickness of the lower uterine wall. Therefore, it may not facilitate complete hemostasis.20,21 These sutures are not effective in achieving hemostasis as their focus was reinforcement of contractions of the myometrial fibers. Inconclusive results have been observed especially in cases involving the lower uterine segment since it is deficient in myometrial fibers because of which the rate of hysterectomy is high.22,23 Also, it can lead to complications such as infection, pyometra, synechiae, obstruction, uterine necrosis, and even infertility because of the restrictive compression and drainage of the uterine cavity. As opposed to the abovementioned anatomical changes and unsatisfying hemostasis effects involved in traditional techniques, Meng et al reported “multifaceted spiral suture” thus achieving significant hemostasis.24
To minimize postpartum hemorrhage and preserve the uterus, aortic balloon occlusion, a minimally invasive technique was performed in 1995.25 Compared with other techniques such as internal iliac balloon occlusion, aortic balloon occlusion helps in prolonging the operator time to achieve hemostasis via curettage and oversewing of the implantation site.26 Hence, the present study was conducted in an effort to decrease maternal morbidity and mortality among women with morbidly adherent placenta that desire fertility-sparing surgery using an operative protocol that includes a spiral suture of the lower uterine segment, intraoperative intermittent abdominal aorta balloon occlusion, and uterine artery embolization, if needed for severe hemorrhage.
Methods
Study Design and Study Population
This was a retrospective study conducted at the Department of Obstetrics and Gynecology in the First Affiliated Hospital with Nanjing Medical University (Nanjing, China). All patients with morbidly adherent placenta treated at our hospital during 2017 to 2020 were included unless they had coagulopathies, serious medical comorbidities such as preeclampsia that would be the risk factor for additional complications, and the cases that showed signs of requiring urgent care. All cases included had previously undergone cesarean delivery or anterior submucosal myomectomy. Expectant management patients were given corticosteroids for lung maturation at 32 weeks and were hospitalized at 34 weeks with delivery planned at 35 to 36 weeks. The ethics committee of the First Affiliated Hospital with Nanjing Medical University approved the study (approval number: 2017-SR-414).
Diagnostic Criteria
Morbidly adherent placentation was diagnosed by ultrasonography (US) (GE Electric Medical Systems, Milwaukee, Wisconsin, USA) and also by magnetic resonance imaging (MRI) with 1.5 T MAGNETOM Amira (Siemens Healthineers, Los Angeles, CA, USA) during 24 to 34 weeks of gestation.27 The diameter of the abdominal aorta was measured by MRI. The ultrasound diagnostic criteria considered were as described by Cali et al,28,29 which include (a) clear space: loss or irregularity of echolucent area located between uterus and placenta; (b) bladder line: thinning or interruption of the hyperechoic interface between anterior uterine serosa and bladder posterior wall; (c) placental lacunae with the turbulent high velocity flow (>15 cm/s), vascular lakes with turbulent flow, increased vascular pattern of the uterine–bladder interface with abnormal vessels linking the placenta to the bladder, and markedly dilated vessels over the peripheral subplacental region. The MRI diagnostic criteria considered for the study included abnormal uterine bulging, dark intraplacental bands on T2-weighted imaging, heterogeneous signal intensity within the placenta, disorganized vasculature of placenta, and disruption of the uteroplacental zone.
Surgical methods
The surgery was performed using intradural anesthesia and was monitored with an arterial line. Using standard techniques, a 7-French (F) balloon catheter of 14 mm × 40 mm (Brad, USA) was inserted through the right femoral artery under digital subtraction angiography guidance. The catheter was placed superior to the abdominal aorta bifurcation and inferior to the renal arteries. About 10 mL of contrast medium was used at a pressure of 6 atm to inflate the balloon to approximately 12 to 14 mm and the correct balloon placement was checked by observing the disappearance of the dorsal artery of the ipsilateral foot. Later, the balloon was deflated and the catheter was secured to prepare for the next stage of the procedure.
Using an abdominal midline incision, the hysterotomy site was selected, which was away from the margin of the placenta and the fetus was delivered. The surgeon then exteriorized the uterus, clamped the incisional margins of the uterus using 8 oval forceps, and the uterine cavity was compressed with gauze packing temporally. Uterotonics were administered after inflating the abdominal aorta balloon for about 10 to 15 minutes. Once the bleeding was controlled due to the aorta balloon, the gauze packing was removed. During the procedure, the aortic balloon inflation was monitored using blood pressure and pulse oxygen measurements in the lower extremities.
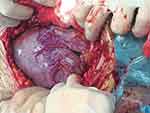
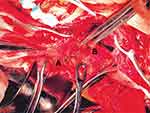
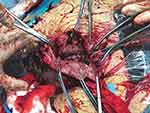
After observing the placental position and the extent of myometrial invasion, the placenta was removed manually as much as possible, followed by blunt and sharp dissection of adherent portions. The internal os was exposed, with palpation of the canal to confirm proper identity. The anterior and posterior edges of the internal os were then elevated into the uterine cavity with either ring or Allis forceps, exposing the lower uterine segment. Using a continuous running polyethicon (1–0) suture, starting around the internal os toward the uterine fundus in a spiral pattern until the placenta bed in the lower uterine segment was sutured (Figures 1–4 and Video S1).
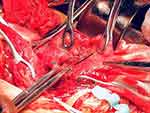 |
Figure 4 Suturing of internal os and anterior uterine wall. Blue line indicates suturing of posterior edges of internal os and black line indicates suturing of anterior wall of uterus. |
The aortic balloon was inflated for a maximum duration of 15 minutes with 2-minute deflation between the inflation periods, as needed to control the bleeding. Additional focal sutures were placed as needed to achieve hemostasis. Once hemostasis was achieved, the hysterotomy was closed using polyethicon (1–0) suture by a double layer technique—a continuous locking technique for the deep myometrial edge with minimal decidua and a continuous running suture for the second layer. Bilateral uterine artery embolization was performed for patients with active bleeding and 1500 mL or more estimated blood loss. Hysterectomy was performed for intractable bleeding or patients with instability. Cystorrhaphy was performed for the placenta infiltrated to the posterior of the bladder. Dissection of the anterior uterine wall from the posterior bladder was accompanied by large posterior cystotomy by the urologist. For bladder repair, a water-tight closure was done using polyethicon (3–0) suture by continuous running double layer technique. The first for the mucosa and muscular layer while the second was used for the serosa layer. Later, a cystoscope was performed and if no leaks were identified upon bladder irrigation, an omental flap was placed between the bladder and the vaginal cuff. A Jackson-Pratt drain and a Foley catheter were placed at least for 2 weeks. The aortic balloon and its sheath were removed prior to the exit from operating room and the patient’s right leg was immobilized for 2 hours. The placentas were sent for final pathologic diagnosis.
Data Collection and Follow-Up
Following the surgical procedure, we recorded the following data: intraoperative blood loss, blood loss in the first 24 hours postoperatively, transfusion volume and type, occurrence of hysterectomy, pattern and duration of occlusion of the abdominal aortic balloon, duration of fluoroscopy, the dosimetry measurements of maternal and fetal radiation exposure, body temperature in case of fever due to infection, status of lochia, blood human chorionic gonadotropin (hCG) levels in 1 week after childbirth, and Apgar score of the neonate and neonatal complications. The Apgar score helps to quickly summarize the health of newborn children against infant mortality using five criteria: A-appearance (skin color), P-pulse (heart rate), G-grimace (response), A-activity (muscle tone), and R-respiration (breathing ability).
After the multidisciplinary operation, postoperative antibiotic therapy was instituted with Augmentin and gentamicin, and cephalexin for 24 to 48 hours for prophylaxis. Postoperative ultrasound images were taken during the sixth to seventh postpartum days to evaluate for uterine involution, retention of placental fragments, hematomas, and embolic complications such as vascular thrombotic disease. However, if patients exhibited any signs or symptoms, including pain or signs of anemia after delivery, they were screened by ultrasound immediately. Using the ultrasound images, we measured the size of the uterus in order to determine the status of the involution. Palpation of the uterine height would be evaluated to check for involution. If there was subinvolution, Oxytocin and Ergonovine were administered as treatment. If hematomas were detected at a size <1.5 cm, they were considered clinically insignificant; however, body temperature, hemoglobin level, and reabsorption were monitored. If the hematomas were detected at a size >1.5 cm, but not increasing in size, antibiotics were administered for prophylaxis. Hematomas that increase in size require immediate reoperation. In case of thrombosis, the 4100 IU of low molecular weight heparins were administrated for at least 1 week and the affected limb was immobilized.
Patients were discharged when they had achieved normal levels of activity and were afebrile. We followed up every patient about the involution of uterus, beta-hCG levels, and menstrual resume in 42 days, 3 months, 6 months, and 1 year at postpartum, respectively. If patients were unable to return to our hospital, we asked their local provider to send their results to our center to be added to their case files.
Statistical Analysis
The demographic and perioperative data were analyzed using statistical software package (SPSS) version 19.0 (IBM, Armonk, NY). Due to relatively low sample number, the statistical normality was tested using Shapiro–wilk test. Normally distributed data are reported as mean and standard deviation (mean ± standard deviation [SD]) while non-normally distributed variables as median (interquartile ranges [IQR] 25th and 75th percentiles) or the full ranges.
Results
Demographics of the Study Population
The study considered 68 cases of morbidly adherent placenta previa cases from medical records retrospectively with age ranging from 23 to 42 years (mean 33.43 ± 4.58 years). Sixty-five of the 68 patients had prior cesarean deliveries: 56 of them had one cesarean, seven of them had two cesareans, and two of them had three prior cesarean deliveries (Table 1). Three women had a history of prior anterior submucous myomectomy. A total of 57 women had a history of uterine curettage, either due to an abortion or miscarriage. Deliveries were electively scheduled from 28+6 weeks to 39+5 weeks. The median gestational age at birth was 35+2 weeks (IQR 34+5, 37+4 weeks). Twenty women included in the study were already pregnant for >36 weeks when they arrived at our hospital.
 |
Table 1 Demographic Characteristics of Patients |
Outcomes of the Study and Follow-Up
During the 3 years of study, the overall incidence of placenta accreta spectrum in our hospital was 1.48% (accreta), 1.20% (increta), and 0.05% (percreta). All 68 women recruited in our cohort had positive ultrasound results, which showed not only previa but also accreta spectrum. The preoperational US results showed accreta, increta, and percreta in 50%, 44%, and 6% of cases, respectively, whereas 58%, 36%, and 6% of cases, respectively, with MRI (Table 2). The results of percreta, both through ultrasound and MRI, were consistent with each other. The results of both imaging modalities, US and MRI, had a consistency rate of 92%. After the surgery and subsequent pathological investigations, it was revealed that four accreta cases were identified as increta. The results of pathological diagnoses post-surgery showed placenta accreta and percreta among 57%, 37%, and 6% of cases, respectively. Antepartum and pathological diagnoses had a concurrence rate of 94%.
 |
Table 2 Category of Placental Invasion During Pre- or Post-Operation |
The median intraoperative blood loss was 1200 mL (IQR 800, 2025 mL) with a mean of 1588 ± 1251 mL. The median blood loss in the first 24 hours of postpartum was 130 mL (IQR 90, 235 mL) with a mean of 124 ± 72 mL. Thirty-five women received blood transfusion with a median transfusion of 800 mL (700, 1200 mL) of packed red cells and 400 mL (400, 800 mL) of plasma (Table 3).
 |
Table 3 Perioperative Data of Patients |
The median duration of abdominal aorta balloon occlusion was 17 minutes (IQR 9, 26 minutes). The mean fetal and maternal radiation doses were 6.98 ± 5.36 mGy and 31.65 ± 39.07 mGy, respectively, while the median fetal exposure and maternal radiation exposure were 5 mGy (IQR 3, 11 mGy) and 12 mGy (IQR 3, 52 mGy), respectively. Bilateral uterine artery embolization was performed in 21 cases of massive hemorrhage over 1500 mL. Hysterectomy was performed in only three cases of intractable hemorrhage. One patient required excision of some bladder tissue due to placental infiltration.
Length of hospital stays ranged from 7 to 10 days. Ultrasonography on the sixth and seventh day of postpartum showed no uterine incisional hematomas or retained placenta. Neither femoral artery catheterization site complications nor venous thromboembolic complications were observed. Serum quantitative beta-hCG levels on day 7 ranged from 1.28 to 267.20 IU/dL with a mean of 24.27 ± 42.24 IU/dL. At 42 days of postpartum, all patients reported lochia consistently with women who had normal deliveries. All patients had negative quantitative beta-hCG levels at 3 months (<0.5 IU/dL). A normal menstrual cycle returned between 3 and 12 months. Around two-thirds of patients’ menstruation resumed in 6 months while the remaining patients’ mensuration resumed by 1 year (Table 4).
 |
Table 4 Short- and Long-Time Observation of Patients |
Complications
In our cohort, 49 of the 68 deliveries were premature. The mean neonatal weight was 264 ± 622 g. Apgar scores of all newborns immediately after birth (within 1 minute) was above 8, except one infant who had mild asphyxia (Apgar score was 5 in 1 minute). This infant’s Apgar score 5 minutes after resuscitation was 8. Forty percent (27/68) of newborns were admitted to the NICU. Eight babies had bronchopulmonary dysplasia, nine had neonatal pneumonia, nine had bloodstream infections, four had transient tachypnea of the newborn, and four had asphyxia. Six of the infants had anemia and hypoglycemia, while one had intracranial hemorrhage, and one had pulmonary hypertension. A baby who was born at 28+6 weeks had necrotizing enterocolitis. All the infants had jaundice and were discharged upon recovery.
Discussion
The currently available techniques for preservation of uterus are mostly effective in achieving hemostasis, and prevent the need for hysterectomy especially with regard to addressing uterine atony, however, are less effective in cases of placenta previa concurrent with invasion into the uterine scar.30 On one hand, defection of the placental insertion site as the source of bleeding could induce subsequent uterus atony; the poor contractile myometrial fibers poorly respond to the use of tocolytics and traditional external compression. On the other hand, these external sutures do not target the placental implantation site or any vessels damaged by the placenta, thus their effectiveness is limited. The latent possibilities of laceration are still a big issue.18,19,31–33 None of these methods have been shown to reduce the cesarean hysterectomy rate, although a trend toward reduced blood loss has been reported.24,34
The present study investigated the combined effect of spiral suture of the lower uterine segment with intraoperative abdominal aortic balloon occlusion in the patients with morbidly adherent placenta previa. In our intrauterine suture method, the running direction was perpendicular to the cervical os and vertical from the lower uterine segment (LUS) to the uterine cavity. Hemostasis is accomplished by directly suturing the bleeding site of the placenta bed, instead of indirect compression sutures compressing the uterine body, furthermore our spiral running suture reinforces the latent bleeding site. Prior to placing the spiral suture, the endometrial surface of the LUS is exposed by pulling the cervical lips using ring forceps or straight Allis. This maneuver temporarily decreases the blood flow to the LUS, providing access to the cervix for removal of peripheral placenta and increases tension on the tissues that stimulate uterine contractions. Additionally, this technique does not permanently change the morphology of the LUS and cervix. It will not necessitate a follow-up treatment, such as a uterine tamponade and uterine artery embolization.35 In addition, during spiral suture, direct bladder injury will be prevented by pushing the bladder inferior away from the sutured area. Only one case of severe penetration through the bladder muscularis was observed in the present study, and the bladder dissection was performed to repair. Direct suturing also ensures no intrusion while implementing other supplementary hemostatic methods such as placement of balloons or uterine artery ligation further reducing the blood loss. Studies have reported reduced bleeding with spiral suturing and aortic balloon occlusion. The present study combines both techniques and thus has an added advantage in achieving hemostasis.24,36 In a meta-analysis on the management of placenta accreta spectrum, the study reported elective or emergent cesarean hysterectomy in 89.7% (208/232) cases,37 whereas in the present study only 4% (3/68) of cases had hysterectomy. The results were consistent with the results reported in a study by Meng et al using spiral suture in patients with placenta accreta spectrum.24
Advanced intervention technology has already been proven effective for the treatment of morbidly adherent placenta previa. Balloon occlusion of the abdominal aorta artery is a widely accepted technique that is implemented to prevent massive hemorrhage during detachment of placenta.38 Compared with occlusion of the internal iliac artery, blocking the abdominal aorta is faster, technically less challenging, and results in lesser radiation exposure for the mother and fetus.26,38 Use of lower abdominal aorta balloon occlusion technique showed a significant reduction in the bleeding amount and blood transfusion volumes [(850 ± 100 mL) and (400 ± 50 mL)], respectively, as compared with the control group [(2500 ± 230 mL) and (1500 ± 100 mL)], respectively (p < 0.05). Also, the hysterectomy rate in placenta previa cases treated using lower abdominal aorta balloon occlusion technique was 5%, which was lower as compared with the control group (30%).36 Utilization of both spiral suture and balloon occlusion, results in a reduced pulse pressure distal to the site and effectively minimizes blood loss during the subsequent stitching procedure.
Building on the work of Sakhavar et al29 and Meng et al,24 we performed a comprehensive procedure with the assistance of balloon occlusion, which resulted in increased time for the dissection and removal of residual placental tissue. The removal of all the placental tissue effectively decreases the risk of any potential secondary hemorrhage and subsequent infection, even sepsis.34,35 Furthermore, continuously running spiral sutures were easily performed at the bleeding site. This suturing technique restores the normal anatomical morphology of the uterus, and by quickly achieving hemostasis.24 In a study by Meng et al, excessive blood loss (>3000 mL) in 15.15% of patients was reported24 whereas in the present study, no patient had blood loss of >3000 mL, which may be due to the combined use of spiral suture with aortic balloon occlusion. Some potential risks of inflating balloon are aortic rupture, abdominal aorta dissection, reperfusion injury, and the inability to deflate or withdraw the balloon through the sheath.25,39 However, the present study reported no such risks or thromboembolic events, which is in par with the previous studies.39,40
To the best of our knowledge, this is the first study to combine spiral suture and intermittent abdomen aorta balloon occlusion. Fertility-sparing surgery, including abdominal aorta balloon occlusion and spiral suture of the LUS, is easy to implement, effective in the minimization of blood loss, as well as reducing many current complications associated with morbidly adherent placenta previa, and decreasing the hysterectomy rate dramatically. The main limitations of our research are the small sample size and the single-institution nature of this study. The long-term complications, especially the effect of our intervention technique is still not very clear and determinate. Additional studies and randomized controlled trials are needed to demonstrate the long-term safety and effectiveness of this technique. The surgical procedure should be adapted and improved to fit patients’ specific medical conditions, with the primary goal of controlling rapid blood loss and protecting adjacent organs. With a high risk of recurrent morbidity adherent placenta previa, and the dangers associated with it, we recommend our patients to practice birth control in the future. If subsequent pregnancy does occur, supplementary clinical expertise and a longer follow-up is needed to ensure the health of the patients.
Conclusion
To conclude, till date the best procedure for morbidly adherent placenta previa has not been fully described. Therefore, efforts should be made to choose the most suitable suture technique on a case-by-case basis. The present study suggested that fertility-sparing surgery for women with morbidly adherent placenta could include abdominal aorta balloon occlusion and spiral suture of the lower uterine segment.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethics Guidelines
The study was approved by the ethics committee of the First Affiliated Hospital with Nanjing Medical University (approval number: 2017-SR-414). The study was performed in accordance with the latest version of the Helsinki Declaration 1964 and its later amendments on human research ethics. As this is a retrospective study, patient consent to participate was not necessary since results of research would have no effect on the subjects. We further confirm that confidentiality of patient information was maintained throughout the present study.
Acknowledgments
Dr. Molly Stout from Maternal and Fetal Medicine of Barnes Jewish Hospital in St. Louis and Brooke Liang B.S. and M.D. Candidate from the Washington University School of Medicine in St. Louis, Dr. Satya Lavanya Jakki and Dr. Amit Bhat (Indegene, Bangalore, India) provided the editorial assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by the Jiangsu Maternal and Child Health Research Project (No: F 201658).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wortman AC, Alexander JM. Placenta accreta, increta, and percreta. Obstet Gynecol Clin North Am. 2013;40(1):137–154. doi:10.1016/j.ogc.2012.12.002
2. Bhide A, Sebire N, Abuhamad A, Acharya G, Silver R. Morbidly adherent placenta: the need for standardization. Ultrasound Obstet Gynecol. 2017;49(5):559–563. doi:10.1002/uog.17417
3. Bartels HC, Postle JD, Downey P, Brennan DJ. Placenta accreta spectrum: a review of pathology, molecular biology, and biomarkers. Dis Markers. 2018;2018:1–11. doi:10.1155/2018/1507674
4. Piñas Carrillo A, Chandraharan E. Placenta accreta spectrum: risk factors, diagnosis and management with special reference to the Triple P procedure. Womens Health. 2019;15:1745506519878081. doi:10.1177/1745506519878081
5. Bailit JL, Grobman WA, Rice MM, et al. Morbidly adherent placenta treatments and outcomes. Obstet Gynecol. 2015;125(3):683–689. doi:10.1097/AOG.0000000000000680
6. Marcellin L, Delorme P, Bonnet MP, et al. Placenta percreta is associated with more frequent severe maternal morbidity than placenta accreta. Am J Obstet Gynecol. 2018;219(2):
7. Viñas MT, Chandraharan E, Moneta MV, Belli AM, Ekici H, Imamoglu M. Effectiveness of segmental resection technique in the treatment of placenta accreta spectrum. J Matern Fetal Neonatal Med. 2021;34(19):3227–3233. doi:10.1080/14767058.2019.1702019
8. El Gelany S, Mosbeh MH, Ibrahim EM, et al. Placenta Accreta Spectrum (PAS) disorders: incidence, risk factors and outcomes of different management strategies in a tertiary referral hospital in Minia, Egypt: a prospective study. BMC Pregnancy Childbirth. 2019;19(1):313. doi:10.1186/s12884-019-2466-5
9. Shazly SA, Hortu I, Shih JC, et al. Prediction of clinical outcomes in women with placenta accreta spectrum using machine learning models: an international multicenter study. J Matern Fetal Neonatal Med. 2021:1–10. doi:10.1080/14767058.2021.1918670
10. Shazly SA, Hortu I, Shih JC, et al. Prediction of success of uterus-preserving management in women with placenta accreta spectrum (CON-PAS score): a multicenter international study. Int J Gynaecol Obstet. 2021;154(2):304–311. doi:10.1002/ijgo.13518
11. Palacios-Jaraquemada JM, Fiorillo A, Hamer J, Martínez M, Bruno C. Placenta accreta spectrum: a hysterectomy can be prevented in almost 80% of cases using a resective-reconstructive technique. J Matern Fetal Neonatal Med. 2020;1–8. doi:10.1080/14767058.2020.1716715
12. Li GT, Li XF, Wu B, Li G. Longitudinal parallel compression suture to control postopartum hemorrhage due to placenta previa and accreta. Taiwan J Obstet Gynecol. 2016;55(2):193–197. doi:10.1016/j.tjog.2016.02.008
13. Sziller I, Hupuczi P, Papp Z. Hypogastric artery ligation for severe hemorrhage in obstetric patients. J Perinat Med. 2007;35(3):187–192. doi:10.1515/JPM.2007.049
14. Ratiu AC, Crisan DC. A prospective evaluation and management of different types of placenta praevia using parallel vertical compression suture to preserve uterus. Medicine. 2018;97(46):e13253. doi:10.1097/MD.0000000000013253
15. Hackethal A, Brueggmann D, Oehmke F, Tinneberg HR, Zygmunt MT, Muenstedt K. Uterine compression U-sutures in primary postpartum hemorrhage after Cesarean section: fertility preservation with a simple and effective technique. Hum Reprod. 2008;23(1):74–79. doi:10.1093/humrep/dem364
16. Mohamed MA, Mohammed AH. Parallel vertical compression sutures to control bleeding in cases of placenta previa and accreta. J Matern Fetal Neonatal Med. 2019;32(4):641–645. doi:10.1080/14767058.2017.1387895
17. Zhou ZC, Hx WCH. Application of early ligation of bilateral uterine artery superior branch in implantable dangerous placenta previa. J Obstet Gynaecol. 2011;27(8):630–632.
18. Li GT, Li XF, Zhang YH, Si Y, Li GR, Xu HM. Ring compression suture for controlling post-partum hemorrhage during cesarean section. J Obstet Gynaecol Res. 2018;44(8):1424–1430. doi:10.1111/jog.13676
19. Li G, Li G, Li X, Wu B. Funnel compression suture: a conservative procedure to control postpartum bleeding from the lower uterine segment. BJOG. 2016;123(8):1380–1385. doi:10.1111/1471-0528.13685
20. Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv. 2007;62(8):540–547. doi:10.1097/01.ogx.0000271137.81361.93
21. Blanc J, Courbiere B, Desbriere R, et al. Uterine-sparing surgical management of postpartum hemorrhage: is it always effective? Arch Gynecol Obstet. 2012;285(4):925–930. doi:10.1007/s00404-011-2083-7
22. Yu L, Hu KJ, Yang HX. A retrospective analysis on the pernicious placenta previa from 2008 to 2014. Zhonghua Fu Chan Ke Za Zhi. 2016;51(3):169–173. doi:10.3760/cma.j.issn.0529-567X.2016.03.002
23. Cho HY, Park YW, Kim YH, Jung I, Kwon JY. Efficacy of intrauterine bakri balloon tamponade in cesarean section for placenta previa patients. PLoS One. 2015;10(8):e0134282. doi:10.1371/journal.pone.0134282
24. Meng Y, Wu P, Deng D, et al. Multifaceted spiral suture: a hemostatic technique in managing placenta praevia or accreta: a retrospective study. Medicine. 2017;96(49):e9101. doi:10.1097/MD.0000000000009101
25. Paull JD, Smith J, Williams L, Davison G, Devine T, Holt M. Balloon occlusion of the abdominal aorta during caesarean hysterectomy for placenta percreta. Anaesth Intensive Care. 1995;23(6):731–734. doi:10.1177/0310057X9502300616
26. Duan X, Chen P, Han X, et al. Intermittent aortic balloon occlusion combined with cesarean section for the treatment of patients with placenta previa complicated by placenta accreta: a retrospective study: cesarean section for placenta previa. J Obstet Gynaecol Res. 2018;44(9):1752–1760. doi:10.1111/jog.13700
27. Varghese B, Singh N, George RAN, Gilvaz S. Magnetic resonance imaging of placenta accreta. Indian J Radiol Imaging. 2013;23(4):379–385. doi:10.4103/0971-3026.125592
28. Calì G, Giambanco L, Puccio G, Forlani F. Morbidly adherent placenta: evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta. Ultrasound Obstet Gynecol. 2013;41(4):406–412. doi:10.1002/uog.12385
29. Cali G, Forlani F, Timor-Trisch I, et al. Diagnostic accuracy of ultrasound in detecting the depth of invasion in women at risk of abnormally invasive placenta: a prospective longitudinal study. Acta Obstet Gynecol Scand. 2018;97(10):1219–1227. doi:10.1111/aogs.13389
30. Fatima T, Kousar S, Mohsin B, Tabassum Z. Effectiveness of single compression suture in management of atonic uterus during C-section. Pakistan J Medical Health Sci. 2018;12:681–683.
31. B-Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104(3):372–375. doi:10.1111/j.1471-0528.1997.tb11471.x
32. Kaya B, Guralp O, Tuten A, Unal O, Celik MO, Dogan A. Which uterine sparing technique should be used for uterine atony during cesarean section? The Bakri balloon or the B-Lynch suture? Arch Gynecol Obstet. 2016;294(3):511–517. doi:10.1007/s00404-016-4015-z
33. Shih JC, Liu KL, Kang J, Yang JH, Lin MW, Yu CU. “Nausicaa” compression suture: a simple and effective alternative to hysterectomy in placenta accreta spectrum and other causes of severe postpartum haemorrhage. BJOG. 2019;126(3):412–417. doi:10.1111/1471-0528.15410
34. Sentilhes L, Kayem G, Chandraharan E, Palacios-Jaraquemada J, Jauniaux E. FIGO placenta accreta diagnosis and management expert consensus panel. FIGO consensus guidelines on placenta accreta spectrum disorders: conservative management. Int J Gynaecol Obstet. 2018;140(3):291–298. doi:10.1002/ijgo.12410
35. Anand A, Gupta D, Prasad A. Reducing intraoperative lower segment blood loss in placenta previa with Ashok Anand stitch. Int J Reprod Contracept Obstet Gynecol. 2013;135–140. doi:10.5455/2320-1770.ijrcog20130605
36. Wei LC, Gong GY, Chen JH, et al. 超声引导下腹主动脉下段球囊阻断术在凶险性前置胎盘剖宫产术中的应用 [Application of lower abdominal aorta balloon occlusion technique by ultrasound guiding during caesarean section in patients with pernicious placenta previa]. Zhonghua Yi Xue Za Zhi. 2018;98(12):930–934. Chinese. doi:10.3760/cma.j.issn.0376-2491.2018.12.011
37. Jauniaux E, Bhide A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217(1):27–36. doi:10.1016/j.ajog.2017.02.050
38. Wang YL, Su FM, Zhang HY, Wang F, Zhe RL, Shen XY. Aortic balloon occlusion for controlling intraoperative hemorrhage in patients with placenta previa increta/percreta. J Matern Fetal Neonatal Med. 2017;30(21):2564–2568. doi:10.1080/14767058.2016.1256990
39. Panici PB, Anceschi M, Borgia ML, et al. Intraoperative aorta balloon occlusion: fertility preservation in patients with placenta previa accreta/increta. J Matern Fetal Neonatal Med. 2012;25(12):2512–2516. doi:10.3109/14767058.2012.712566
40. Masamoto H, Uehara H, Gibo M, Okubo E, Sakumoto K, Aoki Y. Elective use of aortic balloon occlusion in cesarean hysterectomy for placenta previa percreta. Gynecol Obstet Invest. 2009;67(2):92–95. doi:10.1159/000164685
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.