Back to Journals » Cancer Management and Research » Volume 11
Spinal Intramedullary Solitary Fibrous Tumor: A Rare and Challenging Diagnosis
Received 14 September 2019
Accepted for publication 3 December 2019
Published 10 December 2019 Volume 2019:11 Pages 10321—10326
DOI https://doi.org/10.2147/CMAR.S231019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Chenlong Yang,1 Yulun Xu,2 Xiaoguang Liu1
1Department of Orthopedics, Peking University Third Hospital, Beijing 100191, People’s Republic of China; 2Department of Neurosurgery, China National Clinical Research Center for Neurological Diseases, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, People’s Republic of China
Correspondence: Xiaoguang Liu; Yulun Xu Email [email protected]; [email protected]
Background: Solitary fibrous tumor (SFT) is a benign neoplasm arising in the soft tissue, which can occur anywhere in the body, while it is predominantly found in the visceral pleura. Spinal SFT is quite uncommon, with limited cases having been reported in the literature; especially, SFT occurring in the intramedullary site is extremely rare.
Case presentation: We present a case of a 35-year-old woman presenting with progressive numbness and weakness in the legs and urinary incontinence. Magnetic resonance imaging (MRI) showed an intramedullary lesion with bright enhancement. A diagnosis of spinal hemangioblastoma was suspected, and thus a three-dimensional computed tomographic angiography reconstruction was requested, which also demonstrated an angiomatous lesion. The tumor was completely resected under neurophysiological monitoring. However, histopathological and immunohistochemical examinations revealed an SFT. No adjuvant radiotherapy or chemotherapy was scheduled. The symptoms were relieved completely, and no recurrence or progression was noted during the follow-up.
Conclusion: Though SFT has been considered similar to malignant hemangiopericytoma and the histological classification has always been controversial, the intramedullary location and benign behavior in the present case add to the current understandings of this extremely rare entity.
Keywords: solitary fibrous tumor, spinal tumor, intramedullary tumor, MRI, CTA, case report
Background
Solitary fibrous tumor (SFT) is a benign mesenchymal neoplasm of putative fibroblastic origin. They can occur anywhere in the human body but arise predominantly in the visceral pleura; SFTs in extraserosal sites, especially those involving the central nervous system, are quite infrequent.1–3 SFTs occurring in the spinal intramedullary locations are extremely rare.4–8 Although it may be challenging on neuroimages to offer a specific diagnosis of this rare entity, knowledge of this tumor is vital as it has benign features and a possibly better prognosis than some more malignant tumors in this location.4,7 It is crucial to accumulate imaging findings and biological behaviors of spinal intramedullary SFTs given the limited number of reported cases.
Herein, we described a rare spinal intramedullary SFT. On neuroimaging, the tumor appeared as an angiomatous lesion; the preoperative diagnosis is challenging. The clinical manifestations, radiological features, histopathological findings, and follow-up data were presented and discussed.
Case Presentation
History
A 35-year-old female presented with an 11-month history of progressive numbness in both of her legs. Over the last month, she developed weakness in her left leg and loss of bladder control. Physical examination showed a loss of sensation below the level of crista iliaca and muscle strength of grade 4/5 in the left lower extremity. There was no remarkable finding in her past medical history, and she denied a family history of central nervous system diseases.
Examinations
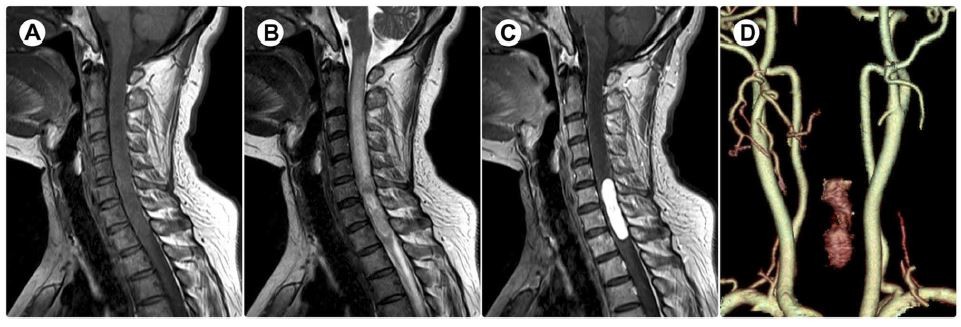
The laboratory data were all in the normal range. Magnetic resonance imaging (MRI) showed an intramedullary well-circumscribed 4.3×0.9×1.3 cm mass at the C6-T1 vertebral level and associated spinal cord edema in the rostral region of the lesion. The lesion was isointense on T1-weighted imaging (Figure 1A) and hyperintense on T2-weighted imaging (Figure 1B). Homogeneous and bright contrast enhancement was noted after intravenous injection of gadolinium-diethylenetriaminepenta-acetate (Gd-DTPA) (Figure 1C). A further three-dimensional computed tomographic angiography (CTA) reconstruction was requested, which demonstrated the mass was remarkably enhanced (Figure 1D), suggesting its rich vascularization; nevertheless, identifying its definite feeding artery is quite challenging.
Operation
The preoperative suspected diagnosis was spinal hemangioblastoma. The tumor was surgically resected via a posterior midline approach, under neurophysiological monitoring with motor and sensory evoked potentials. Intraoperative findings revealed an intramedullary white-yellowish tumor. There was no cleavage plane between the tumor and the parenchyma of the spinal cord. A gross total resection was achieved.
Pathological Findings
Histological examination revealed uniform spindle cells arranged in interlacing fascicles, with intermingled collagen fiber bundles and a dense reticulin fiber network. The nuclei were oval to elongated without pleomorphism. Mitotic activity and necrosis were absent. There were no psammoma bodies or whorls. Immunohistochemical analysis revealed strong and diffuse immunopositivity with vimentin and CD34, while the tumor was negative for S-100, glial fibrillary acidic protein (GFAP), epithelial membrane antigen (EMA), and CD56. The Ki–67 labeling index was low (<1%). Based on the immunohistochemical profiles and the histopathological fascicular spindle cell morphology, the lesion was diagnosed as SFT (Figure 2A).
Postoperative Course and Follow Up
The postoperative MRI confirmed a complete resection (Figure 2B–D), and the postoperative course was uneventful. The paresthesia and weakness in the lower extremities and dysuria were completely relieved seven months postoperatively. The patient was asymptomatic over the subsequent 23-month follow-up. The latest MRI showed no evidence of recurrence.
Discussion
SFTs were initially described in 1931 by Klemperer and Rabin as a mesenchymal tumor arising from the pleura.9 The visceral pleura is the predominant location, while the central nervous system involvement is quite infrequent; SFTs occurring within the spinal cord is extremely rare, with only 18 cases previously reported in the literature.4–8,10-21 The relevant literature review was summarized in Table 1. We analyzed the clinical and radiological characteristics of intramedullary SFTs in 18 reported cases together with the current case. The average age at diagnosis was 48.95 ± 17.08 years (range, 17~83 years). The female-to-male ratio was 1.25:1. The clinical manifestations were nonspecific, including local pain and sensorimotor disturbances. The duration between onset and diagnosis ranged from 1 month to 3 years (mean 15.72 ± 11.35 months). These tumors were predominantly located in the cervical (n=8) and thoracic (n=9) spine, and only one intramedullary SFT in the lumbar spine was reported. Approximately half of these tumors (n=9) were exophytic to the extramedullary compartment. Fifteen patients underwent completely surgical resection, among which tumor recurrent was noted only in one patient during the follow-up; four patients underwent incompletely surgical resection, and one of them experienced tumor recurrence.
 |
Table 1 Previously Reported Cases of Intramedullary Solitary Fibrous Tumor |
SFTs must be differentiated from hemangioblastoma, hemangiopericytoma, and fibrous meningioma; nevertheless, the preoperative identification is difficult as they can show similar appearance in imaging studies. The confident diagnosis still relies on histopathology. The typical microscopic characteristics of SFTs are fascicular spindle cells, dense collagenous band, and absence of specific structural features such as well-formed lobules, whorls, or psammoma bodies.16 Immunoreactivity of SFTs to CD34 and negative to S-100 and EMA distinguish them from the other spindle cell tumors.7 Additionally, hemangiopericytomas can also be positive for CD34 antigen; nevertheless, these tumors are typically hypercellular with a higher mitotic rate and presence of necrosis; hemangiopericytomas exhibit patchy and focal expression of CD34, whereas SFTs show strong and diffuse reactivity.6
The natural history and biological behavior of SFTs are as yet unclear, and there appears to be a variation in tumor growth rates and recurrence;1,2,4,6,7,16,22–24 however, it usually has an indolent course as reflected in the WHO classification of the tumors of the central nervous system. According to the 2007 classification criteria, SFT was classified as an independent benign pathological variation corresponding to grade I.25 However, SFTs show many similarities to more malignant hemangiopericytomas histopathologically; over the past decade, many scholars assume a common spectrum of these tumors, whereas neuropathologists have retained the distinction of these two entities given the historical understanding and distinct clinicopathologic correlations. Hemangiopericytoma is generally considered with high recurrence rates and long-term risk of systemic metastasis.4,26 In the updated WHO classification of CNS tumors (2016 version), these two pathological items were replaced by a new combined term “solitary fibrous tumor/hemangiopericytoma”, and a grading system was adopted to identify the low-grade SFT and the higher-grade lesions including hemangiopericytoma and anaplastic hemangiopericytoma.27 Herein, we highlight the importance of making the correct diagnosis in terms of management and prognosis.
SFTs are indolent neoplasms that can be cured by complete surgical resection. As to spinal intramedullary lesions, a surgical strategy including laminotomy and gross total tumorectomy is the optimal option. Surgical resection should be performed carefully under neurophysiological monitoring as these tumors are usually well-circumscribed but can present with a firm spinal cord attachment. Considering the benign nature of SFTs, no adjuvant radiotherapy or chemotherapy is needed in these cases. Recurrence is extremely rare after gross total removal but has been reported after subtotal resection.4 Chemotherapy has been administrated in some recurrent cases, while the therapeutic efficacy remains indefinite.23 SFTs seem to be insensitive to radiotherapy and chemotherapy.23,24,26,28 Though SFTs with malignant transformation has been rarely reported in the literature, expression of Ki-67 can help determine the possibility of malignant transformation; malignant transformation of SFT has only been reported in cases with high expression of Ki-67 (up to 10%).22
Conclusions
The rarity and the differential diagnosis of this diagnostically challenging tumor should be highlighted. It is crucial for the clinicians to be aware of intraspinal SFT as it can be easily mistaken for more common angiomatous tumors occurring at this site.
Consent for Publication
Institutional Review Board approval was granted to publish this case. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgements
The authors would like to thank the patient and her family who trusted us with the care, and all of the physicians and staff who helped in this study.
Disclosure
The authors have no financial interests or potential conflicts of interest to report in this work.
References
1. Kim JH, Yang KH, Yoon PH, Kie JH. Solitary fibrous tumor of central nervous system: a case report. Brain Tumor Res Treat. 2015;3(2):127–131. doi:10.14791/btrt.2015.3.2.127
2. Wen G, Li M, Xu L, et al. Solitary fibrous tumor of the central nervous system: report of 2 cases and review of literature. Int J Clin Exp Pathol. 2014;7(6):3444–3448.
3. Tihan T, Viglione M, Rosenblum MK, Olivi A, Burger PC. Solitary fibrous tumors in the central nervous system. A clinicopathologic review of 18 cases and comparison to meningeal hemangiopericytomas. Arch Pathol Lab Med. 2003;127(4):432–439. doi:10.1043/0003-9985(2003)127<0432:SFTITC>2.0.CO;2
4. Bruder M, Tews D, Mittelbronn M, Capper D, Seifert V, Marquardt G. Intramedullary solitary fibrous tumor–A benign form of hemangiopericytoma? Case report and review of the literature. World Neurosurg. 2015;84(1):189e187–189 e112. doi:10.1016/j.wneu.2015.02.036
5. Ishii K, Nakamura M, Matsumoto M, Mukai M, Toyama Y, Chiba K. Intramedullary solitary fibrous tumor of the spinal cord. J Orthop Sci. 2009;14(4):450–454. doi:10.1007/s00776-009-1339-6
6. Ciappetta P, D’Urso PI, Cimmino A, et al. Intramedullary solitary fibrous tumor of dorsal spinal cord. Neuropathology. 2010;30(3):273–278.
7. Hwang US, Kim SB, Jo DJ, Kim SM. Intramedullary solitary fibrous tumor of cervicothoracic spinal cord. J Korean Neurosurg Soc. 2014;56(3):265–268. doi:10.3340/jkns.2014.56.3.265
8. Walker CT, Amene CS, Pannell JS, et al. Hemorrhagic intramedullary solitary fibrous tumor of the conus medullaris: case report. J Neurosurg Spine. 2015;23(4):438–443. doi:10.3171/2015.1.SPINE13915
9. Klemperer P, Rabin C. Primary neoplasms of the pleura: a report of five cases. Arch Pathol Lab Med. 1931;11:385–412.
10. Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol. 1996;106(2):217–224. doi:10.1093/ajcp/106.2.217
11. Alston SR, Francel PC, Jane JA
12. Mordani JP, Haq IU, Singh J. Solitary fibrous tumour of the spinal cord. Neuroradiology. 2000;42(9):679–681. doi:10.1007/s002340000360
13. Kawamura M, Izawa K, Hosono N, Hirano H. Solitary fibrous tumor of the spinal cord: case report and review of the literature. Neurosurgery. 2004;55(2):433. doi:10.1227/01.NEU.0000130037.45768.84
14. Bohinski RJ, Mendel E, Aldape KD, Rhines LD. Intramedullary and extramedullary solitary fibrous tumor of the cervical spine. Case report and review of the literature. J Neurosurg. 2004;100(4Suppl Spine):358–363. doi:10.3171/spi.2004.100.4.0358
15. Pizzolitto S, Falconieri G, Demaglio G. Solitary fibrous tumor of the spinal cord: a clinicopathologic study of two cases. Ann Diagn Pathol. 2004;8(5):268–275. doi:10.1016/j.anndiagpath.2004.07.002
16. Jallo GI, Roonprapunt C, Kothbauer K, Freed D, Allen J, Epstein F. Spinal solitary fibrous tumors: a series of four patients: case report. Neurosurgery. 2005;57(1):
17. Fargen KM, Opalach KJ, Wakefield D, Jacob RP, Yachnis AT, Lister JR. The central nervous system solitary fibrous tumor: a review of clinical, imaging and pathologic findings among all reported cases from 1996 to 2010. Clin Neurol Neurosurg. 2011;113(9):703–710. doi:10.1016/j.clineuro.2011.07.024
18. Mariniello G, Napoli M, Russo C, et al. MRI features of spinal solitary fibrous tumors. A report of two cases and literature review. Neuroradiol J. 2012;25(5):610–616. doi:10.1177/197140091202500516
19. Robert T, Duc C, San Millan Ruiz D, Morard M. Solitary fibrous tumour with intramedullary component: case report and review of the literature. Neurol Neurochir Pol. 2014;48(2):144–149. doi:10.1016/j.pjnns.2013.09.006
20. Wang Q, Hu X, Wang Y, You C, Chen H. A huge intramedullary solitary fibrous tumor. Neurology. 2016;87(20):2171–2172. doi:10.1212/WNL.0000000000003338
21. Mansilla Fernandez B, Roman de Aragon M, Paz Solis JF, Garcia Feijoo P, Roda Frade J, Regojo Zapata MR. Solitary fibrous tumor: a clinical case. Neurocirugia (Astur). 2019;30(1):33–37. doi:10.1016/j.neucir.2018.01.003
22. Munoz E, Prat A, Adamo B, et al. A rare case of malignant solitary fibrous tumor of the spinal cord. Spine (Phila Pa 1976). 2008;33(12):E397–E399. doi:10.1111/j.1440-1789.2009.01056.x
23. Brigui M, Aldea S, Bernier M, Bennis S, Mireau E, Gaillard S. Two patients with a solitary fibrous tumor of the thoracic spinal cord. J Clin Neurosci. 2013;20(2):317–319. doi:10.1016/j.jocn.2012.02.031
24. Nagano A, Ohno T, Nishimoto Y, Oshima K, Shimizu K. Malignant solitary fibrous tumor of the lumbar spinal root mimicking schwannoma: a case report. Spine J. 2014;14(1):e17–20. doi:10.1016/j.spinee.2013.07.463
25. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi:10.1007/s00401-007-0243-4
26. Bouvier C, Metellus P, de Paula AM, et al. Solitary fibrous tumors and hemangiopericytomas of the meninges: overlapping pathological features and common prognostic factors suggest the same spectrum of tumors. Brain Pathol. 2012;22(4):511–521. doi:10.1111/bpa.2012.22.issue-4
27. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi:10.1007/s00401-016-1545-1
28. Metellus P, Bouvier C, Guyotat J, et al. Solitary fibrous tumors of the central nervous system: clinicopathological and therapeutic considerations of 18 cases. Neurosurgery. 2007;60(4):
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


