Back to Journals » Infection and Drug Resistance » Volume 12
Spinal brucellosis in Hulunbuir, China, 2011–2016
Authors Liang C, Wei W , Liang X, De E, Zheng B
Received 21 January 2019
Accepted for publication 7 May 2019
Published 6 June 2019 Volume 2019:12 Pages 1565—1571
DOI https://doi.org/10.2147/IDR.S202440
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Chen Liang,1,2,* Wei Wei,3,* Xiuwen Liang,1,2 Enjin De,1,2 Beiwen Zheng4
1Department of Brucellosis, Hulunbuir People’s Hospital, Hulunbuir, People’s Republic of China; 2School of Medicine, Inner Mongolia University for Nationalities, Hulunbuir, People’s Republic of China; 3Hulunbuir Center for Disease Control and Prevention, Hulunbuir, People’s Republic of China; 4Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Purpose: To investigate the demographic, epidemiological, clinical, and laboratory characteristics; treatment options; and outcome of human brucellosis with spine involvement at a major hospital in Hulunbuir, a brucellosis epidemic region of China.
Patients and methods: A total of 842 patients with human brucellosis treated in the Department of Brucellosis, Hulunbuir People’s Hospital from January 2011 to December 2016 were included and analyzed in this study. The results of 67 brucellar spondylodiscitis (BS) cases were compared with those that were negative for spine involvements.
Results: The mean age of spinal brucellosis patients was 50.5±10.2 years (43 males and 24 females; age range 29–70). The risk factors for transmission are direct contact with animals, such as working in the farm, and consumption of unpasteurized milk or daily products. Back pain (92.5%), fever (85.1%), sweating (62.7%), and fatigue (52.8%) were the most common symptoms. Magnetic resonance imaging (MRI) was performed in all the patients with spondylodiscitis. The sites of involvement were lumbar (81.2%), thoracic (8.7%), cervical (4.3%), thoracolumbar (2.9%), and lumbosacral (2.9%). All isolates from blood culture were identified as Brucella melitensis, with 61% biovar 3 and 39% biovar 1 isolates. The antimicrobial therapy for BS lasted for at least 3 months. In the presence of paravertebral or epidural abscess, longer treatment was conducted to avoid possible sequelae.
Conclusion: In endemic areas such as Hulunbuir, BS should be considered in patients with back pain and fever. MRI is a highly sensitive imaging modality that can be used to differentiate BS from other spinal infections. This study will be helpful to establish strategies for prevention, surveillance, and management of spinal brucellosis in China.
Keywords: brucellosis, spine, spondylitis, brucella melitensis, treatment outcome
Introduction
Human brucellosis is a disease that causes substantial morbidity of global importance with more than 500,000 new cases annually.1,2 Brucellosis is transmitted to humans directly or indirectly. Direct transmission may course through contact with animals that carry the pathogenic bacteria Brucella or infectious material during animal husbandry and meat processing.2 By contrast, indirect transmission is achieved through the consumption of unpasteurized dairy products.2
Human brucellosis is endemic in the Arabian Peninsula, Turkey, Mediterranean region, India, and Central and South America.3–5 This disease also remains a serious public health problem in many developing countries, including China.6 In China, the epidemiology of human brucellosis became a real threat during the past decades,7–10 although China is among the few countries where vaccines were used to prevent human brucellosis (Brucella Abortus 104 M).11 The epidemiology of human brucellosis was aggravated in China during the period of 2005–2010.12 A total of 104,125 new cases of brucellosis were reported from 2015 to 2016.13. It is worthy to note that Inner Mongolia is the most severe endemic area in China,14–16 partly due to the well-developed animal (cattle and sheep) husbandry production in Inner Mongolia.12 However, studies report the detail clinical characteristics of human brucellosis from Inner Mongolia are lacking.
Spondylodiscitis is the foremost cause of the debilitating and disabling complications of brucellosis, and the axial skeleton is the most frequently involved site.17 Brucellar spondylodiscitis (BS) is the involvement of the vertebral column, interspinal spaces, and/or paraspinal areas. In China, only limited reports described the spinal brucellosis thus far, which were mainly focused on the application of MRI imaging in the diagnosis of BS.18–21 In this study, we analyzed the epidemiologic, clinical, and laboratory data, as well as the treatment and outcome characteristics of 67 cases with BS for the first time.
Patients and methods
Hospital and participants
This retrospective study was conducted at the Hulunbuir People’s Hospital (HPH), Inner Mongolia. HPH is the largest hospital in Hulunbuir with 1,255-bed and serves whole region with 2.53 million residents, providing free health care to approximately 35,000 inpatients a year. From January 2011 to December 2016, the data of 842 patients with human brucellosis admitted to the Department of Brucellosis were reviewed. Patients younger than 18 years old and pregnant patients were excluded.
Diagnosis of spine brucellosis
The diagnosis of spinal brucellosis was based on the clinical symptoms and signs compatible with the disease (back pain, fever, sweats, fatigue, and hepatosplenomegaly).17,22 The diagnosis was also based on the presence of specific antibodies at significant titers (Standard Tube Agglutination Test, STA test for Brucella ≥1/100) and/or isolation of Brucella species in blood samples. Species identification was performed by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Other criteria include a duration of more than 1 year or STA test for Brucella ≥1/50 and infection in vertebra or intervertebral disc on magnetic resonance imaging (MRI). All the demographic data, physical examination findings, and laboratory analyses (complete blood count, urine analysis, blood biochemical examination, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)) were recorded on brucellosis follow-up forms. All blood cultures, STA tests, rose-Bengal Plate Agglutination Tests, radiological imaging findings, and therapy combinations were also documented on the forms. Given the duration of the symptoms, the cases were classified as acute (<3 months), sub-acute (3–6 months), and chronic (>6 months) based on the diagnostic criteria for brucellosis (health standard no. WS 269–2007) used in China. MRI was performed in the patients with back pain and positive serological tests of brucellosis. MRI was performed using a 1.5-T MRI scanner (General Electric Signa Excite High-speed Scanner, Milwaukee, USA) using appropriate coils. Vertebral bodies, endplates, intervertebral discs, paravertebral soft tissue, and epidural spaces were assessed for the diagnosis of BS.
Treatment
For the treatment, amikacin (0.4 g/day intravenously), tobramycin (0.16 g/day intravenously), etimicin (0.2 g/day intravenously), or cefoperazone sodium and sulbactam sodium (4.5 g/day intravenously) were used in combination with levofloxacin (0.4g/day intravenously) for 2 weeks. The patients were given doxycycline (0.2 g/day oral) and rifampin (0.6 g/day oral) or levofloxacin capsule (0.5 g/day oral) for at least 12 weeks. For the patients with bone destruction, drugs for regulating bone metabolism, as well as neurotrophic agent and nonsteroidal anti-inflammatory drug, were administered in addition to the antimicrobial treatment. Relapse was considered as the recurrence or exacerbation of pain, unexplained fever, night sweats, weight loss, and re-increase in ESR and CRP, new vertebral lesions, and recurrent bacteremia.
Data analysis
All statistical analyses were performed using the SPSS software (version 22.0; SPSS, Chicago, IL, USA). The distribution of variables in the groups was compared with the Chi-squared test or Fisher’s exact test for categorical variables. By contrast, the Mann–Whitney test was used for continuous variables. P-values <0.05 were considered significant.
Ethics
The ethics committee of HPH approved the study. Written informed consent has been obtained from all patients in accordance with the Declaration of Helsinki.
Results
Patients’ characteristics
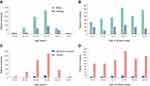
During the study period, a total of 842 brucellosis patients were enrolled in this study. All patients reported the history of direct contact with animals or consumption of unpasteurized dairy products. Most cases occurred in males with the male:female ratio of 2.86:1 (Figure 1A). Additionally, 41.7% (351/842) of the cases were reported in patients with age of 45–54 years. The epidemic peaked in 2014 with 197 reported cases. Although the annual incidence rate fluctuated during the study period, the gradually increased incidence rate was observed (Figure 1B). Our data are in line with the previous observations at county levels.7 Spinal brucellosis was most common in adults aged 45–54 years, but uncommon in patients older than 55 years (Figure 1C). The case distribution was similar by year between BS and other brucellosis (Figure 1D).
Prevalence of spinal brucellosis
In our study, 19 patients were with cardiovascular involvement, 24 cases with neurological involvement, 47 presents with urogenital involvement, 10 reports respiratory system involvement, 167 with liver damage, and 56 with renal damage. It is worth noting that 8% (67/842) patients present with spinal involvement, which is a significant cause of mortality associated with therapeutic failure. We further compared the epidemiologic, clinical, and laboratory data, as well as the treatment and outcome characteristics of BS cases with other brucellosis cases (Table 1). The mean age of BS patients was 50.5±10.2 years (43 males and 24 females; age range 29–72). Age was statistically significantly higher in the patients with BS. The clinical presentations of the BS patients were mostly acute (76.1%) or sub-acute (13.4%). Back pain (92.5%), fever (85.1%), and sweating (62.7%) represent the most common symptoms in BS group. Significant differences in back pain were observed between two groups (Table 1). When compared the laboratory findings, no significant differences in hematological values, erythrocyte sedimentation rate, CRP level, Brucella STA with Coombs serum, and ratios of growth in blood culture were observed between the two groups. Blood culture results demonstrated a low detection rate both in BS group (7, 14.9%) and BS-negative group (67, 8.6%). MALDI-TOF-MS method identified that 47 isolates were Brucella melitensis biovar 3 and 30 isolates were B. melitensis biovar 1 (Table 1).
 | Table 1 Comparison of demographical, clinical, and laboratory features of 842 cases of human brucellosis, Hulunbuir, China, 2011–2016 |
MRI scan
Among 67 spinal brucellosis cases, MRI technique revealed that 56 patients presented with lumbar involvement, 4 with thoracic involvement, and 3 with cervical vertebral involvement (Table 2). Meanwhile, 2 presented with both thoracic and lumbar involvements, and 2 suffered from both lumbar and sacral vertebral involvements. The most prominent involvement was L3-L4 of the lumbar spine (73.1%). Additionally, 14 cases presented with paravertebral abscess formation, 12 patients found cord compression, and 7 with epidural abscess formation.
 | Table 2 MRI findings of 67 patients with brucellar spondylodiscitis |
Follow-up
Among 67 spinal brucellosis patients, 63 were followed up and 4 cases were lost contact. After 12-weeks treatment, the effective rate was 90.5% (57/63). Cord compression was still found in 3 cases and underwent the surgical treatment with no relapse, and 2 patients changed of therapeutic regimen owing to the liver damage of rifampin. However, the therapeutically failure and relapses were not reported in our study after 24-weeks treatment.
Discussion
Human brucellosis cases mainly appear in particular key cities in the endemic areas of China, including Hulunbuir, Inner Mongolia. In Hulunbuir, comprehensive procedures for controlling the source of infected animals are lacking, and the knowledge transfer from the doctors to the high-risk population is inadequate. These reasons may primarily explain the high incidence of the disease in this region. Although many organs and systems might be involved, the osteoarticular disease is the most common complication in brucellosis.12
Spondylodiscitis more frequently occurs in adults and the elderly than in other populations. The mean age of patients in this study was 50.5±10.2 (range 29–72) years, which is consistent with previous studies.20,23,24 This is probably explained by the increased involvement in livestock breeding with increased exposure rate. Of note, all of the blood culture positive isolates were B. melitensis, with 61% biovar 3 isolates and 39% biovar 1 isolates. These data were consistent with previous studies where human brucellosis was mainly caused by B. melitensis biovar 3 and biovar 1 in Inner Mongolia.25
The patients with BS were significantly older than those without spinal involvement (P=0.002). Male patients predominated in the population (64.2%). Given that the greatest risk factor for transmission was direct contact with animals (91.0%), the male predominance likely reflects the exposure pattern. That is, the occupation of males is usually livestock in a pastoral economy, whereas females are less exposed to livestock in their domestic duties. Our cases mainly include the herdsman and peasants. The clinical presentations of the patients were mostly acute (76.1%) or sub-acute (13.4%). Given the widespread use of imaging techniques, such as MRI, and timely treatment, most patients are diagnosed in the early stages of the disease. This finding differs from those of previous studies reporting chronic presentations.
The most common symptom in patients with BS is back pain (33–85.9%),17,20,26 followed by fever (44.3–92%) and sweating (27–69%).20,27
Our study results are compatible with earlier reports. However, our ratio of back pain (92.5%) was higher, and most of patients in our hospital complained of back pain. The isolation of the pathogen in culture is the gold standard of diagnosis for brucellosis. Blood culture sensitivities range widely from 17% to 85%, depending on the strain involved, disease phase, and previous antibiotic treatment.28 In this work, a low isolation rate (14.9%) of Brucella isolates from blood cultures was associated with the experimental antibiotic treatment prescribed by physicians before obtaining cultures or the inadequate microbiological techniques and prolonged blood culture time.
The lumbar spine is the most frequently affected level, followed by the thoracic and cervical segments.17,20,28 The lumbar segment was also the most frequently involved region in our case series, with a rate of 81.2%. However, multiple-level involvement was described in 9% to 20% of the cases.28 In our study, only four patients were involved. The involvement of multiple vertebral bodies and the presence of skip lesions occur mostly in tuberculosis spondylodiscitis.27 Although the intervertebral disc spaces are not generally affected in metastatic diseases or in tuberculosis spondylodiscitis, these cases are narrowed in patients with BS. The irregular and thick enhancement of the abscess and abnormal signal in the paraspinal regions are suggestive of BS.
MRI is useful in differentiating BS from other spinal pathologies, including tuberculous spondylitis, pyogenic spondylitis, postoperative changes, spinal degenerative diseases, and vertebral metastases.17,23,27 This technique exhibits a high sensitivity for detecting spinal brucellosis in the early stages. The method also provides an excellent definition of paravertebral and epidural extension and follow-up of the disease.
The management of spinal brucellosis has not been standardized, and the duration of antibiotic therapy, selection of drugs, and the role of surgical intervention remain controversial. According to the World Health Organization, the treatment regimen for brucellosis consists of a combination of doxycycline and rifampicin (both drugs administered for 6 weeks) or doxycycline plus streptomycin.29,30 However, the treatment results of this study implied that doxycycline and rifampin with or without levofloxacin are more effective than other drug combinations for BS (unpublished data).
The therapy duration varied greatly depending on clinical response and ranged between 2 and 6 months.12,31.In our case series, all patients received treatment with a combination of 2 or 3 antibiotics, and the therapy duration lasted for at least 14 weeks. Cases of severe neurologic deficit and incapacitating back pain often necessitate surgical intervention. Given the good response to antibiotic treatment, only three patients received surgical treatment because of cord compression. Our findings are similar to those of other studies.32,33 Surgery is the last option for treatment and is given for persistent spinal instability, cord compression, and severe muscle weakness.33–36
Conclusion
This report describes the epidemiological and clinical characteristics of 842 cases of human brucellosis in Hulunbuir, China. Given that brucellosis is still endemic in this region, brucellar spondylodiscitis should be considered in elderly patients with back pain and fever. MRI is recommended for early diagnosis and attention should be given to the prolonged duration of patient treatment with paravertebral and epidural abscesses. This study will be helpful to establish strategies for prevention, surveillance, and management of spinal brucellosis in China.
Acknowledgments
This study was supported by funding from the State Key Clinical Specialty Construction Project; the Science and Technology Major Project of Inner Mongolia (No. 2060901); the Science and Technology Major Project of Hulunbuir; and the Science and Technology Planning Project of Inner Mongolia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rubach MP, Halliday JE, Cleaveland S, Crump JA. Brucellosis in low-income and middle-income countries. Curr Opin Infect Dis. 2013;26(5):404–412. doi:10.1097/QCO.0b013e3283638104
2. Atluri VL, Xavier MN, de Jong MF, Den Hartigh AB, Tsolis RM. Interactions of the human pathogenic brucella species with their hosts. Annu Rev Microbiol. 2011;65:523–541. doi:10.1146/annurev-micro-090110-102905
3. Mantur BG, Amarnath SK. Brucellosis in India - a review. J Biosci. 2008;33(4):539–547.
4. Tan KK, Tan YC, Chang LY, et al. Full genome SNP-based phylogenetic analysis reveals the origin and global spread of brucella melitensis. BMC Genomics. 2015;16:93. doi:10.1186/s12864-015-1294-x
5. Al-Adsani W, Ahmad A, Al-Mousa M. A case of brucella melitensis endocarditis in a patient with cardiovascular implantable electronic device. Infect Drug Resist. 2018;11:387–390. doi:10.2147/IDR.S152771
6. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91–99. doi:10.1016/S1473-3099(06)70382-6
7. Lai S, Zhou H, Xiong W, et al. Changing epidemiology of human brucellosis, China, 1955–2014. Emerg Infect Dis. 2017;23(2):184–194. doi:10.3201/eid2311.170833
8. Chen S, Zhang H, Liu X, et al. Increasing threat of brucellosis to low-risk persons in urban settings, China. Emerg Infect Dis. 2014;20(1):126–130. doi:10.3201/eid2001.130324
9. Zheng R, Xie S, Lu X, et al. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. Biomed Res Int. 2018;2018:5712920. doi:10.1155/2018/5712920
10. Zhang J, Yin F, Zhang T, et al. Spatial analysis on human brucellosis incidence in mainland China: 2004–2010. BMJ Open. 2014;4(4):e004470. doi:10.1136/bmjopen-2013-004470
11. Deqiu S, Donglou X, Jiming Y. Epidemiology and control of brucellosis in China. Sci Direct. 2002;90(1–4):165–182.
12. Zhong Z, Yu S, Wang X, et al. Human brucellosis in the People’s Republic of China during 2005–2010. Int J Infect Dis. 2013;17(5):e289–292. doi:10.1016/j.ijid.2013.02.015
13. YJ LS S, Chen QL, Mu D, et al. Analysis on the epidemiological features of human brucellosis in northern and southern areas of China, 2015–2016. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(4):435–440. doi:10.3760/cma.j.issn.0254-6450.2017.04.023
14. Zhang WY, Guo WD, Sun SH, et al. Human brucellosis, Inner Mongolia, China. Emerg Infect Dis. 2010;16(12):2001–2003. doi:10.3201/eid1612.091081
15. Chen Y, Ke Y, Wang Y, et al. Changes of predominant species/biovars and sequence types of brucella isolates, Inner Mongolia, China. BMC Infect Dis. 2013;13:514. doi:10.1186/1471-2334-13-514
16. Liu ZG, Di DD, Wang M, et al. In vitro antimicrobial susceptibility testing of human brucella melitensis isolates from Ulanqab of Inner Mongolia, China. BMC Infect Dis. 2018;18(1):43. doi:10.1186/s12879-018-3109-6
17. Ulu-Kilic A, Karakas A, Erdem H, et al. Update on treatment options for spinal brucellosis. Clin Microbiol Infect. 2014;20(2):O75–82. doi:10.1111/1469-0691.12742
18. Tu L, Liu X, Gu W, et al. Imaging-assisted diagnosis and characteristics of suspected spinal brucellosis: a retrospective study of 72 cases. Med Sci Monit. 2018;24:2647–2654. doi:10.12659/MSM.909288
19. Zhao YT, Yang JS, Liu TJ, He LM, Hao DJ. Sclerosing vertebra in the spine: typical sign of spinal brucellosis. Spine J. 2015;15(3):550–551. doi:10.1016/j.spinee.2014.10.008
20. Yang X, Zhang Q, Guo X. Value of magnetic resonance imaging in brucellar spondylodiscitis. Radiol Med. 2014;119(12):928–933. doi:10.1007/s11547-014-0416-x
21. Li T, Li W, Du Y, et al. Discrimination of pyogenic spondylitis from brucellar spondylitis on MRI. Medicine (Baltimore). 2018;97(26):e11195. doi:10.1097/MD.0000000000011195
22. Sheng ZM, Chertow DS, Morens DM, Taubenberger JK. Fatal 1918 pneumonia case complicated by erythrocyte sickling. Emerg Infect Dis. 2010;16(12):2000–2001. doi:10.3201/eid1612.101376
23. Buzgan T, Karahocagil MK, Irmak H, et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis. 2010;14(6):e469–478. doi:10.1016/j.ijid.2009.06.031
24. Oztekin O, Calli C, Adibelli Z, Kitis O, Eren C, Altinok T. Brucellar spondylodiscitis: magnetic resonance imaging features with conventional sequences and diffusion-weighted imaging. Radiol Med. 2010;115(5):794–803. doi:10.1007/s11547-010-0530-3
25. Liu ZG, Di DD, Wang M, et al. MLVA genotyping characteristics of human brucella melitensis isolated from Ulanqab of Inner Mongolia, China. Front Microbiol. 2017;8:6.
26. Kaptan F, Gulduren HM, Sarsilmaz A, et al. Brucellar spondylodiscitis: comparison of patients with and without abscesses. Rheumatol Int. 2013;33(4):985–992. doi:10.1007/s00296-012-2491-4
27. Ulu-Kilic A, Sayar MS, Tütüncü E, Sezen F, Sencan I. Complicated brucellar spondylodiscitis: experience from an endemic area. Rheumatol Int. 2012;33(11):2909–2912. doi:10.1007/s00296-012-2555-5
28. Erdem H, Elaldi N, Batirel A, et al. Comparison of brucellar and tuberculous spondylodiscitis patients: results of the multicenter “Backbone-1 Study”. Spine J. 2015;15(12):2509–2517. doi:10.1016/j.spinee.2014.10.008
29. Tali ET, Koc AM, Oner AY. Spinal brucellosis. Neuroimaging Clin N Am. 2015;25(2):233–245. doi:10.1016/j.nic.2015.01.004
30. Bozgeyik Z, Aglamis S, Bozdag PG, Denk A. Magnetic resonance imaging findings of musculoskeletal brucellosis. Clin Imaging. 2014;38(5):719–723. doi:10.1016/j.clinimag.2014.04.007
31. Alp E, Doganay M. Current therapeutic strategy in spinal brucellosis. Int J Infect Dis. 2008;12(6):573–577. doi:10.1016/j.ijid.2008.03.014
32. Yoon YK, Jo YM, Kwon HH, et al. Differential diagnosis between tuberculous spondylodiscitis and pyogenic spontaneous spondylodiscitis: a multicenter descriptive and comparative study. Spine J. 2015;15(8):1764–1771. doi:10.1016/j.spinee.2014.10.008
33. Colmenero JD, Ruiz-Mesa JD, Plata A, et al. Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Oxford. 2008;46(3):426–433.
34. Ioannou S, Karadima D, Pneumaticos S, et al. Efficacy of prolonged antimicrobial chemotherapy for brucellar spondylodiscitis. Clin Microbiol Infect. 2011;17(5):756–762. doi:10.1111/j.1469-0691.2010.03298.x
35. Solera J. Update on brucellosis: therapeutic challenges. Int J Antimicrob Agents. 2010;36(Suppl 1):S18–20. doi:10.1016/j.ijantimicag.2010.06.003
36. Turan H, Serefhanoglu K, Karadeli E, Togan T, Arslan H. Osteoarticular involvement among 202 brucellosis cases identified in Central Anatolia Region of Turkey. Internal Med. 2011;50(5):421–428. doi:10.2169/internalmedicine.50.4700
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.