Back to Journals » Cancer Management and Research » Volume 11
Sp1 contributes to radioresistance of cervical cancer through targeting G2/M cell cycle checkpoint CDK1
Authors Deng YR, Chen XJ, Chen W, Wu LF, Jiang HP, Lin D, Wang LJ, Wang W , Guo SQ
Received 9 January 2019
Accepted for publication 21 May 2019
Published 28 June 2019 Volume 2019:11 Pages 5835—5844
DOI https://doi.org/10.2147/CMAR.S200907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Yuan-Run Deng, 1* Xiao-Jing Chen, 2* Wei Chen, 1 Lan-Fang Wu, 1 Hui-Ping Jiang, 1 Dan Lin, 1 Li-Jing Wang, 1 Wei Wang*, 2, 3 Sui-Qun Guo 1,
1Department of Obstetrics and Gynecology, The Third Affiliated Hospital, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Obstetrics and Gynecology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 3Department of Obstetrics and Gynecology, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Background/aims: Radioresistance remains a significant obstacle in the therapy of cervical cancer, and the mechanism of it is still unclear. We aimed to investigate the role of specificity protein 1 (Sp1) in radioresistance of cervical cancer.
Methods: Sp1 was examined immunohistochemically on tissues from 36 human cervical cancer patients. We used RT-qPCR and Western blot to examine the expression of Sp1 in irradiated cervical cancer cell lines SiHa and HeLa. The role of Sp1 in radioresistance of cervical cancer cells was assessed by colony-formation assay and cell cycle analysis. Dual-luciferase reporter assay was performed to detect the downstream of Sp1.
Results: High Sp1 expression was positively correlated with advanced International Federation of Gynecology and Obstetrics (FIGO) stage, lymph node metastasis, and lymphovascular space invasion (LVSI) of cervical cancer. The expression of Sp1 was dose-dependently increased in irradiated cervical cancer cell lines at both mRNA and protein levels. Colony-formation assay showed that alteration of Sp1 expression affected the survival of cervical cancer cells with radiotherapy (RT) treatment. Knockdown of Sp1 significantly strengthened the cellular response to radiation by inducing G2/M arrest in cervical cancer cells. Overexpression of Sp1 significantly decreased G2/M arrest in cervical cancer cells, which was related to upregulation of CDK1 expression. Dual-luciferase reporter assay showed the direct effect of Sp1 on the transcriptional activation of CDK1.
Conclusion: Sp1 may contribute to radioresistance through inhibiting G2/M phase arrest by targeting CDK1, and be considered as a potential therapeutic target to promote the effect of RT for patients with cervical cancer.
Keywords: cervical cancer, Sp1, radioresistance, G2/M phase, CDK1
Corrigendum for this paper has been published
Introduction
Cervical cancer is the second most frequent gynecological malignancy worldwide.1 Radiotherapy (RT) is one of the main adjuvant therapies of cervical cancer, but the therapeutic efficacy of radiation is limited by the occurrence of radioresistance.2 Identifying novel molecules involved in radioresistance of cervical cancer cells can provide promising prospects for the advancement of RT.
Specificity protein 1 (Sp1), initially identified as a transcription factor, plays a crucial role in normal biological processes, neoplastic development, and tumor migration.3,4 Sp1 expression is found to be upregulated in multiple tumor types, and its high level is often related to tumor progression and poor prognosis.5,6 Previous studies demonstrated that Sp1 is a critical mediator that controls HPV18 transforming activity in HPV-induced cervical carcinogenesis7 and is closely related with the progression, metastasis, and recurrence of cervical cancer.8,9
As we all know, the response of cells to radiation is concerned with their cell-cycle phase. The G2/M phase cells were the most sensitive cells to radiation, whereas during the latter part, the S phase cells were the most radiation tolerant.10–13 As an important transcription factor, Sp1 has been shown to be associated with transcriptional regulation of ataxia-telangiectasia mutated (ATM), a key regulator in the cell-cycle regulation.14 Moreover, downregulation of Sp1 protein sensitizes human osteosarcoma and leukemia cells to radiation-induced DNA double-strand breaks (DSBs).15,16 We speculated that Sp1 may serve as a crucial player in the radioresistance of cervical cancer.
In the present study, we found that Sp1 plays an important role in the process of cervical cancer RT, which contributes to radioresistance by decreasing cells arrest at G2/M through targeting cyclin-dependent kinase 1 (CDK1). Our findings help to provide a new strategy for improving the therapeutic effects of treatments for cervical cancer patients with radioresistance.
Materials and methods
Cell culture and RT treatment
The cervical cancer cell lines SiHa (ATCC HTB-35TM) and HeLa (ATCC CCL-2TM) were purchased from the American Type Culture Collection (ATCC, Manassas, VA , USA). All cells were cultured in DMEM (Invitrogen, Waltham, MA, USA) supplemented with 10% FBS at 37°C with 5% CO2.
Cells were exposed to X-ray irradiation at a single dose of 0, 2, 4, 6, 8, and 10 Gy at 200 cGy/min by the use of the Varian 2100C accelerator with 6 MV-photons at room temperature. Cells were then returned to the incubator.
Clinical specimens and immunohistochemistry (IHC)
Cervical cancer specimens were obtained from the Department of Gynecological Oncology of the Third Affiliated Hospital, Southern Medical University between 2013 and 2014. The study was approved by the Institutional Research Ethics Committee. Detailed information on clinical specimens is summarized in Table 1. Tissue sections were subjected to immunohistochemistry (IHC) analysis as previously described.17 The counting was done using the H score algorithm.18 The median H score values were selected for distinction between the groups of low and high Sp1 expression.
 |
Table 1 Association of the clinicopathologic variables with the expression levels of Sp1 |
Western blot assay
Total proteins were extracted using RIPA extraction reagents (Solarbio, Beijing, China), and the protein concentration was checked by BCA Protein Assay Kit (Beyotime, Shanghai, China). A total of 50 μg of protein was separated by 10% SDS-PAGE and subsequently transferred onto polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with 5% BSA for 1 hr at room temperature, incubated with the primary antibody at 4°C overnight, and subsequently incubated with horseradish peroxidase (HRP)-labeled secondary antibody (ZSGB-Bio, Beijing, China, 1: 5,000) for 1 hr at room temperature before detection by ECL (Thermo, Waltham, MA, USA). The information of antibodies was as follows: anti-Sp1 (Santa Cruz, Dallas, TX, USA, 1:1,000), anti-CDK1 (Abcam, Cambridge, UK, 1:1,000), and anti-GAPDH (Abcam, 1:1,000) .
RNA extraction and real-time quantitative PCR (rt-qPCR)
Total RNA was extracted with trizol reagent (Invitrogen). For the mRNA analysis, cDNA was synthesized from 1.0 μg of total RNA using the Prime Script (R) RT reagent kit (TaKaRa Bio Inc, Dalian, China). Real-time PCR was subsequently performed in triplicate with a 1:4 dilution of cDNA using the SYBR Premix Ex Taq kit (TaKaRa Bio Inc) on an ABI Prism 7,500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) following the manufacturer’s protocol. The data were normalized against GAPDH transcript as a reference gene, and levels of RNA expression were determined with the 2−ΔΔCt method. Specific primer sets for Sp1, CDK1, cyclinA, cyclinB, and GAPDH were purchased from Invitrogen Inc. The primer sequences are shown in Table 2. Briefly, for the PCR, the same conditions for analyzed genes were applied: denaturing at 95°C for 10 mins, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Each measurement was performed in duplicate.
 |
Table 2 Primers for real-time RT-PCR |
Establishment of Sp1 silenced and overexpressed stable lines
All recombinant lentiviruses (LV3-h1-GFP/puro-shSP1 and LV5-EF1a-GFP/Puro-SP1) were purchased from the GenePharma Inc (Shanghai, China). Lentiviral infection (MOI=10) was carried out according to the manufacturer’s instructions. Lentivirus-mediated Sp1-silenced and Sp1–overexpressed stable cells were selected by puromycin (2 μg/mL).
Colony-formation assay
Different mounts of cells (500, 1,000, 2,000, 3,000, 4,000, 5,000 cells) were seeded into six-well plates and exposed to indicated doses of IR (0, 2, 4, 6, 8, 10 Gy) for 24 h. After 14 days of incubation, cells were fixed with 10% paraformaldehyde and stained with 1 x Giemsa stain (LEGANE, Beijing, China). Colonies containing 50 cells or more were counted. PE (Plating efficiency)= the number of clones/the number of cells seeded×100%. Surviving fraction= PEs of treated cells/PEs of control cells. The surviving fraction is a recognized indicator used to determine radiosensitivity.
Transwell migration assay
The cell migration assays were performed after exposure to 10 Gy irradiation using transwell inserts (8-μm pore size, Millipore, Billerica, MA, USA) in 24-well dishes. 1×105 tumor cells in serum-free DMEM were added to the upper compartment of the chamber. Medium containing 10% serum was used as a chemoattractant in the lower chambers. After incubation for 48 hrs, the noninvasive cells were removed with a cotton swab. Invading cells on the undersides of membranes were stained with hematoxylin and counted under a light microscope in five random visual fields (×200). Each experiment was repeated at least three times independently.
Wound-healing assay
SiHa and HeLa cells were harvested and seeded in a six-well plate after exposure to 10 Gy irradiation at a density of 5×105 cells/well. A scratch wound was generated using sterile 10-μL pipet tip, and floating cells were removed by washing with PBS. Images of the scratches were taken using an inverted microscope at ×100 magnification at 0 hr and 48 hrs of incubation. The percentage of healed wound area was measured as a ratio of occupied area to the total area using Image Olympus IX71 (Olympus Inc, Tokyo, Japan).
Transient transfection
SiHa and HeLa were transfected with 10 μM CDK1 siRNA or a negative control siRNA (GenePharma, China) with Lipofectamine™ 2000 (Invitrogen) according to the manufacturer’s instructions. SiHa and HeLa were also transfected with 4 μg plasmids (pcDNA3.1-Sp1 or pGL3-CDK1-promoter) with Lipofectamine™ 2000 (Invitrogen) the same way as siRNA. The gene silencing efficiency of both cells was more than 80%, as confirmed by the detection of RT-qPCR.
Cell cycle analysis
Cells were harvested by trypsinization and collected in tubes at 1×106 cells/tube. After washing twice with PBS, cells were fixed in ice-cold 70% ethanol overnight at 4°C. Cells were then resuspended in 50 mg/mL of propidium iodide (PI) and 100 mg/mL of RNAse A as manufacturer’s instructions (KeyGEN, Suzhou, China) at room temperature in the dark for 20 mins. Finally, cells were assayed by flow cytometry (Beckman Coulter, CA, USA).
Luciferase activity assay
The expression plasmid of human Sp1 was purchased from GenePharma Inc, which was sequenced to confirm its accuracy. The pGL3.CDK1 promoter luciferase vector was generated by inserting CDK1 promoter sequence. SiHa and HeLa cells (5*104 cells/well) were plated into 24-well plates and incubated for 24 hrs. Promoter vectors were cotransfected with either pcDNA3.1-Sp1 or pcDNA3.1-NC plasmid into cells using Lipofectamine 2000 (Invitrogen). Luciferase activity was measured 48 hrs after transfection by the Dual-Luciferase Reporter Assay System. Each assay was repeated in 3 independent experiments.
Statistical analysis
SPSS (version 20.0) (IBM Corporation, Armonk, NY, USA) software package was used for statistical analysis. Results were expressed as mean value±SEM and interpreted by t-test or Chi-squared test. Differences were considered to be statistically significant when P<0.05.
Results
High Sp1 expression is correlated with the malignant progression of cervical cancer
To explore the correlation between Sp1 expression and the malignant progression of cervical cancer, we performed IHC staining on tissues from 36 human cervical cancer patients. As shown in Table 1, high Sp1 expression was positively correlated with advanced FIGO stage, lymph node (LN) metastasis, and lymphovascular space invasion (LVSI) of cervical cancer (all P<0.05). These findings suggest that Sp1 may become a new therapeutic target of cervical cancer.
Sp1 is an irradiation-associated protein in cervical cancer
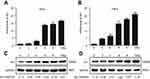
We further analyzed the influence of radiation on Sp1 expression in cervical cancer cells (SiHa and HeLa). RT-qPCR revealed that the expression of Sp1 in SiHa and HeLa cells dose-dependently increased at transcriptional level and reached a peak at 10 Gy X-rays (*P<0.05; **P<0.01; ***P<0.001; Figure 1A for SiHa, Figure1B for HeLa). Consistently, data obtained in the Western blot revealed that the expression of Sp1 was highly upregulated in response to radiation at the translational level (Figure 1C for SiHa, Figure1D for HeLa). Collectively, these data suggest that radiation-mediated upregulation of Sp1 may serve as a crucial player in radioresistance.
Sp1 is related to the radioresistance of cervical cancer cells
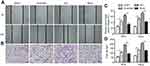
To detect whether Sp1 is related to the radiation response in cervical cancer, we suppressed Sp1 gene expression by shRNA targeting Sp1 in SiHa and HeLa cells. We also generated stable cell lines overexpressing Sp1 in SiHa and HeLa cells. As shown in Figure 2A and B, downregulation of Sp1 induced a significant decrease of surviving fraction in both cell lines compared to the control-scramble-infected cells (*P<0.05; **P<0.01; ***P<0.001). This result shows that downregulation of Sp1 sensitizes cervical cancer cells to a radiation-induced decrease in cell survival. Consistently, upregulation of Sp1 caused a significant increase of surviving fraction in both cell lines compared to control-mock-infected cells (*P<0.05; **P<0.01; ***P<0.001; Figure 2C and D). As shown in Figure 3, transwell migration and wound-healing assays revealed that Sp1 overexpression significantly increased the migration and invasion in cervical cancer cells in response to radiation, whereas Sp1 depletion had the opposite effect (*P<0.05). Thus, alteration of Sp1 expression affects the survival and invasiveness of cervical cancer cells with RT treatment.
Sp1 promotes radioresistance by affecting G2/M phase cell-cycle arrest in cervical cancer cells
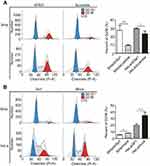
Cell-cycle phase plays an important role in the radioresistance of cancer.10 We next analyzed the role of Sp1 in the cell cycle. The G2/M phase arrest was more obviously in the Sp1-silenced SiHa and HeLa cells than control silenced cervical cancer cells (**P<0.01; Figure 4A). As well, G2/M phase arrest was ameliorated more in Sp1-overexpressed SiHa and HeLa cells compared to the control cells (**P<0.01; Figure 4B). This observation suggests that Sp1 increases radioresistance by decreasing cervical cancer cell arrests at the G2/M phase.
Sp1 targets CDK1 directly in cervical cancer cells
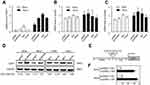
To investigate the molecular mechanism of Sp1-mediated radioresistance of cervical cancer cells, we assessed the mRNA levels of CDK1, cyclinA, and cyclinB in cervical cancer cell lines with altered expression of Sp1. Our data show that Sp1 overexpression markedly enhances the mRNA levels of CDK1 in cervical cancer cells, and Sp1 silencing greatly inhibits the mRNA levels as compared with the control (all P<0.05; Figure 5A). There was no obvious change in the expression of cyclinA and cyclinB after altering the expression of Sp1 (all P>0.05; Figure 5B and C). We also found that the protein level of CDK1 was similar to the mRNA level (Figure 5D).
To uncover the molecular mechanisms of Sp1-promoted CDK1 expression, JASPAR 2018 database was used for analysis. As shown in Figure 5E, RNA sequence of the predicted Sp1-binding site to CDK1 was CGCCCGCCCTC, which suggested Sp1 was a putative upstream transcription factor of CDK1. To further examine the regulation of CDK1 by Sp1, we performed luciferase reporter assays using the pGL3.CDK1 vector carrying the CDK1 promoter region. The pGL3.CDK1 and pcDNA3.1-Sp1 vectors were transiently cotransfected into SiHa and HeLa cells. As shown in Figure 5F, the CDK1 promoter-reporter activity was significantly affected when Sp1 was introduced into the cells (**P<0.01). Sp1 could directly target CDK1 promoter in cervical cancer cells.
Knockdown of CDK1 reverses radioresistance induced by Sp1
To assess whether the radioresistant effect of Sp1 was dependent on CDK1, we silenced CDK1 expression in SiHa/Sp1 and HeLa/Sp1 cells using siRNAs followed by treatment with RT (Figure 6A). The rescue experiments showed that the upregulation of CDK1 by Sp1 could be reversed by siCDK1; meanwhile, the increase of surviving fraction in SiHa/SP1, HeLa/SP1 could be supressed by inhibiting CDK1 (*P<0.05; **P<0.01; Figure 6B and C). These results show that Sp1 mediates resistance to radiation-induced cell death through cell-cycle-related molecules CDK1.
Discussion
Radioresistance is mainly responsible for treatment failure and mortality in cervical cancer patients who receive radical radiation therapy.19 However, the mechanisms underlying this resistance are only partially defined. Therefore, there is an urgent need to discover new markers and therapeutic targets to overcome this problem. Sp1 is an importanrt transcription factor found to have significant differential expression in cervical carcinoma and adjacent tissue.20 We further found that high Sp1 expression is correlated with the malignant progression of cervical cancer. However, the biological role and underlying mechanism of Sp1 in RT response has remained undefined. In this study, we found that the expression of Sp1 in cervical cancer cells showed a dose-dependent increase after irradiation. As such, Sp1 may be associated with radiation-resistant cervical cancer as a clinical biomarker and therapeutic target.
Subsequently, we investigated the role of Sp1 in radioresistance of cervical cancer cells. Silenced or overexpressed expression of Sp1 in cervical cancer cell lines resulted in decreased or increased survival and invasiveness of cervical cancer cells after irradiation. So Sp1 may have potential as a radioresistant gene in RT of cervical cancer, besides its aggressive effect in malignant transformation of cervical cancer cells.7–9 Sp1 is also an important mediator of radiation-associated gene expression in human head and neck squamous cell carcinoma.21 Several studies have reported that radiation can phosphorylate Sp1, which increases its transcriptional activity,22 while Sp1 DNA-binding activity is increased in a transient and reversible manner in response to RT.23 Evidence above further confirmed that inhibiting the expression of Sp1 may be a novel strategy for reducing radioresistance.
Mechanisms contributing to RT resistance in cancers are complex, including arrested cell-cycle progression in the G1 and G2 phases,24 apoptosis,25 DNA damage repair,26 and so on, especially G2/M arrest, which plays an important role in the radiation sensitivity of cancer. It has become clear that cells are blocked in the phase of G2/M during the course of DNA damage, and the cells are more susceptible to the toxic effects of radiation in the G2/M phase.27 Recent evidence suggests T0070907, a PPAR γ inhibitor, could upregulate the radiosensitivity of cervical cancer by arresting cells in the G2/M phase.28 Therefore, targeting the G2/M checkpoint may be an important strategy for RT of cancer. In this study, we demonstrated that Sp1 was a cell-cycle regulator. Knockdown of Sp1 accelerated and overexpression of Sp1 inhibited the G2/M phase arrest in cervical cancer cells. These results provide evidence that downregulation of Sp1 can mediate radiosensitivity by blocking cells at G2/M, the most radiosensitive phase of the cell cycle.
It has been reported that CDK1, cyclinA, and cyclinB are critical determinants of the G2-to-M transition, and various factors decreased the expression of them accompanied by G2/M cell-cycle arrest.29 Inhibition of CDKs can lead to a halt of cell-cycle progression and a stay at different phases or checkpoints.30 Our data indicate that Sp1 exhibits its biological function through directly enhancing the expression of CDK1 in cervical cancer cells. CDK1, the main target molecule of the G2/M cell cycle checkpoint,31 plays an important role in the cellular response to radiation and mediates cell-cycle arrest in response to DNA damage. Meanwhile, CDK1 has been identified as a clinically useful prognostic marker in cancers, including cervical cancer,32 colorectal cancer,30 breast cancer,33 and so on. In addition, CDK1 plays an important role in the radioresistance of numerous cancers.34 Our study found that the expression of CDK1 changed consistently with the Sp1 level. Sp1 was observed to occupy the CDK1 promotor using a luciferase reporter assay. In addition, the increase of surviving fraction in SiHa/SP1, HeLa/SP1 could be suppressed by inhibiting CDK1. These results clearly demonstrate that CDK1 is a key downstream effector in mediating the effects of Sp1 in cell cycle.
In conclusion, we have identified a new mechanism of acquired radioresistance in cervical cancer cells. Sp1 is highly expressed in radioresistant cervical cancer and functions as a regulator of radiation resistance by targeting the G2/M cell-cycle checkpoint mediator CDK1. Based on our findings, the inhibition of Sp1 may represent a novel and more effective approach for cervical cancer treatment, especially for those patients who are resistant to RT.
Statement of ethics
Compliance with ethical standards.
Acknowledgments
This work was supported by the National Key Reaseach and Development Program of China (No. 2016YFC1302901), the Science and Technology Planning Project of Guangdong Province, China (No. 2017ZC0100; No. 2017ZC0096) and the Science and Technology Planning Project of Tianhe District (No. 201604KW010).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
2. Ning S, Nemeth JA, Hanson RL, Forsythe K, Knox SJ. Anti-integrin monoclonal antibody CNTO 95 enhances the therapeutic efficacy of fractionated radiation therapy in vivo. Mol Cancer Ther. 2008;7(6):1569–1578. doi:10.1158/1535-7163.MCT-08-0288
3. Vizcaino C, Mansilla S, Portugal J. Sp1 transcription factor: a long-standing target in cancer chemotherapy. Pharmacol Ther. 2015;152:111–124. doi:10.1016/j.pharmthera.2015.05.008
4. Li L, Davie JR. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann Anat. 2010;192(5):275–283. doi:10.1016/j.aanat.2010.07.010
5. Beishline K, Azizkhan-Clifford J. Sp1 and the ‘hallmarks of cancer’. Febs J. 2015;282(2):224–258. doi:10.1111/febs.13148
6. Safe S, Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer. 2005;41(16):2438–2448. doi:10.1016/j.ejca.2005.08.006
7. Hoppe-Seyler F, Butz K. Activation of human papillomavirus type 18 E6-E7 oncogene expression by transcription factor Sp1. Nucleic Acids Res. 1992;20(24):6701–6706. doi:10.1093/nar/20.24.6701
8. Wang F, Li Y, Zhou J, et al. miR-375 is down-regulated in squamous cervical cancer and inhibits cell migration and invasion via targeting transcription factor SP1. Am J Pathol. 2011;179(5):2580–2588. doi:10.1016/j.ajpath.2011.07.037
9. Choi ES, Nam JS, Jung JY, Cho NP, Cho SD. Modulation of specificity protein 1 by mithramycin A as a novel therapeutic strategy for cervical cancer. Sci Rep. 2014;4:7162. doi:10.1038/srep07162
10. Eriksson D, Lofroth PO, Johansson L, Riklund KA, Stigbrand T. Cell cycle disturbances and mitotic catastrophes in HeLa Hep2 cells following 2.5 to 10 Gy of ionizing radiation. Clin Cancer Res. 2007;13(18 Pt 2):5501s–5508s. doi:10.1158/1078-0432.CCR-07-0980
11. Montenegro MF, Sanchez-del-Campo L, Fernandez-Perez MP, Saez-Ayala M, Cabezas-Herrera J, Rodriguez-Lopez JN. Targeting the epigenetic machinery of cancer cells. Oncogene. 2015;34(2):135–143. doi:10.1038/onc.2013.605
12. Sewing A, Wiseman B, Lloyd AC, Land H. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol Cell Biol. 1997;17(9):5588–5597. doi:10.1128/mcb.17.9.5588
13. Cho HJ, Oh YJ, Han SH, et al. Cdk1 protein-mediated phosphorylation of receptor-associated protein 80 (RAP80) serine 677 modulates DNA damage-induced G2/M checkpoint and cell survival. J Biol Chem. 2013;288(6):3768–3776. doi:10.1074/jbc.M112.401299
14. Tao S, Meng S, Zheng X, Xie L. ATM participates in the regulation of viability and cell cycle via ellipticine in bladder cancer. Mol Med Rep. 2017;15(3):1143–1148. doi:10.3892/mmr.2017.6141
15. Iwahori S, Yasui Y, Kudoh A, et al. Identification of phosphorylation sites on transcription factor Sp1 in response to DNA damage and its accumulation at damaged sites. Cell Signal. 2008;20(10):1795–1803. doi:10.1016/j.cellsig.2008.06.007
16. Nishida Y, Mizutani N, Inoue M, et al. Phosphorylated Sp1 is the regulator of DNA-PKcs and DNA ligase IV transcription of daunorubicin-resistant leukemia cell lines. Biochim Biophys Acta. 2014;1839(4):265–274. doi:10.1016/j.bbagrm.2014.02.004
17. Chen XJ, Han LF, Wu XG, et al. Clinical significance of CD163+ and CD68+ tumor-associated macrophages in high-risk HPV-related cervical cancer. J Cancer. 2017;8(18):3868–3875. doi:10.7150/jca.21444
18. Barash U, Lapidot M, Zohar Y, et al. Involvement of heparanase in the pathogenesis of mesothelioma: basic aspects and clinical applications. J Natl Cancer Inst. 2018;110(10):1102–1114. doi:10.1093/jnci/djy032
19. Dizon DS, Mackay HJ, Thomas GM, et al. State of the science in cervical cancer: where we are today and where we need to go. Cancer. 2014;120(15):2282–2288. doi:10.1002/cncr.28722
20. Zhang L, Liu SK, Song L, Yao HR. SP1-induced up-regulation of lncRNA LUCAT1 promotes proliferation, migration and invasion of cervical cancer by sponging miR-181a. Artif Cells Nanomed Biotechnol. 2019;47(1):556–564. doi:10.1080/21691401.2019.1575840
21. Pore N, Gupta AK, Cerniglia GJ, et al. Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66(18):9252–9259. doi:10.1158/0008-5472.CAN-06-1239
22. Criswell T, Leskov K, Miyamoto S, Luo G, Boothman DA. Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene. 2003;22(37):5813–5827. doi:10.1038/sj.onc.1206680
23. Yang CR, Wilson-Van PC, Planchon SM, et al. Coordinate modulation of Sp1, NF-kappa B, and p53 in confluent human malignant melanoma cells after ionizing radiation. Faseb J. 2000;14(2):379–390. doi:10.1096/fasebj.14.2.379
24. Wang X, Li Q, Jin H, et al. miR-424 acts as a tumor radiosensitizer by targeting aprataxin in cervical cancer. Oncotarget. 2016;7(47):77508–77515. doi:10.18632/oncotarget.12716
25. Ke G, Liang L, Yang JM, et al. MiR-181a confers resistance of cervical cancer to radiation therapy through targeting the pro-apoptotic PRKCD gene. Oncogene. 2013;32(25):3019–3027. doi:10.1038/onc.2012.323
26. Basu AK, Damage DNA. Mutagenesis and Cancer. Int J Mol Sci. 2018;19(4). doi:10.3390/ijms19040970
27. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(4):928–942. doi:10.1016/j.ijrobp.2004.03.005
28. An Z, Muthusami S, Yu JR, Park WY. T0070907, a PPAR gamma inhibitor, induced G2/M arrest enhances the effect of radiation in human cervical cancer cells through mitotic catastrophe. Reprod Sci. 2014;21(11):1352–1361. doi:10.1177/1933719114525265
29. Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344(6266):503–508. doi:10.1038/344503a0
30. Sung WW, Lin YM, Wu PR, et al. High nuclear/cytoplasmic ratio of Cdk1 expression predicts poor prognosis in colorectal cancer patients. Bmc Cancer. 2014;14:951. doi:10.1186/1471-2407-14-951
31. Chen S, Chen X, Xiu YL, Sun KX, Zhao Y. MicroRNA-490-3P targets CDK1 and inhibits ovarian epithelial carcinoma tumorigenesis and progression. Cancer Lett. 2015;362(1):122–130. doi:10.1016/j.canlet.2015.03.029
32. Kori M, Yalcin AK. Potential biomarkers and therapeutic targets in cervical cancer: insights from the meta-analysis of transcriptomics data within network biomedicine perspective. PLoS One. 2018;13(7):e200717. doi:10.1371/journal.pone.0200717
33. Jayanthi V, Das AB, Saxena U. Grade-specific diagnostic and prognostic biomarkers in breast cancer. Genomics. 2019. doi:10.1016/j.ygeno.2019.03.001
34. Alexandrou AT, Li JJ. Cell cycle regulators guide mitochondrial activity in radiation-induced adaptive response. Antioxid Redox Signal. 2014;20(9):1463–1480. doi:10.1089/ars.2013.5684
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.