Back to Journals » OncoTargets and Therapy » Volume 12
SOX5 promotes cell invasion and metastasis via activation of Twist-mediated epithelial–mesenchymal transition in gastric cancer
Authors You J, Zhao Q, Fan X, Wang J
Received 5 December 2018
Accepted for publication 15 February 2019
Published 3 April 2019 Volume 2019:12 Pages 2465—2476
DOI https://doi.org/10.2147/OTT.S197087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Jianxiong You, Qing Zhao, Xindong Fan, Jingbing Wang
Department of Interventional Radiotherapy, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
Background: Sex-determining region Y-box protein 5 (SOX5) has been demonstrated to be implicated in oncogenic function in various types of cancers. However, the role of SOX5 in gastric cancer (GC) remains poorly elucidated. Herein, we investigated the role and the underlying mechanism of SOX5 in GC progression.
Methods: SOX5 mRNA and protein expression were detected by quantitative real-time PCR (qRT-PCR), Western blot and immunohistochemistry in human GC specimens, and their clinical significance was evaluated. The effects of SOX5 knockdown or overexpression on GC cell behavior were determined by proliferation, wound-healing and transwell assays in vitro, and metastasis assays in vivo; and epithelial–mesenchymal transition (EMT)-related markers were detected by qRT-PCR, Western blot and immunofluorescence staining.
Results: The up-regulated expression of SOX5 in GC specimens was significantly correlated with clinical metastasis and poor prognosis for patients with GC. Besides, SOX5 promoted GC cell migration and invasion in vitro, as well as GC cell metastasis in vivo. Mechanically, Twist-mediated EMT was likely involved in SOX5-facilitated GC cell behavior.
Conclusion: SOX5 has an important function in GC progression. In addition, SOX5 promotes GC cell invasion and metastasis via activation of Twist-mediated EMT, thus providing a potential therapeutic target for GC metastasis.
Keywords: SOX5, gastric cancer, epithelial–mensenchymal transition
Introduction
Gastric cancer (GC) is one of the most common malignant tumors, with the fifth highest incidence and the third highest mortality of all malignant tumors.1 Despite significant progress having been made in the treatment of GC in recent decades, metastasis after curative resection remains a major challenge for GC treatment.2 The underlying molecular mechanisms responsible for GC metastasis have yet to be sufficiently elucidated. Therefore, the identification of novel metastasis genes and the molecular mechanisms underlying the metastatic progression may provide potential therapeutic strategies for GC.
Growing evidence has demonstrated that epithelial–mesenchymal transition (EMT) plays a key role in the initial stage of cancer metastatic progression, endowing cancer cells with migratory and invasive properties.3 EMT describes the process whereby epithelial cells lose cell–cell adhesion and develop a mesenchymal phenotype that defines the shape of the cells and reprograms gene expression. This switch increases the motility of individual cells and enables the development of an invasive phenotype, which contributes to the spread of cancer cells to distant organs during cancer metastasis.4 Molecularly, EMT involves multiple complex changes in the distribution and function of proteins, including the inactivation of the epithelial marker E-cadherin and the activation of the mesenchymal marker vimentin. This process is regulated by a complex network of interconnected signaling cascades, as well as transcription factors such as Snail, Slug, ZEB1, ZEB2 and Twist.5
Sex-determining region Y-box protein 5 (SOX5), a member of the SOX family of transcription factors, has an important function in the regulation of embryonic development and determination of cell fate.6 Recent studies of SOX5 function have focused mainly on tumor progression in various cancers, including hepatocellular carcinoma (HCC), glioma and breast cancer.7–9 For example, research shows that SOX5 contributes to prostate cancer (PCa) metastasis via TGF-β-mediated EMT, resulting in an unfavorable prognosis in patients with PCa. In PCa, SOX5 is observably increased in primary PCa tissues compared with that in matched non-tumor normal tissue, and has an instrumental role in PCa invasion and metastasis.10 In HCC, gain- and loss-of-function studies reveal that SOX5 promotes HCC cell migration and invasion, describing SOX5 as a potential therapeutic target for HCC metastasis.9 Furthermore, SOX5 is found to display a remarkable up-regulation expression in primary lung cancer tissues, and act as a tumor metastasis-promoting gene in lung cancer, as evidenced by its overexpression facilitating cell invasion and metastasis, whereas its down-regulation led to the opposite outcome.11 However, to our knowledge, the expression and biological role of SOX5 in GC have not been characterized, and even less is known about the mechanisms of SOX5 with respect to GC invasion and metastasis.
Herein, we examined the expression of SOX5 in GC tissues and the correlation to the clinicopathological features. In addition, we investigated the effects of SOX5 on GC cell invasion and metastasis, as well as the regulatory mechanism of SOX5-induced GC progression.
Materials and methods
Tissue specimens and cell lines
A total of 96 primary GC tissues and adjacent normal tissues were obtained from patients with gastric adenocarcinoma at Shanghai Ninth People’s Hospital (Shanghai, People’s Republic of China). All participants who underwent radical resection had not received preoperative chemotherapy or radiotherapy before enrollment. Among them, 48 cases were evaluated for SOX5 mRNA expression by quantitative real-time PCR (qRT-PCR) and SOX5 protein expression by Western blot analysis. All samples were subjected to immunohistochemical staining. Written informed consent was obtained from each patient in accordance with the Declaration of Helsinki, and this study was approved by the Ethics Committee of Shanghai Ninth People’s Hospital.
The human GC cells (MGC803, SGC7901, BGC823, AGS, HGC27, KATO-III and MKN45) and normal gastric epithelial GES-1 cells were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). KATO-III cells were cultured in 80% Iscove’s Modified Dulbecco’s Medium (IMDM) (ATCC, Manassas, VA, USA) supplemented with 20% FBS (Thermo Fisher Scientific, Waltham, MA, USA). The other cell lines were maintained in 90% RPMI-1640 (Thermo Fisher Scientific) supplemented with 10% FBS (Thermo Fisher Scientific). All cells were propagated at 37°C in a humidified atmosphere with 5% CO2.
Lentivirus infection
SOX5-specific short hairpin RNA (shRNA) was chemically synthesized (Genecopoeia, Rockville, MD, USA) to knockdown endogenous SOX5 expression in GC cells. Negative control-shRNA (shNC) was used as a control. Cells were transfected using Lipofectamine 2000 according to the manufacturer’s protocol (Thermo Fisher Scientifc). Stable overexpression of SOX5 was determined using the lentiviral expression system (Thermo Fisher Scientific).9 The empty vector was employed as a control. The infection efficiency was evaluated by Western blot analysis.
qRT-PCR
Total RNA was extracted from GC tissues or GC cells using the TRIzol reagent (Thermo Fisher Scientific) and reverse transcribed to cDNA using the PrimeScript RT Reagent Kit (TaKaRa, Shanghai, People’s Republic of China) according to the manufacturer’s protocol. qRT-PCR was performed to determine the mRNA expression levels of SOX5 and EMT-related markers using the SYBR Green assay kit (Takara, Kusatsu, Japan) on a 7500 RT-PCR system (Applied Biosystems, Waltham, MA, USA). Primers are summarized in Table S1.
Western blot
In brief, equal amounts of protein extracted from tissue or cell lysate were separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). Transferred blots were incubated with a primary antibody at 4°C overnight, followed by a secondary antibody. Immunoreactive bands were detected by the enhanced chemiluminescence detection reagent (Thermo Fisher Scientific). The primary antibody against SOX5 was obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Primary antibodies against E-cadherin, vimentin, Snail, Slug, ZEB1, ZEB2 and Twist were purchased from Cell Signaling Technology (Danvers, MA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was purchased from Bioworld Technology (St Louis Park, MN, USA).
Immunohistochemistry (IHC)
In brief, IHC analysis was carried out to detect SOX5 expression in GC tissues and matched normal tissues. The paraffin-embedded slides were deparaffinized, rehydrated and heat-treated for antigen retrieval. Then, slides were blocked with hydrogen peroxide and blocking serum and incubated with a primary antibody at 4°C overnight, followed by biotin-conjugated anti-IgG serum (Boster Biological Technology, Pleasanton, CA, USA) and a streptavidin–biotin complex (SABC) solution. Finally, slides were observed under the microscope in a blinded manner.
IHC staining of SOX5 was evaluated by the sum of the intensity scores and the area scores. The staining intensity was scaled as 0 for no IHC signal, 1 for weak, 2 for moderate and 3 for strong. The staining area was set as 0 for 0%–5%, 1 for 6%–25%, 2 for 26%–50% and 3 for >50%. Sum scores <3 points were defined as negative, while sum scores ≥3 points were considered positive.
Wound-healing assay
Cells without serum were seeded in a six-well plate to grow into a confluent monolayer. An artificial linear wound was created using a sterile 10 μL plastic tip, followed by a wash to remove detached cells. Photomicrographs were taken at the appropriate time-points (0 and 24 hours) to assess the remaining distance. The wound-healing percentage = (0-hour width − 24-hour width)/0-hour width ×100%.
Cell migration and invasion assays
For the migration assay, cells were seeded into the top chamber of a 24-well 8 μm pore-size transwell plate (Corning Incorporated, Corning, NY, USA). For the invasion assay, cells were seeded into a Matrigel-coated chamber (BD Biosciences, San Jose, CA, USA). Medium supplemented with 20% FBS served as the chemoattractant and was added to the bottom chamber. After 24 hours of culture, cells on the lower surface of the filters were fixed with 4% paraformaldehyde and stained with 0.05% crystal violet solution. Average cells from five random fields were counted under an inverted light microscope.
Proliferation assay
In brief, GC cells (1×104 cells/well) were seeded in a six-well plate. At the indicated times (0, 1, 2, 3 and 4 days after culture), 10 μL of Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) solution was added to each well and cultured for another 80 minutes. Then, the absorbance was measured at 450 nm to calculate cell growth rates following the manufacturer’s protocol.
Immunofluorescence staining
In brief, cells were fixed in 4% paraformaldehyde for 15 minutes and permeabilized with Triton X-100 for 30 minutes. Then, cells were blocked with 5% BSA for 1 hour at room temperature, followed by incubation with primary antibodies against E-cadherin and vimentin at 4°C overnight. Subsequently, cells were incubated with a secondary antibody for 30 minutes and stained with DAPI for another 10 minutes. Finally, images were captured under a fluorescence microscope.
Animal experiments
To determine the function of SOX5 in tumor metastasis in vivo, MGC803 cells (shNC/shSOX5) or AGS cells (Vector/SOX5) were injected intravenously into the tail vein of male BALB/c nude mice (aged 6 weeks). At 30 days after the injection, the mice were killed and their lungs were examined for tumor metastases using H&E staining. The in vivo experiments were approved by the Ethics Committee of Shanghai Ninth People’s Hospital, and were conducted according to the Declaration of Helsinki.
Statistical analyses
SOX5 expression and clinicopathological features were compared using the chi-squared test. The Kaplan–Meier survival curve was assessed by the log-rank test. Other data are presented as mean±SD, and were analyzed by Student’s t-test. All statistical analyses were performed using SPSS 21.0 software (IBM Corporation, Armonk, NY, USA). P<0.05 was considered statistically significant.
Results
High SOX5 expression predicts poor outcomes in patients with GC
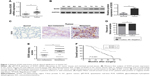
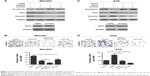
To explore the function of SOX5 in the development of GC, we investigated SOX5 expression in GC samples. As shown in Figure 1A, the qRT-PCR result revealed that the relative SOX5 mRNA expression was significantly higher in tumor samples compared with matched normal tissues in 48 pairs of cases. The Western blot analysis of SOX5 protein expression indicated a similar outcome (Figure 1B). Meanwhile, IHC analysis of SOX5 in all cases demonstrated that SOX5 expression was markedly increased in tumor tissues compared to normal tissues (Figure 1C and D), and its expression was significantly higher in GC patients with lymph-node metastasis (Figure 1C and E), suggesting that SOX5 may have an important function in metastasis. In addition, as shown in Table 1, SOX5 expression was significantly associated with T stage, pTNM stage and lymph-node metastasis (P<0.05). Statistical analyses further demonstrated that high SOX5 expression correlated with poor overall survival (P<0.05) (Figure 1F).
SOX5 expression in GC cell lines
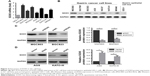
To select the appropriate GC cells for subsequent studies, we examined the expression of SOX5 in GC cell lines (MGC803, SGC7901, BGC823, AGS, HGC27, KATO-III and MKN45) and normal gastric epithelial GES-1 cells. Compared to GES-1 cells, SOX5 mRNA and protein expression was distinctly elevated in GC cells. Among them, MGC803 and BGC823 cells exhibited the highest level of SOX5 mRNA and protein expression. AGS and KATO-III cells showed the opposite results (Figure 2A and B). Subsequently, MGC803 and BGC823 cells were selected for transfection with shRNA lentivirus vector toward SOX5 (Figure 2C). AGS and KATO-III cells were selected for transfection with SOX5-overexpression vector (Figure 2D).
SOX5 promotes GC cell migration and invasion in vitro and metastasis in vivo
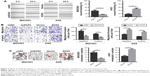
To investigate the potential effects of SOX5 on GC cell invasion and metastasis, we established stable SOX5 knockdown (MGC803-shSOX5) and overexpression (AGS-SOX5) cells for subsequent studies. The wound-healing assay indicated that SOX5-overexpressing AGS cells exhibited a significantly more extensive wound closure area, compared with the respective control, whereas SOX5-silencing MGC803 cells showed weaker migration capability (Figure 3A). This result was confirmed by the transwell migration assay (Figure 3B). As expected, the transwell invasion assay revealed a similar effect on GC cell invasion (Figure 3B). Thus, we concluded that SOX5 facilitated GC cell migration and invasion in vitro.
To further examine the in vivo effect of SOX5 on GC cell metastasis, the lung metastasis model was established via the injection of GC cells into the tail vein in nude mice. We found that SOX5 silencing suppressed lung metastasis, as evidenced by a significantly lower number of lung metastatic nodules. Conversely, SOX5 overexpression led to the opposite effect (Figure 3C). Taken together, these findings were consistent with the in vitro results, suggesting that SOX5 promoted GC cell metastasis in vivo.
To exclude the possibility that the effects of SOX5 on cell migration and invasion were attributable to different proliferation rates, the cellular growth rates in the two groups were compared. As shown in Figure 4A, all cells exhibited similar growth rates under the same conditions in vitro. Consistently, the in vivo results showed that SOX5 knockdown or overexpression did not affect GC cell growth (Figure 4B), suggesting that SOX5 has little impact on GC cell proliferation.
SOX5 promotes GC cell invasion through Twist-induced EMT
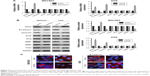
Since the activation of EMT has been demonstrated to strongly facilitate tumor cell metastasis in several cancers, including GC,5,12 we examined the association between SOX5 expression and EMT. The qRT-PCR result revealed that depletion of SOX5 in MGC803 cells led to the decreased expression of vimentin and Twist, and the increased expression of E-cadherin. Conversely, overexpression of SOX5 in AGS cells resulted in the opposite outcome (Figure 5A). In addition, Western blot and immunofluorescence staining further confirmed that SOX5 negatively correlated with E-cadherin but positively correlated with Twist and vimentin in GC cells (Figure 5B and C). Thus, we reasoned that SOX5 may contribute to GC cell migration and invasion via activation of EMT.
Next, to further investigate the interval molecular mechanism of SOX5 involving EMT, the transcription factor Twist was stably down-regulated in GC cells. The results demonstrated that shTwist enhanced shSOX5-induced up-regulation of E-cadherin and down-regulation of vimentin, but did not alter SOX5 expression (Figure 6A). Meanwhile, shTwist substantially potentiated shSOX5-inhibited MGC803 cell invasion (Figure 6B). Besides, shTwist resulted in a noticeable reversal of SOX5-mediated EMT and reverted SOX5-facilitated AGS cell invasion (Figure 6C and D). Collectively, these data suggest that SOX5 could promote GC cell invasion via activation of Twist-mediated EMT.
Discussion
GC remains a highly prevalent disease with poor clinical outcomes, and most afflicted individuals do not survive owing to tumor metastasis.2,13 Given that the transition of cancer cells from a relatively immobile type to a more invasive cell phenotype is increasingly accepted as a major cause in tumor metastasis, novel therapeutic strategies are urgently needed to control the metastatic dissemination of cancer cells.4,14 Therefore, the identification of cancer-specific cellular targets and a better understanding of the underlying mechanisms involved in cancer cell metastasis may represent such a strategy. Recent studies demonstrated that SOX5 was highly expressed in many human malignancies and was significantly related to invasion and metastasis of tumors.7–10 Nevertheless, there is little evidence to elucidate the clinical significance and the role of SOX5 in GC tissues.
In the current study, we presented evidence that SOX5 may serve as a tumor metastasis-promoting gene in patients with GC. We showed that SOX5 had an important function in the aggressive behavior of GC, and our data suggested that patients with high SOX5 expression were more likely to suffer from metastases with a lower progression-free survival. In addition, we found that depletion of endogenous SOX5 expression attenuated GC cell invasion and metastasis, while SOX5 overexpression dramatically reversed these events. Mechanistically, we demonstrated that SOX5 accelerated metastatic effects, at least partly via induction of EMT through the up-regulation of Twist.
As mentioned in the Introduction, EMT is a process involved in the early stage of the metastatic cascade, conferring the cellular conversion to a more invasive cell phenotype in numerous human malignancies. In light of the clinical importance of this step, the need to develop novel therapeutic strategies to restrain this process in cancer is apparent. Nevertheless, key questions remain regarding effective strategies for targeting the EMT pathway to prevent cancer metastasis. Hence, great efforts have been made to identify novel targeted biomarkers that promote cancer metastasis via the aberrant activation of EMT.15,16 For example, our findings implicated SOX5 as a crucial regulator of EMT in GC cell invasion and metastasis. This conclusion is based on the results that SOX5 silencing resulted in the down-regulation of vimentin and the up-regulation of E-cadherin, whereas SOX5 overexpression indicated the opposite effects. In addition, a well-known function of transcription factors (such as Snail, Slug, ZEB1, ZEB2 and Twist) is that they serve as key regulators of EMT in many types of human cancer and are frequently overactivated, thus controlling cell invasion and metastasis.17 Notably, the present study proved that SOX5 knockdown could significantly decrease Twist expression and in turn reverse EMT, thereby suppressing GC cell migration and invasion. Conversely, SOX5 overexpression led to the opposite outcomes. However, no changes in the expression of the other EMT transcription factors (Snail, Slug, ZEB1 and ZEB2) were observed when SOX5 expression was changed.
As confirmation that the transcription factor Twist was involved in SOX5-mediated EMT, endogenous Twist expression was stably silenced in GC cells, which not only weakened SOX5-induced EMT and cell invasion, but also strengthened shSOX5-inhibited EMT and cell invasion. Taking these results together, it seems reasonable that SOX5 promotes GC cell invasion and metastasis, at least partly via Twist-regulated EMT. Although Twist served as a downstream substrate in the process of SOX5-mediated GC cell migration and invasion, the accurate mechanism of how SOX5 exerts an influence on Twist remains to be thoroughly characterized. Hence, it is worth exploring the complex interaction between SOX5 and Twist in future research.
Conclusion
In summary, our data demonstrated that SOX5 was significantly overexpressed in GC tissues, and associated with poor pathological characteristics and an unfavorable prognosis in patients with GC. Our findings also indicated that SOX5 facilitated GC cell invasion and metastasis via activation of Twist-mediated EMT, thus possibly providing a valuable candidate for treatment to suppress GC metastasis.
Acknowledgment
This work was supported in part by funding from the National Natural Science Foundation of China (No. 81871458) and the State Key Laboratory of Molecular Engineering of Polymers at Fudan University (No. K2017-03).
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442 | ||
Gurzu S, Jung I, Orlowska J, et al. Hereditary diffuse gastric cancer-an overview. Pathol Res Pract. 2015;211(9):629–632. doi:10.1016/j.prp.2015.06.003 | ||
Heerboth S, Housman G, Leary M, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6. doi:10.1186/s40169-015-0048-3 | ||
Davis FM, Stewart TA, Thompson EW, Monteith GR. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends Pharmacol Sci. 2014;35(9):479–488. doi:10.1016/j.tips.2014.06.006 | ||
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi:10.1038/nrm3758 | ||
She ZY, Yang WX. SOX family transcription factors involved in diverse cellular events during development. Eur J Cell Biol. 2015;94(12):547–563. doi:10.1016/j.ejcb.2015.08.002 | ||
Renjie W, Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015;356(2Pt B):568–578. doi:10.1016/j.canlet.2014.10.003 | ||
Ueda R, Yoshida K, Kawase T, Kawakami Y, Toda M. Preferential expression and frequent IgG responses of a tumor antigen, SOX5, in glioma patients. Int J Cancer. 2007;120(8):1704–1711. doi:10.1002/ijc.22472 | ||
Wang D, Han S, Wang X, Peng R, Li X. SOX5 promotes epithelial-mesenchymal transition and cell invasion via regulation of Twist1 in hepatocellular carcinoma. Med Oncol. 2015;32(2):461. | ||
Hu J, Tian J, Zhu S, et al. Sox5 contributes to prostate cancer metastasis and is a master regulator of TGF-β-induced epithelial mesenchymal transition through controlling Twist1 expression. Br J Cancer. 2018;118(1):88–97. doi:10.1038/bjc.2017.372 | ||
Chen X, Fu Y, Xu H, et al. SOX5 predicts poor prognosis in lung adenocarcinoma and promotes tumor metastasis through epithelial-mesenchymal transition. Oncotarget. 2017;9(13):10891–10904. doi:10.18632/oncotarget.22443 | ||
Li J, Zhen L, Zhang Y, et al. Circ-104916 is downregulated in gastric cancer and suppresses migration and invasion of gastric cancer cells. Onco Targets Ther. 2017;10:3521–3529. doi:10.2147/OTT.S136347 | ||
Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Gastric cancer. Crit Rev Oncol Hematol. 2009;71(2):127–164. doi:10.1016/j.critrevonc.2009.01.004 | ||
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi:10.1126/science.1203543 | ||
Huang Y, Chen Y, Lin X, Lin Q, Han M, Guo G. Clinical significance of SLP-2 in hepatocellular carcinoma tissues and its regulation in cancer cell proliferation, migration, and EMT. Onco Targets Ther. 2017;10:4665–4673. doi:10.2147/OTT.S144638 | ||
Su D, Liu Y, Song T. Knockdown of IQGAP1 inhibits proliferation and epithelial-mesenchymal transition by Wnt/β-catenin pathway in thyroid cancer. Onco Targets Ther. 2017;10:1549–1559. doi:10.2147/OTT.S128564 | ||
Goossens S, Vandamme N, Van Vlierberghe P, Berx G. EMT transcription factors in cancer development re-evaluated: beyond EMT and MET. Biochim Biophys Acta Rev Cancer. 2017;1868(2):584–591. doi:10.1016/j.bbcan.2017.06.006 |
Supplementary material
  | Table S1 Primers designed for qRT-PCR |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.