Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 8
Sotagliflozin improves glycemic control in nonobese diabetes-prone mice with type 1 diabetes
Authors Powell D , Doree D, Jeter-Jones S, Ding Z, Zambrowicz B, Sands A
Received 24 October 2014
Accepted for publication 2 December 2014
Published 26 February 2015 Volume 2015:8 Pages 121—127
DOI https://doi.org/10.2147/DMSO.S76342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ming-Hui Zou
David R Powell, Deon Doree, Sabrina Jeter-Jones, Zhi-Ming Ding, Brian Zambrowicz, Arthur Sands
Lexicon Pharmaceuticals, The Woodlands, TX, USA
Purpose: Oral agents are needed that improve glycemic control without increasing hypoglycemic events in patients with type 1 diabetes (T1D). Sotagliflozin may meet this need, because this compound lowers blood glucose through the insulin-independent mechanisms of inhibiting kidney SGLT2 and intestinal SGLT1. We examined the effect of sotagliflozin on glycemic control and rate of hypoglycemia measurements in T1D mice maintained on a low daily insulin dose, and compared these results to those from mice maintained in better glycemic control with a higher daily insulin dose alone.
Materials and methods: Nonobese diabetes-prone mice with cyclophosphamide-induced T1D were randomized to receive one of four daily treatments: 0.2 U insulin/vehicle, 0.05 U insulin/vehicle, 0.05 U insulin/2 mg/kg sotagliflozin or 0.05 U insulin/30 mg/kg sotagliflozin. Insulin was delivered subcutaneously by micro-osmotic pump; the day after pump implantation, mice received their first of 22 once-daily oral doses of sotagliflozin or vehicle. Glycemic control was monitored by measuring fed blood glucose and hemoglobin A1c levels.
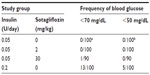
Results: Blood glucose levels decreased rapidly and comparably in the 0.05 U insulin/sotagliflozin-treated groups and the 0.2 U insulin/vehicle group compared to the 0.05 U insulin/vehicle group, which had significantly higher levels than the other three groups from day 2 through day 23. A1c levels were also significantly higher in the 0.05 U insulin/vehicle group compared to the other three groups on day 23. Importantly, the 0.2 U insulin/vehicle group had, out of 100 blood glucose measurements, 13 that were <70 mg/dL compared to one of 290 for the other three groups combined.
Conclusion: Sotagliflozin significantly improved glycemic control, without increasing the rate of hypoglycemia measurements, in diabetic mice maintained on a low insulin dose. This sotagliflozin-mediated improvement in glycemic control was comparable to that achieved by raising the insulin dose alone, but was not accompanied by the increased rate of hypoglycemia measurements observed with the higher insulin dose.
Keywords: insulin, glucose, hypoglycemia, hemoglobin A1c
Introduction
In the US, 30,000 people are diagnosed with type 1 diabetes (T1D) each year, and the incidence in children is steadily increasing.1,2 T1D results from profound insulin deficiency secondary to autoimmune destruction of insulin-producing pancreatic β-cells and insulin replacement can return these patients to a euglycemic state.2 However, the majority of T1D patients are not achieving blood hemoglobin A1c (A1c) targets chosen to minimize diabetic complications, thus risking the microvascular and macrovascular side effects that accompany chronic hyperglycemia.2,3 The main reason for this failure to optimize glycemic control is the lack of simple regimens that allow delivery of sufficient insulin to maintain euglycemia without significantly increasing the risk of severe hypoglycemic events; such events increase with age and duration of diabetes, and are responsible not only for significant morbidity but also for the death of between 4% and 10% of individuals with T1D,2–9 observations that underscore the need to develop these new regimens.
Sotagliflozin, also known as LX4211, is an orally available small molecule that has consistently improved glycemic control in patients with T2D in previous clinical studies.10–12 Sotagliflozin lowers blood glucose by inhibiting both SGLT1 to delay glucose absorption by the intestine, and SGLT2 to decrease glucose reabsorption by the kidney.10,11,13,14 By working through these two insulin-independent pathways, sotagliflozin may have the ability to improve glycemic control and decrease the frequency of extreme blood glucose excursions in patients with T1D, and potentially achieve these effects with a lower but still metabolically adequate daily dose of bolus insulin. This study was designed to test whether LX4211 could improve glycemic control in nonobese diabetes-prone (NOD) mice with poorly controlled T1D on a low daily dose of insulin, and how the improved glycemic control and frequency of hypoglycemic measurements compared to that observed in NOD mice with better-controlled T1D on a higher daily insulin dose.
Materials and methods
Mouse care and study
All mouse studies were performed at Lexicon Pharmaceuticals, and were approved by the Lexicon Institutional Animal Care and Use Committee. All institutional and national guidelines for the care and use of laboratory animals were followed. Female NOD/ShiLtJ mice (001976; The Jackson Laboratory, Bar Harbor, ME, USA), obtained at 5 weeks of age, were housed four per cage at 24°C on a fixed 12-hour light/12-hour dark cycle, and had ad libitum access to water and rodent chow (5001; Purina, St Louis, MO, USA). Body weight was measured on day –1 (the day before the first sotagliflozin dose), and then daily throughout the study.
Induction of diabetes
Cyclophosphamide has been shown to rapidly promote a high incidence of diabetes in NOD mice.15,16 At 11 weeks of age, 70 mice had blood drawn for baseline A1c, and then received 200 mg/kg of cyclophosphamide (Sigma-Aldrich, St Louis, MO, USA) by intraperitoneal injection. All mice were screened for blood glucose levels three times weekly; by 13 weeks of age, 25 mice with two consecutive blood glucose levels >300 mg/dL were diagnosed as diabetic and entered into the study, and the remaining mice received a second 200 mg/kg intraperitoneal dose of cyclophosphamide. By 15 weeks of age, an additional 14 mice (39 total) had two consecutive blood glucose levels >300 mg/dL, were diagnosed as diabetic, and were entered into the study.
Sotagliflozin treatment
Diabetic mice were randomized by body weight and blood glucose level into four treatment groups, as shown in Table 1. On day –1, while under isoflurane anesthesia, all mice were implanted subcutaneously with an Alzet micro-osmotic pump (model 1004, 4 weeks’ duration; Durect, Cupertino, CA, USA) delivering insulin in the form of Humulin R (Eli Lilly, Indianapolis, IN, USA) at doses of either 0.05 U/day or 0.2 U/day; these doses were determined in preliminary studies (data not shown). On the next day (study day 1), all mice received their first daily dose of either vehicle (0.1% Tween 80 in water) alone or vehicle containing sotagliflozin, chemical structure (2S,3R,4R,5S,6R)-2-(4-chloro-3-[4-ethoxybenzyl]phenyl)-6-(methylthio)tetrahydro-2H-pyran-3,4,5-triol, which was synthesized by Lexicon Pharmaceuticals.10 Sotagliflozin was delivered once in the afternoon by oral gavage at a dose of either 2 mg/kg or 30 mg/kg in a 10 mL/kg total volume; these doses were chosen because in past mouse studies, 2 mg/kg had a half-maximal effect and 30 mg/kg a maximal effect on urinary glucose excretion.14
Blood-chemistry analyses
All blood samples were collected on conscious, unanesthetized mice. Glucose levels were measured by an Accu-Chek Aviva glucometer (Roche Diagnostics, Indianapolis, IN, USA) on whole blood samples obtained by tail nick; off-scale high readings were assigned a glucose level of 601 mg/dL. A1c was measured on whole blood samples collected by retro-orbital bleed, using the A1c Now+ System (Bayer HealthCare, Sunnyvale, CA, USA) as described previously.14 Serum samples, obtained from whole blood samples collected by retro-orbital bleed, were assayed for insulin using the Ultra Sensitive Mouse Insulin ELISA Kit (90080; Crystal Chem, Downers Grove, IL, USA) which recognizes endogenous and exogenous insulin, and for β-hydroxybutyrate using the β-Hydroxybutyrate LiquiColor Kit (SBHR-100; Stanbio Laboratory, Boerne, TX, USA).
Statistics
Results are presented as mean ± standard deviation. Analyses of A1c values were performed by one-way analysis of variance (ANOVA), with post hoc analysis performed by the Bonferroni method. Daily glucose levels and daily change from baseline body weight were analyzed by repeated-measures ANOVA, with post hoc analysis performed by the Bonferroni method. Insulin and β-hydroxybutyrate levels were analyzed using the Kruskal–Wallis test, with post hoc analysis performed with Dunn’s multiple-comparison test. All statistical tests were performed using Prism 4.03 (GraphPad). Differences were considered statistically significant when P<0.05.
Results
Preliminary studies using micro-osmotic pumps to deliver insulin to NOD mice with T1D showed: 1) all mice receiving at least 0.02 U insulin/day appeared healthy and survived the 4-week study, while >40% of mice that did not receive insulin were euthanized because they appeared quite ill; 2) mice receiving 0.05 U insulin/day maintained comparable high blood glucose levels, but with less body weight loss, when compared to mice receiving 0.02 U insulin/day; and 3) mice receiving 0.2 U insulin/day had lower blood glucose levels than mice receiving 0.1 U insulin/day, and had far fewer measurements of blood glucose <70 mg/dL and <50 mg/dL than mice receiving 0.25 U insulin/day (data not shown). Based on these data, we delivered insulin doses of 0.05 U/day to maintain diabetic NOD mice in poor glycemic control, and 0.2 U/day to maintain diabetic NOD mice in good glycemic control.
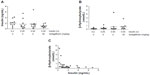
Of the 70 NOD mice treated with cyclophosphamide, 39 developed diabetes and were randomized to one of the four experimental groups (Table 1). As shown in Figure 1, body weight was stable over the course of the study in the 0.2 U insulin/vehicle group designed to maintain good glycemic control, but was significantly decreased in the 0.05 U insulin/vehicle group designed to maintain poor glycemic control. Decreased body weight was also observed in the two groups receiving 0.05 U insulin and sotagliflozin, relative to the 0.2 U insulin/vehicle group; this decrease was significant in the group receiving 2 mg/kg, but not in the group receiving 30 mg/kg, of sotagliflozin. All mice survived to the end of the 23-day study.
Fed blood glucose levels were measured frequently over the course of the study. As shown in Figure 2, glucose levels remained quite high throughout the study for the 0.05 U insulin/vehicle group. In contrast, glucose levels in the 0.2 U insulin/vehicle group were significantly lower than those of the poorly controlled 0.05 U insulin/vehicle group by day 2 of insulin treatment, and remained significantly lower throughout the study. Among the three groups of mice receiving 0.05 U insulin/day, glucose levels were significantly lower in the two groups receiving sotagliflozin compared to the poorly controlled group receiving vehicle by day 2 of insulin treatment, and remained significantly lower throughout the study. In general, glucose levels appeared lowest and roughly comparable for the 0.2 U insulin/vehicle group and the 0.05 U insulin/30 mg/kg sotagliflozin group, and appeared somewhat higher for the group receiving 0.05 U insulin/2 mg/kg sotagliflozin; however, there were no significant differences in glucose levels among these three groups on any study day. To evaluate the rate of low blood glucose measurements among the four groups of mice, all glucose measurements <70 mg/dL and <50 mg/dL were counted for each group. As shown in Table 2, measurements <70 mg/dL occurred 13 of 100 times in the 0.2 U insulin/vehicle group and only one of 290 times in the other three groups, while measurements <50 mg/dL occurred five of 100 times in the 0.2 U insulin/vehicle group and zero of 290 times in the other three groups.
The effect of the four treatments on A1c levels is shown in Figure 3. Over the course of the study, A1c levels rose significantly more in the 0.05 U insulin/vehicle group than in each of the other three groups. There was no difference in final A1c levels, or in the change in A1c levels over the course of the study, among the 0.2 U insulin/vehicle group and the two groups receiving 0.05 U insulin and sotagliflozin.
The relative stability of glucose levels over time for all groups suggests that the micro-osmotic pumps delivered insulin throughout the study consistent with the presence of measurable insulin levels in all mice on the last study day (Figure 4A). β-Hydroxybutyrate levels were variable, within the normal range, and not significantly different among the four groups (Figure 4B). In the single mouse with β-hydroxybutyrate level >3.8 nmol/L on the last study day (day 23), the accompanying serum insulin level of 0.28 ng/mL was the lowest value recorded in all four groups, and the blood glucose level of 510 mg/dL was among the highest values recorded. As shown in Figure 4B and C, the three mice with β-hydroxybutyrate levels >1 nmol/L on day 23 had very low accompanying insulin levels.
Discussion
Sotagliflozin significantly lowered fed blood glucose levels in NOD mice with poorly controlled T1D. The response was rapid, appearing to be maximal by day 2, and lasted through the remainder of the study. These data on fed blood glucose are consistent with the significantly slower rise in A1c observed when NOD mice with poorly controlled T1D were treated with sotagliflozin, and are reminiscent of the ability of sotagliflozin to lower blood glucose and A1c levels in humans and mice with T2D.10–12,14 These results are also reminiscent of the resistance of mice lacking SGLT2 to develop hyperglycemia despite insulin deficiency brought on by exposure to the β-cell toxin streptozotocin.17,18 In mice receiving 0.05 U insulin/day, the improved glycemic control that accompanied sotagliflozin treatment was not associated with an increased rate of hypoglycemia measurements. In addition, this improved glycemic control of sotagliflozin-treated mice receiving 0.05 U insulin/day was similar to the glycemic control of vehicle-treated mice receiving higher-dose insulin (0.2 U insulin/day), but was without the higher rate of hypoglycemia measurements observed in vehicle-treated mice receiving 0.2 U insulin/day. These results are consistent with the ability of sotagliflozin to lower blood glucose through two insulin-independent pathways: 1) by inhibiting renal SGLT2, which increases urinary glucose excretion, and 2) by inhibiting intestinal SGLT1, which delays glucose absorption.10,11,13,14
The NOD mice used in this study had a fairly typical response to cyclophosphamide, with 56% developing diabetes.14 The diabetes that develops in NOD mice has been shown to have an autoimmune basis similar to that present in humans with T1D, making the NOD-mouse model a mainstay for T1D research.19 Indeed, the NOD mice used in the present study required insulin treatment to be reasonably healthy, suggesting that this model can provide insight into the efficacy of sotagliflozin in patients with T1D. This contrasts with past data generated by studying the effects of sotagliflozin in KKAy mice, because KKAy mice are in fact hyperinsulinemic and represent a valuable rodent model of T2D, not T1D.14
Delivering insulin by micro-osmotic pump at a rate of 0.2 U/day over 23 days provided better control of body weight and levels of fed blood glucose and A1c than did delivery at a rate of 0.05 U/day, consistent with our preliminary data. In NOD mice with T1D, the blood glucose levels and rate of low blood glucose measurements observed when we delivered insulin at 0.2 U/day were very similar to those recently observed by others delivering insulin by micro-osmotic pump at the same rate;20 in addition, it was observed that delivering insulin at a rate of 0.25 U/day led to lower glucose levels, but more hypoglycemia measurements, than a rate of 0.2 U/day,20 similar to our experience. The insulin dose of 0.2 U/day appears to be a good compromise between normalizing glucose levels and minimizing the frequency of hypoglycemia measurements.
The greater body-weight loss of the three groups receiving 0.05 U insulin/day relative to the group receiving 0.2 U insulin/day stabilized over the last week of the study, and was not associated with increased levels of β-hydroxybutyrate, suggesting that the greater weight loss was not in general due to a deteriorating metabolic state associated with ketoacidosis. At the end of the study, all mice with β-hydroxybutyrate levels >1 nmol/L were receiving the lower insulin dose of 0.05 U/day and had very low serum insulin levels associated with high blood glucose levels; this observation suggests that the higher β-hydroxybutyrate levels were due to inadequate insulin release from the micro-osmotic pumps in these particular mice. The mouse with the lowest insulin level had the highest β-hydroxybutyrate level of 4.4 nmol/L, which suggests the presence of diabetic ketoacidosis.21,22 The fact that this mouse was receiving sotagliflozin suggests that sotagliflozin will not protect individuals with T1D maintained on an inadequate insulin dose from developing diabetic ketoacidosis; however, the very high blood glucose level of 510 mg/dL measured on the same day indicates that sotagliflozin was not masking the state of profound insulin deficiency present in this mouse.
The present work is limited by the small numbers of mice studied and by the short study duration. Also, we did not measure glucagon levels in the current study, because we did not observe a significant increase in glucagon levels in sotagliflozin-treated patients with T2D.10 However, data showing that treatment with selective SGLT2 inhibitors was associated with elevated glucagon levels in patients with T2D,23,24 published after our study was completed, suggest that future studies must carefully evaluate the effect of sotagliflozin on glucagon levels in individuals with T1D and T2D. In addition, we did not investigate the relative contribution of renal SGLT2 inhibition versus intestinal SGLT1 inhibition on the ability of sotagliflozin to improve glycemic control; in this study, we wanted to remain focused on whether sotagliflozin could improve glycemic control without increasing the frequency of hypoglycemia events in NOD mice with T1D, to help inform us of the value of proceeding with clinical trials of sotagliflozin in patients with T1D.
Conclusion
Sotagliflozin significantly improved the poor glycemic control of NOD mice with T1D maintained on a low insulin dose without increasing the rate of hypoglycemia measurements. This sotagliflozin-mediated improvement in glycemic control was comparable to that achieved by treating T1D NOD mice with a higher dose of insulin alone, but was not accompanied by the increased rate of hypoglycemia measurements observed with the higher insulin dose. Despite the limitations noted, the data presented here clearly support further study of sotagliflozin-mediated dual SGLT1/SGLT2 inhibition as a novel mechanism of action to improve glycemic control, while minimizing the frequency of hypoglycemic events in patients with T1D.
Acknowledgments
The authors wish to thank Paul Strumph, MD for many helpful discussions regarding our data, and Kristi Boehm, MS, ELS for her help in preparing the figures and tables. Sponsorship for this study and article-processing charges were provided by Lexicon Pharmaceuticals, Inc. These data were presented in part on June 15, 2014 at the 74th Annual Meeting of the American Diabetes Association held in San Francisco, CA, and on September 18, 2014 at the 50th Annual Meeting of the European Association for the Study of Diabetes held in Vienna, Austria.
Author contributions
All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosure
DRP is an employee of Lexicon Pharmaceuticals and owns stock; DD, SJJ and ZMD were employees of Lexicon Pharmaceuticals at the time the studies were performed; BZ is an employee of Lexicon Pharmaceuticals and owns stock; and AS was an employee of Lexicon Pharmaceuticals at the time the studies were performed, is currently a consultant for Lexicon Pharmaceuticals, and owns stock.
References
Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, 2011. | |
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. | |
Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA. The T1D Exchange clinic registry. J Clin Endocrinol Metab. 2012;97:4383–4389. | |
Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384–1395. | |
Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188–2195. | |
Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49:298–305. | |
Patterson CC, Dahlquist G, Harjutsalo V, et al. Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. Diabetologia. 2007;50:2439–2442. | |
Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group, Jacobson AM, Musen G, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842–1852. | |
Feltbower RG, Bodansky HJ, Patterson CC, et al. Acute complication and drug misuse are important causes of death for children and young adults with type 1 diabetes. Diabetes Care. 2008;31:922–926. | |
Zambrowicz B, Freiman J, Brown PM, et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther. 2012;92:158–169. | |
Zambrowicz B, Ding ZM, Ogbaa I, et al. Effects of LX4211, a dual SGLT1/SGLT2 inhibitor, plus sitagliptin on postprandial active GLP-1 and glycemic control in type 2 diabetes. Clin Ther. 2013;35: 273–285. | |
Rosenstock J, Cefalu W, Lapuerta P, et al. LX4211, a dual inhibitor of sodium glucose transporters SGLT1 and SGLT2, lowers A1c and improves cardiovascular risk factors in patients with type 2 diabetes: impact of LX4211 on diabetes CV risk factors. Diabetes Care. Epub 2014 Sep 11. | |
Powell DR, Smith M, Greer J, et al. LX4211 increases serum GLP-1 and PYY levels by reducing SGLT-1-mediated absorption of intestinal glucose. J Pharmacol Exp Ther. 2013;345:250–259. | |
Powell DR, DaCosta CM, Smith M, et al. Effects of LX4211 on glucose homeostasis and body composition in preclinical models. J Pharmacol Exp Ther. 2014;350:232–242. | |
Harada M, Makino S. Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia. 1984;27: 604–606. | |
Faleo G, Fotino C, Bocca N, et al. Prevention of autoimmune diabetes and induction of β-cell proliferation in NOD mice by hyperbaric oxygen therapy. Diabetes. 2012;61:1769–1778. | |
Powell DR, DaCosta C, Gay J, et al. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol. 2013;304:E117–E130. | |
Vallon V, Rose M, Gerasimova M, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol. 2013;304:F156–F167. | |
Chaparro RJ, DiLorenzo TP. An update on the use of NOD mice to study autoimmune (type 1) diabetes. Expert Rev Clin Immunol. 2010;6: 939–955. | |
Grant CW, Duclos SK, Moran-Paul CM, et al. Development of standardized insulin treatment protocols for spontaneous rodent models of type 1 diabetes. Comp Med. 2012;62:381–390. | |
Guerci B, Tubiana-Rufi N, Bauduceau B, et al. Advantages to using capillary blood beta-hydroxybutyrate determination for the detection and treatment of diabetic ketosis. Diabetes Metab. 2005;31:401–406. | |
Bresciani F, Pietra M, Corradini S, Giunti M, Fracassi F. Accuracy of capillary blood 3-β-hydroxybutyrate determination for the detection and treatment of canine diabetic ketoacidosis. J Vet Sci. 2014;15: 309–316. | |
Ferrannini E, Muscelli E, Frascerra S, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124:499–508. | |
Merovci A, Solis-Herrera C, Daniele G, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124:509–514. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.