Back to Journals » Drug Design, Development and Therapy » Volume 16
Sorafenib Attenuates Fibrotic Hepatic Injury Through Mediating Lysine Crotonylation
Received 30 March 2022
Accepted for publication 28 June 2022
Published 3 July 2022 Volume 2022:16 Pages 2133—2144
DOI https://doi.org/10.2147/DDDT.S368306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Xiao-Feng Chen, Shaoxiu Ji
School of Basic Medical Sciences, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
Correspondence: Xiao-Feng Chen, School of Basic Medical Sciences, Chengdu University of Traditional Chinese Medicine, No. 1166, Liutai Avenue, Wenjiang District, Chengdu, People’s Republic of China, Tel +86-28-61800219, Email [email protected]
Background: Liver fibrosis is an independent contributor of chronic liver diseases, and regressing liver fibrosis is considered a potential therapeutic target for chronic liver diseases. We aimed to explore the effects and mechanism of sorafenib in liver fibrosis.
Methods: Male Sprague Dawley (SD) rats were subjected to subcutaneous injection of carbon tetrachloride (CCl4) for 8 weeks to induce liver fibrosis and then treated with sorafenib. The degree of liver fibrosis was analyzed by hematoxylin–eosin (H&E) staining, Masson staining, and Picrosirius red (PSR) staining. Serum biochemical indexes were detected by fully automatic biochemical analyzer or enzyme-linked immunosorbent assay (ELISA). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to detect the expression of pro-fibrotic genes. Immunohistochemical staining and Western blotting were carried out to evaluate the levels of lysine crotonylation.
Results: Liver index was reduced with oral sorafenib in CCl4-induced rats. Serum liver function (alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL)) and fibrosis indicators (type III procollagen (PC-III), hyaluronic acid (HA), and laminin (LN)) were attenuated with sorafenib treatment. Sorafenib improved the hepatic structure and fibrotic progression. The expression of fibrosis-related genes was remarkely reduced with sorafenib treatment. Meanwhile, sorafenib inhibited α-SMA and collagen I cumulation induced by CCl4 injection. Besides, protein lysine crotonylation especially the crotonylated H2BK12 (H2BK12cr) and crotonylated H3K18 (H3K18cr) were reversed by sorafenib, which were decreased in response to CCl4 treatment. Spearman correlation analysis shown lysine crotonylation expression was negatively correlated with serum fibrotic indicators. Conversely, crotonylation-regulated enzymes, which negatively regulate protein crotonylation, were increased in response to CCl4 treatment, while sorafenib reduced their expression.
Conclusion: Sorafenib exerts significant anti-fibrotic effects through mediating crotonylation-regulated enzymes and protein crotonylation in fibrotic rats.
Keywords: liver fibrosis, sorafenib, crotonylation, epigenetic regulation
Introduction
Liver fibrosis is a wound healing reaction caused by viral hepatitis, alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), etc.1 The long term of hepatic fibrosis could develop into cirrhosis and even hepatocellular carcinoma (HCC), which threatens life span and life quality of people.2 Chronic liver injury leads to the overdeposition of extracellular matrix (ECM) in the liver, the sign of liver fibrosis. The hepatic stellate cells (HSCs) are the primary cells producing extracellular matrix (ECM).3 Various pathological stimuli activate quiescent hepatic stellate cells (HSCs) to generate superabundant ECM. Moreover, sustained inflammatory reaction and fibrotic-related signaling pathway are involved in the activation of liver fibrogenesis.4 Therefore, interrupting associated targets to remove superfluous ECM is a promising strategy to regress liver fibrosis.4 However, there are no effective anti-fibrotic drugs in clinical. More researches need to explore the underlying mechanism in liver fibrosis to enhance our understanding of liver fibrosis.
Lysine crotonylation is a novel posttranslational modification (PTM) of proteins, initially identified on histones and subsequently found also on non-histones.5,6 Lysine crotonylation is widely involved in the pathophysiological processes such as cardiac remolding,7,8 endoderm differentiation,9 DNA repair,10 and spermatogenesis.11 Accumulating evidence has found crotonylation-regulated enzymes were directly or indirectly involved in regulating protein crotonylation.12 For example, class I histone deacetylases (HDACs) including HDAC1 and HDAC3 are major histone decrotonylases to diminish protein crotonylation.13,14 Besides, chromodomain Y-like protein (CDYL) serves as crotonyl-CoA hydratase to regulate the levels of crotonyl-CoA, the direct donor of protein crotonylation, to negatively modulate protein crotonylation.10,11,15 Surprisingly, a great deal of crotonylated proteins is identified in normal mouse liver. These proteins are mainly distributed in the nucleus and cytoplasm participating in metabolism-related biological processes.16 Moreover, the level of lysine crotonylation is lower in hepatocellular carcinoma and increasing lysine crotonylation is effective to prevent proliferation and migration of hepatocellular carcinoma cells.17 Thus, lysine crotonylation might take part in the initiation and progression of liver fibrosis.
Sorafenib, a multikinase inhibitor, is approved for treating hepatocellular and renal cell carcinomas and reportedly possesses hepatoprotective and anti-fibrotic roles.18 Sorafenib prevents liver fibrosis supported by inhibiting the activation of hepatic stellate cells (HSCs), repressing epithelial-mesenchymal transition (EMT), and suppressing transforming growth factor β1 (TGFβ1) signaling pathway.19–21 Recently, evidences have demonstrated sorafenib could induce hepatic stellate cells (HSCs) ferroptosis to retard liver fibrosis.22 In addition, epigenetic modifications are critically involved in the liver fibrosis. The well-studied protein acetylation has been demonstrated to participate in the modulating liver fibrosis.23,24 Nevertheless, the roles of lysine crotonylation underlying the liver fibrosis and sorafenib treatment remain indistinct.
In the present study, we found sorafenib significantly prevented the progression of CCl4-induced liver fibrosis. The lysine crotonylation levels were decreased in CCl4-treated rats, especially the crotonylated H2BK12 (H2BK12cr) and crotonylated H3K18 (H3K18cr), while sorafenib promoted the abundance of lysine crotonylation. Meanwhile, protein lysine crotonylation expression was negatively correlated with serum fibrotic indicators. Collectively, these findings might uncover the mystery of lysine crotonylation in liver fibrosis.
Materials and Methods
Animals
Thirty-eight male Sprague Dawley (SD) rats (160–200g) were purchased from Chengdu Dossy Experimental Animals Co., Ltd. (Chengdu, China). The rats were housed in a standard 12 h light–dark cycle and were allowed for free access to water and food. All animal protocols were approved by the Committee on Laboratory Animal Care of Chengdu University of Traditional Chinese Medicine and the rat experiments were performed according to the guidelines of the National Institutes of Health (USA).
Animal Treatments
The rats were randomly divided into three groups: with 8 in the control group (Ctr); 14 in the CCl4 group (CCl4); 16 in the CCl4 plus sorafenib group (CCl4+S). Liver fibrosis was induced by subcutaneous injection of 50% CCl4 olive oil twice a week for 15 weeks. Oral sorafenib (1mg/kg, dissolved in Cremophor EL) was performed every day begin at ninth week for 7 weeks.25 Finally, the rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and the serum samples were collected from the abdominal aortic blood of the rats by centrifuging at 3500 r/min, 4 °C for 10 min, and then stored at −80 °C for further analyses. Rats were then euthanized and the fresh liver was collected to dissect into pieces for further analyses.
Histopathological staining
Four percent paraformaldehyde was used to fix the rats liver. Paraffin was implemented to embed the liver and then embedded liver was dissected into 5µm section. Hematoxylin-eosin (H&E) was performed to analyze the structure of the liver. Masson staining and Picrosirius red (PSR) staining were carried out to detect the collagen deposition. Histopathological staining results were analyzed through Image-Pro Plus 6.0.
Serum Biochemistry Analysis
A fully automatic biochemical analyzer (Chemray 800, Rayto) was used to detect the level of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL) in the blood according to the manufacturer’s instructions. The concentration of serum hyaluronic acid (HA), laminin (LN), and type III procollagen (PC-III) were detected via enzyme-linked immunosorbent assay (ELISA) according to the instructions.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA of rat liver was collected through TRIzol (Invitrogen), then the first-strand cDNA Synthesis kit (New England Biolabs, #M0277) was used to synthesize the first-strand cDNA. The relative mRNA levels of indicated genes were performed with the SYBR Green Master Mix (TaKaRa, #R820A) by qRT-PCR.26 Sequences for qRT-PCR primers are presented in Table 1.
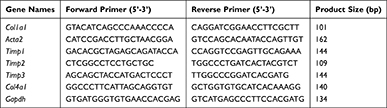 |
Table 1 mRNA Primer Sequences Used in This Study |
Immunohistochemical Staining Analysis
Xylene was used to deparaffinize the paraffin-embedded liver section, then the sections were rehydrated with gradient ethanol. Three percent hydrogen peroxide (H2O2) was used to quench endogenous peroxidases for 30min. Antigen retrieval was performed with EDTA-Tris (pH 8.0) to boil the section for 3min. The section was blocked with goat serum and incubated with indicated primary antibodies H2BK12cr (PTM Biolabs, PTM-509), H3K18cr (PTM Biolabs, PTM-517), pan anti-Kcr (PTM Biolabs, PTM-501), α-SMA (Servicebio, GB111364) and collagen I (Servicebio, GB11022) overnight at 4°C. Eventually, diaminobenzidine (DAB) staining was enforced and counterstained with hematoxylin.
Western Blotting
RIPA lysis buffer (Beyotime, #P0013B) supplemented with protease inhibitor cocktail and phosphatase inhibitor was used to extract liver protein according to previous published paper.26 Briefly, homogenized hepatic tissues were sonicated and centrifuged at 4 °C for 15 minutes to obtain the supernatant for Western blotting. Then, the BCA Protein Assay Reagent (Thermo, # 23227) was used to detect the content of each supernatant protein. The supernatant protein was mixed with SDS-PAGE Sample Loading Buffer (Beyotime Biotechnology, # P0015) and boiled in water to denature protein. Twenty micrograms of protein was subjected to SDS-PAGE and transferred to a PVDF membrane. Five percent non-fat milk was used to block the PVDF membrane. Then, the membrane was incubated with indicated primary antibodies overnight at 4 °C. Subsequently, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (ZSGB-BIO; #ZB2301, #ZB2305), and protein expression was detected by Pierce ECL Western Blotting Substrate (Thermo, #32106). Antibodies to α-SMA (Servicebio, GB111364), Col1a1 (Servicebio, GB11022), H2BK12cr (PTM Biolabs, PTM-509), H3K18cr (PTM Biolabs, PTM-517), pan anti-Kcr (PTM Biolabs, PTM-501), H3 (Abcam, ab1791), HDAC1 (Proteintech, 10197-1-AP), HDAC3 (CST, 3949), CDYL (Proteintech, 17763-1-AP), GAPDH (Abcam, ab8245) were used in the present study.
Statistical Analysis
The data were typically shown as mean±SEM. One-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test was implemented to analyze the significant differences between groups. P values <0.05 were considered statistically significant. All statistical analyses were performed with GraphPad Prism 8.0.
Results
Sorafenib Improved the Liver Index of CCl4-Induced Rats
To investigate the effect of sorafenib on liver index in fibrotic rats, the body weight and liver weight of the rats were documented. As shown in Figure 1A, the body weights of CCl4-treated rats were significantly decreased compared to the control group and the body weights of CCl4 and CCl4+S rats were comparable. Notably, CCl4 increased the liver weight of rats, while sorafenib could reversed the increase of liver weight induced by CCl4 (Figure 1B). Then, according to the body weight and liver weight of rats, we figured out the liver index of rats. CCl4 treatment remarkably enhanced the liver index of rats, whereas sorafenib decreased the liver index of rats (Figure 1C). These data implicate that sorafenib could improve the liver index of fibrotic rats.
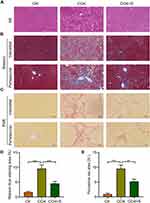
Sorafenib Relieved Liver Fibrosis in CCl4-Induced Rats
To explore the effects of sorafenib on the liver fibrosis in rats treated by CCl4. Hematoxylin–eosin (H&E) staining was used to analyze the histopathological changes of liver induced by CCl4. The results indicated CCl4 treatment promoted hepatocellular necrosis and inflammatory infiltration, while sorafenib improved these phenotypes (Figure 2A). As for the degree of liver fibrosis, Masson trichrome results indicated perivascular and interstitial fibrosis significantly increased in rats induced by CCl4. Conversely, sorafenib treatment displayed notable decrease in perivascular and interstitial fibrosis in rats (Figure 2B and D). Consistently, Picrosirius red (PSR) staining assay also revealed that hepatocellular collagen accumulation was remarkably inhibited with sorafenib (Figure 2C and E). These results indicate sorafenib retards the progression of liver fibrosis.
Sorafenib Improved Liver Function and Liver Fibrosis Index of Fibrotic Rats
The concentration of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (TBIL) is common indicators to evaluate the liver function in clinical. Therefore, we evaluated the roles of sorafenib on these indicators. CCl4 treatment increased the levels of serum ALT, AST, and TBIL, whereas sorafenib treatment dramatically reversed the serum ALT, AST, and TBIL levels (Figure 3A–C). These findings indicate that sorafenib could improve liver function of CCl4-induced rats.
The non-invasive indicators for evaluating the liver fibrosis are the content of hyaluronic acid (HA), type III procollagen (PC III), laminin (LN). To analyze the effects of sorafenib on liver fibrosis indexes, we performed enzyme-linked immunosorbent assay (ELISA) and results showed the concentration of serum PC-III, HA, and LN were significantly up-regulated in CCl4-induced rats. In contrast, these indexes were decreased in response to sorafenib (Figure 3D–F). These data demonstrate that sorafenib improves serum liver fibrosis indexes in CCl4-induced rats.
Sorafenib Repressed HSC Activation and ECM Accumulation
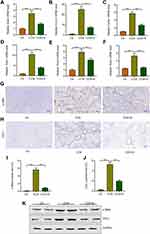
During liver injury, persistent liver injury stimuli lead to transform the resting hepatic stellate cells into activated hepatic stellate cells, which would generate excess extracellular matrix (ECM).27,28 To detect the roles of sorafenib on the activation of HSCs, we analyzed the expression of actin alpha 2, smooth muscle (Acta2, also called α-SMA in mice) in fibrotic livers, the indicator of HSCs activation. The qRT-PCR results demonstrated the expression of Acta2 was markedly increased in response to CCl4 and reduced by sorafenib treatment (Figure 4A). Moreover, the expression of collagen type I alpha 1 chain (Col1a1) was also up-regulated in CCl4 group and down-regulated in the sorafenib group (Figure 4B). The expression tendency of another collagen-related gene called collagen type IV alpha 1 chain (Col4a1) was consistent to Col1a1 (Figure 4C). Besides, TIMPs (tissue inhibitors of metalloproteinases) play important role in maintaining homeostasis of ECM, which prevent metalloproteinases to inhibit the degradation of ECM.29 Consistently, in our study, CCl4 treatment promoted the expression of Timp1, Timp2, and Timp3 and sorafenib reversed the high expression of TIMPs (Figure 4D–F). To further investigate the effects of sorafenib on HSC activation and ECM accumulation, immunohistochemical staining and Western blotting were performed to analyze the protein changes of α-SMA and collagen I. Similarly, α-SMA and collagen I accumulation induced by CCl4 were improved upon sorafenib treatment (Figure 4G–K). These results indicate sorafenib could improve HSC activation and ECM accumulation.
Lysine Crotonylation Mediated Anti-Fibrotic Effect of Sorafenib
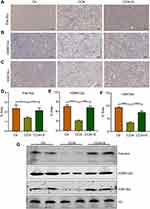
Lysine crotonylation is a novel post-translational modification of proteins widely participating in many biological processes.5,30 Wan et al have uncovered lysine crotonylation expression was decreased in hepatocellular carcinoma and closely associated with hepatoma cell migration and proliferation.17 However, the roles of lysine crotonylation in liver fibrosis remain indistinct. To explore the underlying mechanism of sorafenib in liver fibrosis, we analyzed the lysine crotonylation expression in our study. Surprisingly, compared to the control group, CCl4 treatment led to a sharply decrease of total lysine crotonylation expression (Pan-Kcr) and sorafenib treatment increased the level of total lysine crotonylation (Figure 5A and D). Especially, the expression of crotonylated H2BK12 (H2BK12cr) and crotonylated H3K18 (H3K18cr) was significantly decreased after CCl4 treatment and increased in response to sorafenib (Figure 5B, C, E and F). Western blotting results also demonstrated Pan-Kcr, H3K18cr and H2BK12cr levels were decreased in fibrotic liver. By contrast, sorafenib treatment increased histone crotonylation in liver (Figure 5G). These findings suggest that lysine crotonylation is important for antifibrotic effect of sorafenib.
To deeply explore the relationship between lysine crotonylation expression and liver fibrosis, we performed Spearman correlation analysis between lysine crotonylation expression and serum biochemical indicators. Heatmap Matrix showed that lysine crotonylation was negatively correlated with serum biochemical indicators (ALT, AST, TBIL, PCIII, HA, and LN) (Figure 6A and Table 2). To further investigate how lysine crotonylation expression was disturbed, the levels of crotonylation-regulated enzymes (HDAC1, HDAC3, and CDYL) were detected by Western blotting. The results indicated the expression of HDAC1, HDAC3, and CDYL was increased in response to CCl4 treatment, while sorafenib reduced their expression (Figure 6B). These data indicated sorafenib attenuated liver fibrosis through regulating crotonylation-regulated enzymes to modulate the level of protein crotonylation.
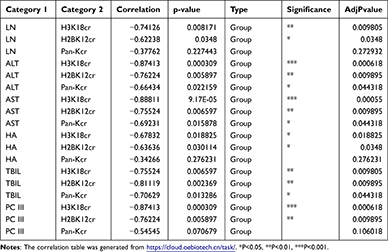 |
Table 2 Correlation Analysis of Lysine Crotonylation and Fibrotic Serum Biochemical Indexes |
 |
Figure 6 Analysis of the relationship between lysine crotonylation expression and each of the indicators of fibrosis and detection protein expression change in fibrotic liver. (A) Correlation analysis of lysine crotonylation and fibrotic serum biochemical indexes. The Heatmap Matrix was generated from https://cloud.oebiotech.cn/task/. (B) Western blotting was used to evaluate the expression of crotonylation-regulated enzymes. |
Discussion
Liver fibrosis is a leading contributor of chronic liver diseases such as cirrhosis and hepatocellular carcinoma.31 Sorafenib was approved to cure hepatocellular and renal cell carcinomas and had the potential to treat liver fibrosis.18 However, the association between protein lysine acylation such as lysine crotonylation and liver fibrosis and whether sorafenib participated in remain unknown. In this study, we found CCl4 treatment induced an increase in liver index, hepatocellular necrosis and inflammatory infiltration, serum liver function and fibrosis indexes, and expression of fibrosis-related genes and proteins in rats. The lysine crotonylation expression was negatively correlated with serum fibrotic indicators. Conversely, sorafenib improved these abnormities to inhibit liver fibrosis. Mechanistically, sorafenib reduced crotonylation-regulated enzymes expression and increased protein lysine crotonylation levels, which contributed to the anti-fibrotic effect of sorafenib.
Serum liver function (ALT, AST, and TBIL) and fibrosis indicators (PC-III, HA, and LN) were used to evaluate the degree of liver disease.32 In our study, we found sorafenib dramatically inhibited the increase of these indicators in fibrotic rats. Besides, histopathological changes were also used to analyze the degree of liver fibrosis.22 H&E, Masson trichrome and PSR were all demonstrated sorafenib repressed the hepatocellular necrosis, inflammatory infiltration and liver fibrosis. Moreover, the typical hallmark of fibrosis was the activation of hepatic stellate cells to induce Acta2 expression.33 We found sorafenib significantly reduced the expression of Acta2 in CCl4-induced rats. In addition, the expression of other fibrogenic genes such as Col1a1, Col4a1, Timp1, Timp2, Timp3 were also inhibited by sorafenib. Collectively, these facts support the notion that sorafenib has the potential to treat liver fibrosis.
Histone short-chain lysine acylations, such as 2-hydroxyisobutyrylation, β-hydroxybutyrylation, glutarylation, and crotonylation, were reportedly to modulate gene expression and widely participate in the various pathophysiological process.5,34–36 Histone lysine crotonylation was a novel short-chain lysine acylation and structurally and functionally distinctive from the well-studied lysine acetylation, which was mainly distributed in active promoters and latent enhancer.5,8 It has been demonstrated lysine crotonylation was increased during acute kidney injury (AKI), which was protective for AKI.37 Moreover, many researches have indicated lysine crotonylation also regulated spermatogenesis,11 endoderm differentiation,9 cardiac homeostasis,7 and tumorigenesis.38 However, the effects of lysine crotonylation on the liver fibrosis remain to explore. The mouse liver lysine crotonylome has been globally profiled and proved crotonylated liver protein primarily influenced metabolism-related biological processes.16 Besides, liver fibrosis would eventually lead to hepatocellular carcinoma, the high morbidity and mortality of malignancy. It has been proved the occurrence and development of hepatocellular carcinoma is accompanied by alteration of total lysine crotonylation.17 Interestingly, we found total lysine crotonylation was decreased in fibrotic liver and reversed by oral sorafenib, especially H2BK12cr and H3K18cr levels. And the lysine crotonylation expression was negatively associated with fibrotic serum biochemical indexes. Besides, interruption of decrotonylases HDAC1 and HDAC3 to enhance the levels of total lysine crotonylation could suppress hepatoma cell proliferation, which suggested the potential role of lysine crotonylation on the liver fibrosis.17 It has been reported that inhibiting HDAC activity could attenuate CCl4-induced liver fibrosis.39 Surprisingly, crotonylation-regulated enzymes (HDAC1, HDAC3, and CDYL), which negatively regulated protein crotonylation expression, were dramatically increased in fibrotic liver and decreased by sorafenib treatment. Therefore, deficiency of lysine crotonylation may be a mechanism that leads to liver fibrosis. However, we just discovered the expression changes and more attentions should be paid to uncover the potential mechanisms contributing to lysine crotonylation in liver fibrosis.
Conclusion
We demonstrate sorafenib is an effective drug for attenuating liver fibrosis. Sorafenib inhibits CCl4-induced liver fibrosis through maintaining homeostasis of hepatic crotonylation-regulated enzymes and protein lysine crotonylation. These findings broaden our knowledge of anti-fibrotic effects of sorafenib and suggest lysine crotonylation may influence liver fibrosis progression.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 82004097); the China Postdoctoral Science Foundation (No. 2020M673163); and the Potential Postdoctoral Program of Chengdu University of Traditional Chinese Medicine (No. BSH2019015).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151–166. doi:10.1038/s41575-020-00372-7
2. Šmíd V. Liver fibrosis. Vnitr Lek. 2020;66(4):61–66. Jaterní fibróza. doi:10.36290/vnl.2020.078
3. Mu M, Zuo S, Wu RM, et al. Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway. Drug Des Devel Ther. 2018;12:4107–4115. doi:10.2147/dddt.S186726
4. Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55. doi:10.1016/j.mam.2018.09.002
5. Tan M, Luo H, Lee S, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146(6):1016–1028. doi:10.1016/j.cell.2011.08.008
6. Xu W, Wan J, Zhan J, et al. Global profiling of crotonylation on non-histone proteins. Cell Res. 2017;27(7):946–949. doi:10.1038/cr.2017.60
7. Tang X, Chen XF, Sun X, et al. Short-chain Enoyl-CoA hydratase mediates histone crotonylation and contributes to cardiac homeostasis. Circulation. 2021;143(10):1066–1069. doi:10.1161/circulationaha.120.049438
8. Chen XF, Chen X, Tang X. Short-chain fatty acid, acylation and cardiovascular diseases. Clin Sci. 2020;134(6):657–676. doi:10.1042/cs20200128
9. Fang Y, Xu X, Ding J, et al. Histone crotonylation promotes mesoendodermal commitment of human embryonic stem cells. Cell Stem Cell. 2021;28(4):748–763.e7. doi:10.1016/j.stem.2020.12.009
10. Yu H, Bu C, Liu Y, et al. Global crotonylome reveals CDYL-regulated RPA1 crotonylation in homologous recombination-mediated DNA repair. Sci Adv. 2020;6(11):eaay4697. doi:10.1126/sciadv.aay4697
11. Liu S, Yu H, Liu Y, et al. Chromodomain protein CDYL acts as a Crotonyl-CoA hydratase to regulate histone crotonylation and spermatogenesis. Mol Cell. 2017;67(5):853–866.e5. doi:10.1016/j.molcel.2017.07.011
12. Ntorla A, Burgoyne JR. The regulation and function of histone crotonylation. Front Cell Dev Biol. 2021;9:624914. doi:10.3389/fcell.2021.624914
13. Wei W, Liu X, Chen J, et al. Class I histone deacetylases are major histone decrotonylases: evidence for critical and broad function of histone crotonylation in transcription. Cell Res. 2017;27(7):898–915. doi:10.1038/cr.2017.68
14. Fellows R, Denizot J, Stellato C, et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat Commun. 2018;9(1):105. doi:10.1038/s41467-017-02651-5
15. Liu Y, Li M, Fan M, et al. Chromodomain Y-like protein-mediated histone crotonylation regulates stress-induced depressive behaviors. Biol Psychiatry. 2019;85(8):635–649. doi:10.1016/j.biopsych.2018.11.025
16. Liu JF, Wu SF, Liu S, et al. Global lysine crotonylation profiling of mouse liver. Proteomics. 2020;20(19–20):e2000049. doi:10.1002/pmic.202000049
17. Wan J, Liu H, Ming L. Lysine crotonylation is involved in hepatocellular carcinoma progression. Biomed Pharmacother. 2019;111:976–982. doi:10.1016/j.biopha.2018.12.148
18. Ma R, Chen J, Liang Y, et al. Sorafenib: a potential therapeutic drug for hepatic fibrosis and its outcomes. Biomed Pharmacother. 2017;88:459–468. doi:10.1016/j.biopha.2017.01.107
19. Chen YL, Lv J, Ye XL, et al. Sorafenib inhibits transforming growth factor β1-mediated epithelial-mesenchymal transition and apoptosis in mouse hepatocytes. Hepatology. 2011;53(5):1708–1718. doi:10.1002/hep.24254
20. Cheng Y, Zheng H, Wang B, Xu W, Xu J, Zhu Y. Sorafenib and fluvastatin synergistically alleviate hepatic fibrosis via inhibiting the TGFβ1/Smad3 pathway. Dig Liver Dis. 2018;50(4):381–388. doi:10.1016/j.dld.2017.12.015
21. Wang Y, Gao J, Zhang D, Zhang J, Ma J, Jiang H. New insights into the antifibrotic effects of sorafenib on hepatic stellate cells and liver fibrosis. J Hepatol. 2010;53(1):132–144. doi:10.1016/j.jhep.2010.02.027
22. Yuan S, Wei C, Liu G, et al. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell Prolif. 2022;55(1):e13158. doi:10.1111/cpr.13158
23. Kim SM, Hur WH, Kang BY, et al. Death-associated protein 6 (Daxx) alleviates liver fibrosis by modulating Smad2 acetylation. Cells. 2021;10(7):1742. doi:10.3390/cells10071742
24. Zhong X, Huang M, Kim HG, et al. SIRT6 protects against liver fibrosis by deacetylation and suppression of SMAD3 in hepatic stellate cells. Cell Mol Gastroenterol Hepatol. 2020;10(2):341–364. doi:10.1016/j.jcmgh.2020.04.005
25. Liu C, Yang Z, Wang L, et al. Combination of sorafenib and gadolinium chloride (GdCl3) attenuates dimethylnitrosamine (DMN)-induced liver fibrosis in rats. BMC Gastroenterol. 2015;15:159. doi:10.1186/s12876-015-0380-5
26. Tang X, Chen XF, Wang NY, et al. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation. 2017;136(21):2051–2067. doi:10.1161/circulationaha.117.028728
27. Chen H, Cai J, Wang J, et al. Targeting Nestin(+) hepatic stellate cells ameliorates liver fibrosis by facilitating TβRI degradation. J Hepatol. 2021;74(5):1176–1187. doi:10.1016/j.jhep.2020.11.016
28. Fan J, Chen Q, Wei L, Zhou X, Wang R, Zhang H. Asiatic acid ameliorates CCl(4)-induced liver fibrosis in rats: involvement of Nrf2/ARE, NF-κB/IκBα, and JAK1/STAT3 signaling pathways. Drug Des Devel Ther. 2018;12:3595–3605. doi:10.2147/dddt.S179876
29. Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis - a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007;46(5):955–975. doi:10.1016/j.jhep.2007.02.003
30. Jiang G, Li C, Lu M, Lu K, Li H. Protein lysine crotonylation: past, present, perspective. Cell Death Dis. 2021;12(7):703. doi:10.1038/s41419-021-03987-z
31. Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020;9(4):875. doi:10.3390/cells9040875
32. Qiao J, Zhao C. Therapeutic effect of adenosylmethionine on viral hepatitis and related factors inducing diseas. Am J Transl Res. 2021;13(8):9485–9494.
33. Campana L, Iredale JP. Regression of Liver Fibrosis. Semin Liver Dis. 2017;37(1):1–10. doi:10.1055/s-0036-1597816
34. Sabari BR, Zhang D, Allis CD, Zhao Y. Metabolic regulation of gene expression through histone acylations. Nat Rev Mol Cell Biol. 2017;18(2):90–101. doi:10.1038/nrm.2016.140
35. Dai L, Peng C, Montellier E, et al. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat Chem Biol. 2014;10(5):365–370. doi:10.1038/nchembio.1497
36. Tan M, Peng C, Anderson KA, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19(4):605–617. doi:10.1016/j.cmet.2014.03.014
37. Ruiz-Andres O, Sanchez-Niño MD, Cannata-Ortiz P, et al. Histone lysine crotonylation during acute kidney injury in mice. Dis Model Mech. 2016;9(6):633–645. doi:10.1242/dmm.024455
38. Xu X, Zhu X, Liu F, Lu W, Wang Y, Yu J. The effects of histone crotonylation and bromodomain protein 4 on prostate cancer cell lines. Transl Androl Urol. 2021;10(2):900–914. doi:10.21037/tau-21-53
39. Liu Y, Wang Z, Wang J, et al. A histone deacetylase inhibitor, largazole, decreases liver fibrosis and angiogenesis by inhibiting transforming growth factor-β and vascular endothelial growth factor signalling. Liver Int. 2013;33(4):504–515. doi:10.1111/liv.12034
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.