Back to Journals » International Journal of General Medicine » Volume 13
Some of the Factors Involved in Male Infertility: A Prospective Review
Authors Babakhanzadeh E, Nazari M, Ghasemifar S, Khodadadian A
Received 4 December 2019
Accepted for publication 23 January 2020
Published 5 February 2020 Volume 2020:13 Pages 29—41
DOI https://doi.org/10.2147/IJGM.S241099
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Emad Babakhanzadeh,1,2 Majid Nazari,1 Sina Ghasemifar,1 Ali Khodadadian1
1Department of Medical Genetics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran; 2Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Correspondence: Ali Khodadadian
Genetic Department, Clinical and Research Centre for Infertility, Bou-Ali Avenue, Safayeh, Yazd, Iran
Tel +98 351 8247085
Fax +98 351 8247087
Email [email protected]
Abstract: Infertility is defined as the inability of couples to have a baby after one year of regular unprotected intercourse, affecting 10 to 15% of couples. According to the latest WHO statistics, approximately 50– 80 million people worldwide sufer from infertility, and male factors are responsible for approximately 20– 30% of all infertility cases. The diagnosis of infertility in men is mainly based on semen analysis. The main parameters of semen include: concentration, appearance and motility of sperm. Causes of infertility in men include a variety of things including hormonal disorders, physical problems, lifestyle problems, psychological issues, sex problems, chromosomal abnormalities and single-gene defects. Despite numerous efforts by researchers to identify the underlying causes of male infertility, about 70% of cases remain unknown. These statistics show a lack of understanding of the mechanisms involved in male infertility. This article focuses on the histology of testicular tissue samples, the male reproductive structure, factors affecting male infertility, strategies available to find genes involved in infertility, existing therapeutic methods for male infertility, and sperm recovery in infertile men.
Keywords: male infertility, spermatogenesis, azoospermia, non-obstructive azoospermia
Introduction
Infertility is defined as the inability of couples to have a baby after one year of regular unprotected intercourse, affecting 10–15 percent of couples.1–4 According to the latest WHO statistics, about 50–80 million people worldwide suffer from infertility.5,6 Large-scale studies have shown that about half of all cases of infertility occur due to female factors, 20 to 30 percent male factors, and 20 to 30 percent due to common causes of both gender.6–8 Recent meta-analysis studies by researchers show that male’s factors are present in 20–70 percent of infertility cases.7,9 These findings are significantly broader than previously reported. However, the wide range of male infertility in meta-analysis studies may not reflect the prevalence of this complication in all parts of the world because of reasons such as the lack of rigorous statistical methods that include bias, heterogeneity in data collection, and cultural constraints. Given the significant contribution of male factors to infertility in couples, as well as high levels of unknown factors in male infertility, a lack of understanding of the underlying mechanisms seems to be one of the most important challenges facing this problem. In this article, we have reviewed the histological studies of testicular tissue specimens, male reproductive structure, factors influencing male infertility, strategies to find genes involved in infertility, available therapeutic methods for male infertility, sperm recovery methods in infertile men, and assisting reproductive method (Figure 1).
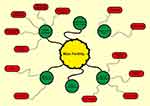 |
Figure 1 Main factors involved in male infertility. |
Male’s Reproductive Organ
In order to better understand the issues and problems associated with infertility, we first discuss some of the key elements involved in male fertility. Human reproductive organs include the primary and secondary organs. Primary reproductive organs include the gonads (responsible for gamete and hormone production), while the secondary organs include the ducts and glands, which play a role in the growth, maturation and transmission of gametes.10,11 The testicles are the primary male reproductive organs enclosed by the tunica albuginea capsule in the testicle sack. Two morphologically and functionally separated parts are in the testis. Tubular components include seminiferous tubules and intercellular portions between seminiferous tubules. The intertubular portions of the seminiferous tubules are involved in providing blood and immune responses.12–14 Leydig cells are one of the most important cells in testis that are the source of testicular testosterone and insulin-like factor 3. In addition to Leydig cells, intercellular components include immune cells, lymphatic and blood vessels, nerves, connective tissue, and fibroblasts.15–18 The seminiferous tubules are functional units in the testis, accounting for 60–80 percent of testicular volume.19–21 These tubes are surround by epithelial tissue and include two types of cells: Sertoli cells and spermatogenic cells. The function of Sertoli cells is to nourish and develop sperm through the stages of spermatogenesis and their mechanical support.22–24 These cells produce two types of inhibin and activin hormone that have positive and negative feedback to FSH.25–27 In addition, Sertoli cells control the stages of sperm release into the lumen, phagocytosis of the degraded germ cells and additional cytoplasm resulting from sperm release. In adulthood, Sertoli cells are meiotically inactive.28–30 Sertoli cell division terminates concurrently with the first meiotic division of the germ cells, giving rise to tight junctions between these cells, known as the Blood-Testis Barrier (BTB) (Figure 2).31,32 The epithelium of seminiferous tubules is divided into two (functionally different) regions by BTB. Two important functions for BTB are: (a) the physical separation of the germ cells that protect them against the immune system; (b) providing an environment for meiosis and sperm development.33–35
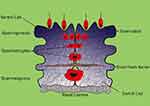 |
Figure 2 Schematic of the Blood-Testis Barrier. |
Spermatogenesis
Spermatogenesis is one of the most crucial stages in male fertility.36–39 The slightest deviation from the natural course of spermatogenesis can lead to infertility in men. The term spermatogenesis is a description of the development of male gametes in the seminiferous epithelial tissue from diploid spermatogonia that results in the release of differentiated haploid germ cells into the seminiferous tubules. Each cycle of spermatogenesis in humans requires 16 days and almost 4.6 cycles for development and differentiation of spermatogenic cells into adult sperm, which takes approximately 74 days in humans.40–42 The regulation of spermatogenesis occurs in two main stages: a) hormonal and endocrine b) paracrine and autocrine. Many studies have shown that testosterone and FSH are required to successfully complete spermatogenesis.22,43 The spermatogenesis process divided into four general phases: 1) mitotic proliferation and spermatogonial differentiation into pre-leptotene spermatocytes (spermatogoniogenesis); 2) Meiotic division of spermatocytes that leads to spermatids (meiosis); 3) Conversion of round spermatids into adult spermatids (spermogenesis); 4) Release of elongated spermatids into the lumen (spermatogenesis) (Figure 3).44 Considering the importance of spermatogenesis and since the disorder at any of its stages can have irreversible consequences, below are some of the most important features of each stage.
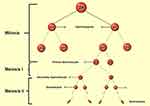 |
Figure 3 Spermatogenesis process. |
Spermatogoniogenesis
The germ cell lines originate from the primary germ cell (PGC).45 In humans, PGCs develop between endoderm cells at the end of the third week of development, and by the fifth week they migrate to the genital tract, where the presence of the Y chromosome results in the proliferation and transformation of the genital tract into primary male sexual organs.46–48 PGCs are commonly called gonocytes during the first trimester of mitosis, then stop in the G3 phase of the cell cycle and remain silent until birth (i.e., when they become spermatogonia).49–51 Spermatogonia remain silent until puberty. Spermatogenesis begins with the mitotic proliferation of spermatogonia after birth.52–54 Spermatogenesis during puberty is probably initiated by the production of bone morphogenetic protein 8B (BMP8B). Mice with lack of Bmp8b do not initiate spermatogenesis at puberty and consequently are infertile.55–57 Two distinct fates await reproductive cells: (a) self-renewal by replication; (b) becomes spermatozoa. Apoptosis in spermatogonia rarely occurs in the human seminiferous epithelial tissue, but the rate of apoptosis is increased in patients with impaired spermatogenesis, especially in spermatocytes and spermatids.58,59
Meiotic Division
Meiosis is the distinction between sexual reproduction and non-sexual reproduction.60,61 Meiosis eventually results in the production of haploid gametes from diploid cells. During mammalian meiosis, nuclear division is done twice in a cycle of DNA replication. Each meiosis division is generally divided into two stages Meiosis I and Meiosis II.62–64 In meiosis I, also called subtractive division, the microtubules are attached to sister chromatids via the kinetochore and transported to opposite poles.65–67 This transition leads to a decrease in the number of chromosomes from diploid to haploid. Meiosis II is an equal division, in which the microtubules attach to the kinetochore of centromere and separate the sister chromatids, resulting in the formation of four daughter haploid cells. Meiosis begins with the production of two pre-leptotene spermatocytes from spermatogonia. In meiosis I, primary spermatocytes become two secondary spermatocytes, and these cells then form spermatids in meiosis II. The result of meiosis is four different (genetically) cell types.68–70
Spermogenesis
Spermogenesis is a process that transforms the meiosis II final product (i.e., spherical spermatids) without splitting into specialized elongated spermatids. This process requires the development of the cytoplasm and nucleus regeneration, which can comprise four distinct phases: the Golgi phase, the capping phase, the acrosomal phase, and the maturation phase.71–73
Spermatogenesis
Sperm production is the final stage of spermatogenesis, which mature spermatids are released from the somatic supporting Sertoli cells into the lumen of the seminiferous tubules. At this stage, the cells are known as spermatozoa and continue their journey to epididymis. Seminiferous spermatozoa have low motility and fertility. Spermatozoa passage through the epididymal duct is crucial for final maturation and ability to move.74–76 A small amount of cytoplasmic content, cytoplasmic droplets remain in the neck region and the middle segment of the spermatozoa, which facilitates the achievement of epididymis. During the transition from epididymis, which takes approximately two weeks, the cytoplasmic droplets move and exit during the spermatozoa tail, which is associated with increased spermatozoa movement. This event is associated with an increase in the movement of spermatozoa.77–79
Main Causes of Infertility
As mentioned, infertility can have a feminine or masculine origin, with the male factor only present in one third of cases. The diagnosis of infertility in men is mainly based on semen analysis. Unusual parameters of semen include: sperm concentration, appearance and motility.80,81 There are seven main cases of semen-related abnormalities. Infertility in men can be due to a variety of causes, however, in almost 40% of infertile men there is no clear etiology. There are various reasons for male infertility, the most important of which are: Hormonal deficits, physical causes, sexually transmitted problems, environment and lifestyle, and genetic factors.82–85
Hormonal Defects
The male reproductive hormone axis is known as the hypothalamic-pituitary-gonadal axis. It consists of 3 major components: the hypothalamic, pituitary and testicular glands (Figure 4).86,87 This axis works very regularly to provide the right concentration of hormones for male sexual development and function. Any abnormality in the system can lead to infertility.88–90 If the brain is unable to produce gonadotropic releasing hormone (GnRH), this disorder results in a lack of testosterone and stopping sperm production.91,92 Lack of GnRH causes a group of disorders known as hypogonadotropic hypogonadism.93,94 One of them is known as Kallmann syndrome, which is associated with a change in sense of smell and immaturity.95 Treatment options for gonadotropin-releasing hormone deficiency include: Use of sex steroids, gonadotropins and injection of gonadotropin releasing hormone. Testosterone injections are mainly used to improve testicular growth, normalize testosterone concentration, and stimulate the development of secondary sexual traits.96–98 Similarly, the pituitary’s inability to produce sufficient amounts of luteinizing hormone and follicular stimulating hormone results in a failure to stimulate the testes and to produce testosterone and sperm.99,100 Patients with pituitary deficiency require long-term hormonal therapy, which can lead to complications such as diabetes mellitus, heart disease and bone defects. Conversely, elevated concentrations of LH and FSH are associated with low concentrations of testosterone, leading to defects in spermatogenesis.101,102 Therefore, using high doses of testosterone and estrogen can be a viable treatment option because it suppresses the production of LH and FSH. Increased prolactin can also lead to reduced sperm production, libido and impotence. Hyperprolactemia leads to infertility in 11% of people with oligospermia.103 In many cases, a dopamine agonist can be a good treatment.
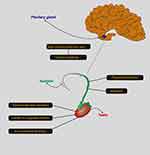 |
Figure 4 Brain-pituitary-testicular axis. |
Physical Reasons
Physical problems can disrupt sperm production and blockage of the ejaculatory pathway. Enlargement of the sperm vessels known as varicocele is one of the most common male infertility problems affecting about 40% of men.104,105 Testicular torsion within the testicle sac can cause testicular damage due to pressure on the sperm vessels and impaired testicular circulation. Chronic and acute genital tract infections can also be common causes of infertility in men.106 Mumps viral infection can lead to testicular atrophy and infertility.107 Sexually transmitted diseases such as Gonorrhea and Chlamydia can also lead to infertility in men due to obstruction in the epididymis.108 In some cases, semen is ejaculated in the bladder, known as recurrent ejaculation, and accounts for about 2% of infertility cases that can be caused by anatomical problems of the bladder sphincter.109
Sexual Problems
Many sexual problems are both physical and psychological. Erectile dysfunction, known as impotence, early ejaculation and inability to ejaculate are examples of intercourse problems.110,111
Environment and Lifestyle
Men exposed to hazardous substances in their workplace, including solvents, insecticides, adhesives, silicones and radiation, exposure to these and similar substances can lead to infertility.82,112,113 Exposure to radiation can lead to reduced sperm production, and exposure to high doses can lead to complete infertility. Overuse of the sun bath can also lead to a temporary decrease in sperm count.114 Occupations that require prolonged sitting (such as driving) or being exposed to high temperatures (such as bakeries) can have negative effects on fertility.115 Concerning alcohol consumption and smoking, there is no definite agreement regarding their effect on sperm parameters and fertility outcomes.116,117 However, progressive degradation in sperm quality may be associated with cigarette smoking and alcohol consumption. Poor nutrition can also play an important role in male infertility. There has been a recent report of a decrease in sperm concentration in men with an increase in saturated fat intake.118 Repeated use of drugs such as cocaine and cannabinoids is associated with a significant decrease in sperm concentration, and urinary testosterone in men.119 In addition, studies have also shown that air pollution in men reduces sperm motility, and the way to deal with and prevent this problem is to continually use antioxidants and vitamin C-containing substances. Moreover the presence of pollutants and sulfur dioxide in the air changes the natural shape of sperm and also has a detrimental effect on sperm motility.120–123
Genetic Factors
Genetic factors are detected in 15% of male infertility cases and can be classified into two groups: chromosomal abnormalities and single-gene mutations.124,125 Any lack or acquisition of unusual rearrangements in genetic material at the chromosomal level is known as chromosomal abnormalities and is one of the major genetic causes involved in male infertility.126 About 14% of men with azoospermia and 2% of men with oligospermia have chromosomal abnormalities, which is much higher than the general population (about 0.6%).127,128 Some chromosomal abnormalities are inherited and some are acquired. The most common genetic cause of azoospermia in the aneuploid sex chromosome is Klinefelter syndrome, which accounts for about 14% of male infertility cases. 47,XYY, chromosomal defects can cause spermatogenesis malfunction due to increased FSH and Y chromosome disomy.129,130 Noonan syndrome in men, such as Turner syndrome in women, which is XO/XY mosaic, can lead to cryptocidism and spermatogenesis deficiency due to increased FSH.131,132 Translocations occur in 3% of patients with severe oligozoospermia, the most important is Robertsonian and bilateral translocation.133,134 Inversion is called chromosomal translocation, in which a fragment of the chromosome is broken and rearrangement within itself.135 Autosomal inversions are eight times more frequent in infertile men,136 although these rearrangements are balanced, in some cases leading to severe oligoasthenoteratozoospermia or azoospermia.137 The role of the Y chromosome was identified by Zofardi and colleagues by karyotype analysis of deletions in the long arm of the Y chromosome in six infertile men, they termed the deletion region as azoospermic factor (AZF).138 This region contains three zones AZFa, AZFb, and AZFc.139 Micro-deletions occur following the recombination of similar fragments in palindromic sequences. Y chromosome microdeletions are present in 10% of infertile men, whereas in oligozoospermic males, the prevalence is 7%.140,141 The most common microdeletion occurs in the AZFc region, accounting for 80% of cases.142 Deletions that encompass the entire AZFa region result in the Sertoli cell phenotype.139 Intra-AZFb deletion usually results in azoospermia.143 Deletion in the AZFc region can lead to a wide range of infertility phenotypes including azoospermia, Sertoli cell syndrome and oligozoospermia.144 Some gene mutations with pathological syndromes can be associated with infertility, such as congenital bilateral absence of the vas deferens (CBAVD), which cause obstructive azoospermia in 80 to 90% of cases.145 This defect is caused by a mutation in the Cystic fibrosis transmembrane regulator (CFTR).146 Primary ciliary defects are an autosomal recessive heterogeneous defect caused by a lack of normal eyelash function and present in half of men with asthenospermia. Little has been known so far about non-syndromic infertility.147
Epigenetic Factor
Acetylation and methylation are two effective factors in epigenetic modifications that cause different expression of genes. Epigenetic factors act a critical role in male infertility, and numerous studies have been devoted to it. During spermatogenesis, germ cells face major epigenetic reprogramming that includes the organization of sex-specific designs in the sperm, which substitution of histone to protamine is one of them.148–150 Numerous experiments have revealed altered epigenetic function in sperm from men with oligozoospermia and oligoasthenoteratozoospermia. Besides, many studies have been reported that hypermethylation in several genes, lead to deficiency in semen parameters or male infertility.151–153
Strategies for Finding Genes Involved in Infertility
There are two general approaches to infertility studies for finding genes involved in infertility: the candidate gene approach and the whole genome approach. A) The candidate gene approach; Identification of genes that lead to impaired fertility in model animals (mostly mice), and assuming that their function is maintained during evolution, these genes are selected and their roles and effects in human infertility are studied. It is important to note that in this method, the function and expression of candidate genes in model animals and their effect on infertility have already been proven, and given the foregoing, it is possible to predict the gene involved in human infertility. B) Whole-genome approach; technological advances in whole-genome studies such as single-nucleotide polymorphism (SNP) microarray, high-throughput sequencing technologies such as exome or whole-genome sequencing, and their use for finding effective genes in infertility has been considered.154,155 In single nucleotide polymorphisms or SNPs, the difference in one nucleotide causes different phenotypes. SNPs are classified into common and non-common groups based on allele frequency. Sequencing technologies enable researchers to perform high-throughput sequencing, which allows millions of pieces of DNA to be sequenced. Exome sequencing is another field that has revolutionized the study of a variety of disorders, including infertility. Exom covers about 1% of the entire human genome but accounts for about 85% of the pathogenic mutations.156 Exome sequencing allows us to identify mutations in the protein coding region. On the other hand, whole-genome sequencing can identify potentially susceptible mutations throughout the genome. Genome-wide association studies (GWAS) have been able to identify different polymorphisms related to defects in spermatogenesis. Until now, however, genetic risk factors identified with this technique have shown poor association.
After introducing some of the influencing factors in infertility and exploring its identification methods, the challenge that remains to be resolved is how to use the information obtained to treat diagnosed infertility. Following some of the most up-to-date and important strategies for treating infertility with a specific cause, are mentioned.
Fertility Assistance Techniques
Although different definitions have been proposed, assisted reproductive techniques refer to a range of methods generally used to treat infertility problems in humans and help infertile couples to have a healthy child. There are three main stages of progressive intervention in this area. A) Stimulation of ovulation during intercourse. B) Stimulation of ovulation and injection of sperm into the female reproductive tract. C) Artificial fertilization in which the egg and sperm are fertilized outside the body and the resulting embryo is transferred into the uterus. Each of these steps is discussed below.
Auxiliary Fertilization
This process involves controlled ovarian stimulation to increase the maturation of several oocytes, egg harvest through follicle aspiration, sperm recovery, laboratory inoculation, and embryo transfer and culture. Although assistant fertilization can be performed in the normal sex cycle, the most common protocol involves the daily injection of recombinant human FSH to stimulate follicle growth to obtain the most oocytes.157,158 The follicles are monitored by serum estradiol and uterine ultrasound. Once the follicles have reached the appropriate size, human chorionic gonadotropin (HCG) is injected to stimulate follicle maturation and is collected 32 to 36 hrs after injection. Although frozen sperm can also be used according to WHO guidelines; Generally, on the day that eggs are collected, semen is also collected by masturbation after a period of 2 to 7 days of abstinence for artificial insemination, After egg stimulation and sperm recovery, two methods of fertilization are used: IVF and ICSI.159,160
Sperm Recovery in Infertile Men
For infertile men, sperm must be recovered directly from the testicles or epididymis. Obstructive azoospermia (OA) and non-obstructive azoospermia51 are two major categories of azoospermia.161 Obstructive azoospermia is the result of physical obstruction of the male genital tract, which may be due to or acquired factors (i.e., infection, vasectomy or physical injury to the genital tract), congenital absence of the vas deferens (congestion of the vas deferens, which accounts for about 60% of men with azoospermia), epididymal obstruction.162 On the other hand, NOA is due to the lack of testicular sperm production in the ejaculate.163,164 The best way to treat NOAs is to extract sperm from the testis (TESE) and done intracytoplasmic sperm injection (ICSI). However, in half of the cases of azoospermia, sperm cannot be found as a result of TESE.165 Unfortunately, serum hormone levels such as FSH and inhibin B and noninvasive assessments such as testicular volume cannot predict sperm recovery and to date only testicular histopathology can be used as a predictor of successful sperm recovery rate (SSR).166 In the conventional TESE method, spermatozoa are extracted from testicular biopsies by local or general anesthesia.167 On the other hand, sperm extraction is much safer and more successful by micro-TESE.168
The purpose of the micro-TESE is to identify the nuclear regions of testicular sperm production based on the size and appearance seminiferous tubules with the aid of a microscope, in which spermatozoa can be recovered from open seminiferous tubules, the whole process being visible under the microscope. Micro-TESE is a better alternative to TESE because of the increased chance of sperm recovery and reduced testicular damage due to the smaller size of the harvested tissue. In general, testicular tissue resection for histopathologic evaluation can potentially eliminate sites that still produce sperm, despite abnormalities.169,170 Testis biopsy before sperm recovery is generally not recommended. Testicular biopsy is usually performed on the day of egg retrieval. Biopsy specimens are examined for the presence of sperm. A small sample is taken from an accessible area and evaluated for histopathology.170–172 Due to the uncertainty of sperm retrieval and failure of sperm retrieval, egg retrieval will be unnecessary. This can cause emotional, economic, and physical stress for couples, so sperm retrieval requires the use of predictive factors, and this will not be possible unless you have in-depth knowledge of all the steps that can lead to Infertility in men.
Discussion
Today, around 10–15% of couples around the world are experiencing infertility (60–80 million people). In half of cases, male infertility is the cause. Disruption of spermatogenesis is a major cause of infertility, and genetic abnormalities affecting spermatogenesis can be the cause of many unknown male infertility cases. Therefore, identifying and presenting prognostic biomarkers as well as finding non-invasive therapeutic techniques seem necessary. In hormonal investigations, the micro-TESE technique can increase the biomarker value of FSH to predict sperm recovery; because the results of hormonal studies show the whole process of spermatogenesis in all areas of the testis. Given that the testicular tissue is heterogeneous, the micro-TESE technique can increase the likelihood of sperm retrieval despite inadequate levels of the FSH hormone, due to sampling small areas of testicular tissue.
Conclusion
Given that male infertility in many cases remains unknown. Therefore, it is necessary to introduce new key factors and diagnostic and noninvasive biomarkers. Over the past few years, the identification and evaluation of small noncoding RNAs in many diseases, including infertility, has helped greatly in understanding the underlying mechanisms of disease. But this alone is not enough, and through increased insight into the complex stages and processes of pregnancy in humans, more key elements must be identified so that infertile couples can enjoy the chance of a natural pregnancy in addition to reducing costs and problems. With the advances in technology and the introduction of new methods and approaches, it is hoped that many of the causes of male infertility will soon be identified and treated.
Abbreviations
BTB, Blood-Testis Barrier; PGC, primary germ cell; GnRH, gonadotropic releasing hormone; AZF, azoospermic factor; CBAVD, congenital bilateral absence of the vas deferens; CFTR, cystic fibrosis transmembrane regulator; SNP, single-nucleotide polymorphism; GWAS, genome-wide association studies; HCG, human chorionic gonadotropin; OA, obstructive azoospermia; NOA, non-obstructive azoospermia; TESE, testicular sperm extraction; ICSI, intracytoplasmic sperm injection; SSR, sperm recovery rate.
Acknowledgment
The authors would like to thank to Dr. S. Azizi, Dr. M. Yousofi, and Mr. Zadrostam for their valuable support.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no conflicts of interest.
References
1. ESHRE Capri Workshop Group, Albertini DF, Anderson R, Bhattacharya S, et al. A prognosis-based approach to infertility: understanding the role of time. Hum Reprod. 2017;32(8):1556–1559. doi:10.1093/humrep/dex214
2. Turchi P. Prevalence, definition, and classification of infertility. In: Cavallini G, Beretta G, editors. Clinical Management of Male Infertility. Springer; 2015:5–11.
3. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. doi:10.1016/j.clinbiochem.2018.03.012
4. Zegers-Hochschild F, Adamson GD, Dyer S, et al. The international glossary on infertility and fertility care, 2017. Hum Reprod. 2017;32(9):1786–1801. doi:10.1093/humrep/dex234
5. Briceag I, Costache A, Purcarea V, et al. Fallopian tubes–literature review of anatomy and etiology in female infertility. J Med Life. 2015;8(2):129.
6. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015;8(4):191. doi:10.4103/0974-1208.170370
7. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13(1):37. doi:10.1186/s12958-015-0032-1
8. Masoumi SZ, Parsa P, Darvish N, Mokhtari S, Yavangi M, Roshanaei G. An epidemiologic survey on the causes of infertility in patients referred to infertility center in Fatemieh Hospital in Hamadan. Iran J Reprod Med. 2015;13(8):513.
9. Qi X, Wang K, Zhou G, Xu Z, Yu J, Zhang W. The role of testicular artery in laparoscopic varicocelectomy: a systematic review and meta-analysis. Int Urol Nephrol. 2016;48(6):955–965. doi:10.1007/s11255-016-1254-7
10. Ampatzidis G, Georgakopoulou D, Kapsi G. Clitoris, the unknown: what do postgraduate students of educational sciences know about reproductive physiology and anatomy? J Biol Educ. 2019;1–10. doi:10.1080/00219266.2019.1679658
11. Carson SA, Buster JE, Cesario M, Woodard SP. Recovery and processing of human embryos formed in vivo. Google Patents; 2019.
12. Li X, Wang Z, Jiang Z, et al. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci. 2016;113(10):2666–2671. doi:10.1073/pnas.1519395113
13. Mehrabani D, Hassanshahi MA, Tamadon A, et al. Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats. J Hum Reprod Sci. 2015;8(2):103. doi:10.4103/0974-1208.158618
14. Orman D, Vardi N, Ates B, Taslidere E, Elbe H. Aminoguanidine mitigates apoptosis, testicular seminiferous tubules damage, and oxidative stress in streptozotocin-induced diabetic rats. Tissue Cell. 2015;47(3):284–290. doi:10.1016/j.tice.2015.03.006
15. Chang W-H, Wu M-H, Pan H-A, Guo P-L, Lee C-C. Semen quality and insulin-like factor 3: associations with urinary and seminal levels of phthalate metabolites in adult males. Chemosphere. 2017;173:594–602. doi:10.1016/j.chemosphere.2017.01.056
16. Chen G, Zhang S, Jin Y, et al. TPP and TCEP induce oxidative stress and alter steroidogenesis in TM3 Leydig cells. Reprod Toxicol. 2015;57:100–110. doi:10.1016/j.reprotox.2015.05.011
17. Pitia AM, Minagawa I, Uera N, et al. Expression of insulin-like factor 3 hormone-receptor system in the reproductive organs of male goats. Cell Tissue Res. 2015;362(2):407–420. doi:10.1007/s00441-015-2206-8
18. Ye L, Li X, Li L, Chen H, Ge R-S. Insights into the development of the adult Leydig cell lineage from stem Leydig cells. Front Physiol. 2017;8:430. doi:10.3389/fphys.2017.00430
19. Bhushan S, Aslani F, Zhang Z, Sebastian T, Elsässer H-P, Klug J. Isolation of Sertoli cells and peritubular cells from rat testes. J Vis Exp. 2016;(108):e53389.
20. Sahin Z, Ozkaya A, Cuce G, Uckun M, Yologlu E. Investigation of the effect of naringenin on oxidative stress‐related alterations in testis of hydrogen peroxide‐administered rats. J Biochem Mol Toxicol. 2017;31(9):e21928. doi:10.1002/jbt.2017.31.issue-9
21. Shah S, Saini N, Ashraf S, et al. Comparative expression analysis of gametogenesis‐associated genes in foetal and adult bubaline (Bubalus bubalis) ovaries and testes. Reprod Domest Anim. 2015;50(3):365–377. doi:10.1111/rda.2015.50.issue-3
22. Duan P, Hu C, Quan C, et al. 4-Nonylphenol induces apoptosis, autophagy and necrosis in Sertoli cells: involvement of ROS-mediated AMPK/AKT-mTOR and JNK pathways. Toxicology. 2016;341–343:28–40. doi:10.1016/j.tox.2016.01.004
23. Yao C, Sun M, Yuan Q, et al. MiRNA-133b promotes the proliferation of human Sertoli cells through targeting GLI3. Oncotarget. 2016;7(3):2201. doi:10.18632/oncotarget.v7i3
24. Zhang L, Chen M, Wen Q, et al. Reprogramming of Sertoli cells to fetal-like Leydig cells by Wt1 ablation. Proc Natl Acad Sci. 2015;112(13):4003–4008. doi:10.1073/pnas.1422371112
25. Cortes D, Clasen-Linde E, Hutson JM, Li R, Thorup J. The Sertoli cell hormones inhibin-B and anti Müllerian hormone have different patterns of secretion in prepubertal cryptorchid boys. J Pediatr Surg. 2016;51(3):475–480. doi:10.1016/j.jpedsurg.2015.08.059
26. Dimitriadis F, Tsiampali C, Chaliasos N, Tsounapi P, Takenaka A, Sofikitis N. The Sertoli cell as the orchestra conductor of spermatogenesis: spermatogenic cells dance to the tune of testosterone. Hormones. 2015;14(4):479–503. doi:10.14310/horm.2002.1633
27. Loveland KL, Hedger MP. Activins and inhibins in Sertoli cell biology: implications for testis development and function. In: Griswold MD, editor. Sertoli Cell Biology. Elsevier; 2015:201–232.
28. Chaichanathong S, Taya K, Watanabe G, et al. Immunohistochemical localization of inhibin/activin subunits in adult Asian elephant (Elephas maximus) testes. J Vet Med Sci. 2018;80(3):549–552. doi:10.1292/jvms.17-0531
29. Li Y, Fortin J, Ongaro L, et al. Betaglycan (TGFBR3) functions as an inhibin A, but not inhibin B, coreceptor in pituitary gonadotrope cells in mice. Endocrinology. 2018;159(12):4077–4091. doi:10.1210/en.2018-00770
30. Winters S, Moore J
31. Gerber J, Heinrich J, Brehm R. Blood-testis barrier and Sertoli cell function: lessons from SCCx43KO mice. Reproduction (Cambridge, England). 2016;151(2):R15–R27. doi:10.1530/REP-15-0366
32. Li N, Mruk DD, Lee WM, Wong CK, Cheng CY. Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis?. Semin Cell Dev Biol. 2016;59:141–156.
33. Cao X-N, Shen L-J, Yan C, et al. Urban fine particulate matter exposure causes male reproductive injury through destroying blood-testis barrier (BTB) integrity. Toxicol Lett. 2017;266:1–12. doi:10.1016/j.toxlet.2016.12.004
34. Fan Y, Liu Y, Xue K, et al. Diet-induced obesity in male C57BL/6 mice decreases fertility as a consequence of disrupted blood-testis barrier. PLoS One. 2015;10(4):e0120775. doi:10.1371/journal.pone.0120775
35. Zhang J, Li Z, Qie M, Zheng R, Shetty J, Wang J. Sodium fluoride and sulfur dioxide affected male reproduction by disturbing blood-testis barrier in mice. Food Chem Toxicol. 2016;94:103–111. doi:10.1016/j.fct.2016.05.017
36. Goh WSS, Falciatori I, Tam OH, et al. piRNA-directed cleavage of meiotic transcripts regulates spermatogenesis. Genes Dev. 2015;29(10):1032–1044. doi:10.1101/gad.260455.115
37. Griswold MD. Spermatogenesis: the commitment to meiosis. Physiol Rev. 2016;96(1):1–17. doi:10.1152/physrev.00013.2015
38. Gunes S, Al-Sadaan M, Agarwal A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online. 2015;31(3):309–319. doi:10.1016/j.rbmo.2015.06.010
39. O’Hara L, Smith LB. Androgen receptor roles in spermatogenesis and infertility. Best Pract Res Clin Endocrinol Metab. 2015;29(4):595–605. doi:10.1016/j.beem.2015.04.006
40. Galdon G, Atala A, Sadri-Ardekani H. In vitro spermatogenesis: how far from clinical application? Curr Urol Rep. 2016;17(7):49. doi:10.1007/s11934-016-0605-3
41. Pieri NCG, Souza A, Mançanares ACF, et al. Immunolocalization of proteins in the spermatogenesis process of canine. Reprod Domest Anim. 2017;52:170–176. doi:10.1111/rda.2017.52.issue-S2
42. Xu H, Shen L, Chen X, et al. mTOR/P70S6K promotes spermatogonia proliferation and spermatogenesis in Sprague Dawley rats. Reprod Biomed Online. 2016;32(2):207–217. doi:10.1016/j.rbmo.2015.11.007
43. Garolla A, Ghezzi M, Cosci I, et al. FSH treatment in infertile males candidate to assisted reproduction improved sperm DNA fragmentation and pregnancy rate. Endocrine. 2017;56(2):416–425. doi:10.1007/s12020-016-1037-z
44. Yao C, Liu Y, Sun M, et al. MicroRNAs and DNA methylation as epigenetic regulators of mitosis, meiosis and spermiogenesis. Reproduction. 2015;150(1):R25–R34. doi:10.1530/REP-14-0643
45. Donovan PJ, de Miguel MP. Turning germ cells into stem cells. Curr Opin Genet Dev. 2003;13(5):463–471. doi:10.1016/j.gde.2003.08.010
46. Hoei-Hansen C, Sehested A, Juhler M, et al. New evidence for the origin of intracranial germ cell tumours from primordial germ cells: expression of pluripotency and cell differentiation markers. J Pathol. 2006;209(1):25–33. doi:10.1002/(ISSN)1096-9896
47. Looijenga LH. Pathogenesis of testicular germ cell tumors. In: Lau YF, CHan WY, editors. The Y Chromosome and Male Germ Cell Biology in Health and Diseases. World Scientific; 2007:263–288.
48. Meyts ERD, Jørgensen N, Brøndum‐Nielsen K, Müller J, Skakkebæk NE. Developmental arrest of germ cells in the pathogenesis of germ cell neoplasia. APMIS. 1998;106(1‐6):198–206. doi:10.1111/j.1699-0463.1998.tb01336.x
49. Andrews PW. Teratocarcinomas and human embryology: pluripotent human EC cell lines. APMIS. 1998;106(1‐6):158–168. doi:10.1111/j.1699-0463.1998.tb01331.x
50. Sasaki K, Nakamura T, Okamoto I, et al. The germ cell fate of cynomolgus monkeys is specified in the nascent amnion. Dev Cell. 2016;39(2):169–185. doi:10.1016/j.devcel.2016.09.007
51. Wong JC, Alon N, Mckerlie C, Huang JR, Meyn MS, Buchwald M. Targeted disruption of exons 1 to 6 of the Fanconi Anemia group A gene leads to growth retardation, strain-specific microphthalmia, meiotic defects and primordial germ cell hypoplasia. Hum Mol Genet. 2003;12(16):2063–2076. doi:10.1093/hmg/ddg219
52. Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci. 1994;91(24):11298–11302. doi:10.1073/pnas.91.24.11298
53. de Kretser DM, Loveland KL, Meinhardt A, Simorangkir D, Wreford N. Spermatogenesis. Hum Reprod. 1998;13(suppl 1):1–8. doi:10.1093/humrep/13.suppl_1.1
54. L’Hernault SW Spermatogenesis. WormBook: the online review of C elegans biology [Internet]: WormBook; 2006.
55. Pellegrini M, Grimaldi P, Rossi P, Geremia R, Dolci S. Developmental expression of BMP4/ALK3/SMAD5 signaling pathway in the mouse testis: a potential role of BMP4 in spermatogonia differentiation. J Cell Sci. 2003;116(16):3363–3372. doi:10.1242/jcs.00650
56. Zhao G-Q, Chen Y-X, Liu X-M, Xu Z, Qi X. Mutation in Bmp7 exacerbates the phenotype of Bmp8a mutants in spermatogenesis and epididymis. Dev Biol. 2001;240(1):212–222. doi:10.1006/dbio.2001.0448
57. Zhao G-Q, Hogan BL. Evidence that mouse Bmp8a (Op2) and Bmp8b are duplicated genes that play a role in spermatogenesis and placental development. Mech Dev. 1996;57(2):159–168. doi:10.1016/0925-4773(96)00543-6
58. Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282(35):25842–25851. doi:10.1074/jbc.M703474200
59. Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays. 2000;22(5):423–430. doi:10.1002/(SICI)1521-1878(200005)22:5<>1.0.CO;2-C
60. Angell R. First-meiotic-division nondisjunction in human oocytes. Am J Hum Genet. 1997;61(1):23–32. doi:10.1086/513890
61. Eberhart CG, Maines JZ, Wasserman SA. Meiotic cell cycle requirement for a fly homologue of human deleted in Azoospermia. Nature. 1996;381(6585):783. doi:10.1038/381783a0
62. Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280. doi:10.1038/35066065
63. Tanaka A, Nagayoshi M, Awata S, Mawatari Y, Tanaka I, Kusunoki H. Completion of meiosis in human primary spermatocytes through in vitro coculture with vero cells. Fertil Steril. 2003;79:795–801. doi:10.1016/S0015-0282(02)04833-1
64. Tesarik J, Mendoza C, Greco E. In-vitro maturation of immature human male germ cells. Mol Cell Endocrinol. 2000;166(1):45–50. doi:10.1016/S0303-7207(00)00296-3
65. Housworth EA, Stahl FW. Crossover interference in humans. Am J Hum Genet. 2003;73(1):188–197. doi:10.1086/376610
66. Sluder G, McCollum D. The mad ways of meiosis. Science. 2000;289(5477):254–255. doi:10.1126/science.289.5477.254
67. Yoon S-R, Dubeau L, de Young M, Wexler NS, Arnheim N. Huntington disease expansion mutations in humans can occur before meiosis is completed. Proc Natl Acad Sci. 2003;100(15):8834–8838. doi:10.1073/pnas.1331390100
68. Dailey T, Dale B, Cohen J, Munné S. Association between nondisjunction and maternal age in meiosis-II human oocytes. Am J Hum Genet. 1996;59(1):176.
69. Lamb NE, Freeman SB, Savage-Austin A, et al. Susceptible chiasmate configurations of chromosome 21 predispose to non–disjunction in both maternal meiosis I and meiosis II. Nat Genet. 1996;14(4):400. doi:10.1038/ng1296-400
70. Rasmussen SW, Holm PB. Human meiosis II. Chromosome pairing and recombination nodules in human spermatocytes. Carlsberg Res Commun. 1978;43(5):275. doi:10.1007/BF02906106
71. Breton B, Billard R. Effects of the temperature and of the photoperiod on trout spermogenesis. Selezione Veterinaria (Italy); 1979.
72. Sapsford C, Rae CA, Cleland K. Ultrastructual studies on spematids and sertoli cells during early spermogenesis in the bandicoot Peramelea nasuta geoffroy (Marsupialia). Aust J Zool. 1967;15(5):881–909. doi:10.1071/ZO9670881
73. Thamer IK, Hussein FA, Khorsheed HH. Study the event cycles of spermatogenesis and spermogenesis in the testes of creeper. Euphrates J Agric Sci. 2016;8(2):1–7.
74. Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science. 1963;140(3563):184–186. doi:10.1126/science.140.3563.184
75. Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc B. 2010;365(1546):1663–1678. doi:10.1098/rstb.2010.0026
76. Sharpe R. Testosterone and spermatogenesis. J Endocrinol. 1987;113(1):1–2. doi:10.1677/joe.0.1130001
77. Eddy EM. Regulation of gene expression during spermatogenesis. Semin Cell Dev Biol. 1998;9(4):451-457.
78. Johnson L. Spermatogenesis and aging in the human. J Androl. 1986;7(6):331–354. doi:10.1002/j.1939-4640.1986.tb00943.x
79. Painter TS. Studies in mammalian spermatogenesis. II. The spermatogenesis of man. J Exp Zool. 1923;37(3):291–336. doi:10.1002/(ISSN)1097-010X
80. De Kretser D, Baker H. Infertility in men: recent advances and continuing controversies. J Clin Endocrinol Metab. 1999;84(10):3443–3450.
81. Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod. 1991;6(6):811–816. doi:10.1093/oxfordjournals.humrep.a137433
82. Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15(6):369–384. doi:10.1038/s41585-018-0003-3
83. Lee JA, Ramasamy R. Indications for the use of human chorionic gonadotropic hormone for the management of infertility in hypogonadal men. Transl Androl Urol. 2018;7(Suppl 3):S348. doi:10.21037/tau.2018.04.11
84. Lotti F, Maggi M. Sexual dysfunction and male infertility. Nat Rev Urol. 2018;15(5):287. doi:10.1038/nrurol.2018.20
85. Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin. 2014;41(1):195–204. doi:10.1016/j.ucl.2013.08.006
86. Corradi PF, Corradi RB, Greene LW. Physiology of the hypothalamic pituitary gonadal axis in the male. Urol Clin. 2016;43(2):151–162. doi:10.1016/j.ucl.2016.01.001
87. Kuiri-Hänninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82(2):73–80. doi:10.1159/000362414
88. Costanzo P, Suárez S, Scaglia H, Zylbersztein C, Litwak L, Knoblovits P. Evaluation of the hypothalamic‐pituitary‐gonadal axis in eugonadal men with type 2 diabetes mellitus. Andrology. 2014;2(1):117–124. doi:10.1111/andr.2013.2.issue-1
89. Tsatsanis C, Dermitzaki E, Avgoustinaki P, Malliaraki N, Mytaras V, Margioris AN. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis. Hormones. 2015;14(4):549–562. doi:10.14310/horm.2002
90. Xiong X, Zhong A, Xu H. Effect of cyanotoxins on the hypothalamic–pituitary–gonadal axis in male adult mouse. PLoS One. 2014;9(11):e106585. doi:10.1371/journal.pone.0106585
91. Lucas X. Clinical use of deslorelin (GnRH agonist) in companion animals: a review. Reprod Domest Anim. 2014;49:64–71. doi:10.1111/rda.2014.49.issue-s4
92. Monaco D, Fatnassi M, Padalino B, et al. Effects of a GnRH administration on testosterone profile, libido and semen parameters of dromedary camel bulls. Res Vet Sci. 2015;102:212–216. doi:10.1016/j.rvsc.2015.08.011
93. Cejko BI, Żarski D, Judycka S, Kucharczyk D, Sarosiek B, Kowalski RK. Effect of two commercial preparations containing different GnRH analogues with dopamine antagonists on barbel Barbus barbus (L.) sperm quantity and quality. Aquac Int. 2014;22(1):97–109. doi:10.1007/s10499-013-9657-2
94. Ullah H, Ambreen A, Ahsan N, Jahan S. Bisphenol S induces oxidative stress and DNA damage in rat spermatozoa in vitro and disrupts daily sperm production in vivo. Toxicol Environ Chem. 2017;99(5–6):953–965. doi:10.1080/02772248.2016.1269333
95. Kotan LD, Hutchins BI, Ozkan Y, et al. Mutations in FEZF1 cause Kallmann syndrome. Am J Hum Genet. 2014;95(3):326–331. doi:10.1016/j.ajhg.2014.08.006
96. Balasubramanian R, Crowley WF
97. Pienkowski C, Tauber M. Gonadotropin-releasing hormone agonist treatment in sexual precocity. In: Bourguignon JP, Parent AS, editors. Puberty from Bench to Clinic. Vol. 29. Karger Publishers; 2016:214–229.
98. Schagen SE, Cohen-Kettenis PT, Delemarre-van de Waal HA, Hannema SE. Efficacy and safety of gonadotropin-releasing hormone agonist treatment to suppress puberty in gender dysphoric adolescents. J Sex Med. 2016;13(7):1125–1132. doi:10.1016/j.jsxm.2016.05.004
99. Kiezun M, Smolinska N, Maleszka A, Dobrzyn K, Szeszko K, Kaminski T. Adiponectin expression in the porcine pituitary during the estrous cycle and its effect on LH and FSH secretion. Am J Physiol Endocrinol Metab. 2014;307(11):E1038–E1046. doi:10.1152/ajpendo.00299.2014
100. Wdowiak A, Raczkiewicz D, Stasiak M, Bojar I. Levels of FSH, LH and testosterone, and sperm DNA fragmentation. Neuroendocrinol Lett. 2014;35(1):73–79.
101. Ekman B, Fitts D, Marelli C, Murray RD, Quinkler M, Zelissen PM. European Adrenal Insufficiency Registry (EU-AIR): a comparative observational study of glucocorticoid replacement therapy. BMC Endocr Disord. 2014;14(1):40. doi:10.1186/1472-6823-14-40
102. Fukuda I, Hizuka N, Muraoka T, Ichihara A. Adult growth hormone deficiency: current concepts. Neurol Med Chir (Tokyo). 2014;54(8);599–605.
103. Marrag I, Hajji K, Braham MY, Dhifallah M, Nasr M. Antipsychotics and hyperprolactinemia: prevalence and risk factors. Ann Psychiatry Ment Health. 2015;3(6):1047.
104. Sengupta P, Agarwal A, Pogrebetskaya M, Roychoudhury S, Durairajanayagam D, Henkel R. Role of Withania somnifera (Ashwagandha) in the management of male infertility. Reprod Biomed Online. 2018;36(3):311–326. doi:10.1016/j.rbmo.2017.11.007
105. Sun X-L, Wang J-L, Peng Y-P, et al. Bilateral is superior to unilateral varicocelectomy in infertile males with left clinical and right subclinical varicocele: a prospective randomized controlled study. Int Urol Nephrol. 2018;50(2):205–210. doi:10.1007/s11255-017-1749-x
106. Fallahi S, Rostami A, Shiadeh MN, Behniafar H, Paktinat S. An updated literature review on maternal-fetal and reproductive disorders of Toxoplasma gondii infection. J Gynecol Obstet Hum Reprod. 2018;47(3):133–140. doi:10.1016/j.jogoh.2017.12.003
107. Davison P, Morris J. Mumps. StatPearls [Internet]. StatPearls Publishing; 2018.
108. Tsoumanis A, Hens N, Kenyon CR. Is screening for chlamydia and gonorrhea in men who have sex with men associated with reduction of the prevalence of these infections? A systematic review of observational studies. Sex Transm Dis. 2018;45(9):615–622. doi:10.1097/OLQ.0000000000000824
109. Omolaoye T, Du Plessis SS. Diabetes mellitus and male infertility. 2018.
110. Katib A. Mechanisms linking obesity to male infertility. Cent European J Urol. 2015;68(1):79. doi:10.5173/ceju.2015.01.435
111. Otunctemur A, Bozkurt M, Besiroglu H, Polat EC, Ozcan L, Ozbek E. Erectile dysfunction is positively correlated with mean platelet volume and platelet count, but not with eosinophil count in peripheral blood. Urol J. 2015;12(5):2347–2352.
112. Mustafa M, Sharifa AM, Hadi J, IIIzam E, Aliya S. Male and female infertility: causes, and management. IOSR-JDMS. 2019;18(9):27-32.
113. Rim K-T. Reproductive toxic chemicals at work and efforts to protect workers’ health: a literature review. Saf Health Work. 2017;8(2):143–150. doi:10.1016/j.shaw.2017.04.003
114. Liu K-S, Pan F, Chen Y-J, Mao X-D. The influence of sperm DNA damage and semen homocysteine on male infertility. Reprod Dev Med. 2017;1(4):228. doi:10.4103/2096-2924.224910
115. Al-Otaibi ST. Male infertility among bakers associated with exposure to high environmental temperature at the workplace. J Taibah Univ Med Sci. 2018;13(2):103. doi:10.1016/j.jtumed.2017.12.003
116. Gaur DS, Talekar MS, Pathak VP. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010;53(1):35. doi:10.4103/0377-4929.59180
117. Petraglia F, Serour GI, Chapron C. The changing prevalence of infertility. Int J Gynecol Obstet. 2013;123:S4–S8. doi:10.1016/j.ijgo.2013.09.005
118. Tsai Y-H, Wang T-W, Wei H-J, et al. Dietary intake, glucose metabolism and sex hormones in women with polycystic ovary syndrome (PCOS) compared with women with non-PCOS-related infertility. Br J Nutr. 2013;109(12):2190–2198. doi:10.1017/S0007114512004369
119. Onyije F. Drug: a major cause of infertility in male. Asian J Med Pharm Res. 2012;2(2):30–37.
120. Carré J, Gatimel N, Moreau J, Parinaud J, Léandri R. Does air pollution play a role in infertility?: a systematic review. Environ Health. 2017;16(1):82. doi:10.1186/s12940-017-0291-8
121. Evenson DP, Wixon R. Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology. 2006;65(5):979–991. doi:10.1016/j.theriogenology.2005.09.011
122. Hammoud A, Carrell DT, Gibson M, Sanderson M, Parker-Jones K, Peterson CM. Decreased sperm motility is associated with air pollution in Salt Lake City. Fertil Steril. 2010;93(6):1875–1879. doi:10.1016/j.fertnstert.2008.12.089
123. Najafi TF, Roudsari RL, Namvar F, Ghanbarabadi VG, Talasaz ZH, Esmaeli M. Air pollution and quality of sperm: a meta-analysis. Iran Red Crescent Med J. 2015;17(4). doi:10.5812/ircmj.23191v2
124. Güney A, Javadova D, Kırac D, et al. Detection of Y chromosome microdeletions and mitochondrial DNA mutations in male infertility patients. Genet Mol Res. 2012;11(2):1039–1048. doi:10.4238/2012.April.27.2
125. Stouffs K, Seneca S, Lissens W. Genetic causes of male infertility. Annales d’endocrinologie. 2014;75(2):109-111.
126. Wang R-X, Fu C, Yang Y-P, et al. Male infertility in China: laboratory finding for AZF microdeletions and chromosomal abnormalities in infertile men from Northeastern China. J Assist Reprod Genet. 2010;27(7):391–396. doi:10.1007/s10815-010-9420-9
127. Mafra FA, Christofolini DM, Bianco B, et al. Chromosomal and molecular abnormalities in a group of Brazilian infertile men with severe oligozoospermia or non-obstructive azoospermia attending an infertility service. Int Braz J Urol. 2011;37(2):244–251. doi:10.1590/S1677-55382011000200011
128. Pylyp LY, Spinenko LO, Verhoglyad NV, Zukin VD. Chromosomal abnormalities in patients with oligozoospermia and non-obstructive azoospermia. J Assist Reprod Genet. 2013;30(5):729–732. doi:10.1007/s10815-013-9990-4
129. Groth KA, Skakkebæk A, Høst C, Gravholt CH, Bojesen A. Klinefelter syndrome—a clinical update. J Clin Endocrinol Metab. 2013;98(1):20–30. doi:10.1210/jc.2012-2382
130. Wikström AM, Dunkel L. Klinefelter syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25(2):239–250. doi:10.1016/j.beem.2010.09.006
131. Roberts AE, Allanson JE, Tartaglia M, Gelb BD. Noonan syndrome. Lancet. 2013;381(9863):333–342. doi:10.1016/S0140-6736(12)61023-X
132. Romano AA, Allanson JE, Dahlgren J, et al. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2010;126(4):746–759. doi:10.1542/peds.2009-3207
133. Bianco B, Christofolini D, Gava M, Mafra F, Moraes E, Barbosa C. Severe oligospermia associated with a unique balanced reciprocal translocation t (6; 12)(q23; q24. 3): male infertility related to t (6; 12). Andrologia. 2011;43(2):145–148. doi:10.1111/and.2011.43.issue-2
134. Choi BH, Kim UH, Lee KS, Ko CW. Various endocrine disorders in children with t (13; 14)(q10; q10) Robertsonian translocation. Ann Pediatr Endocrinol Metab. 2013;18(3):111. doi:10.6065/apem.2013.18.3.111
135. Furuta Y, Kawai M, Yahara K, et al. Birth and death of genes linked to chromosomal inversion. Proc Natl Acad Sci. 2011;108(4):1501–1506. doi:10.1073/pnas.1012579108
136. Neto FTL, Bach PV, Najari BB, Li PS, Goldstein M. Genetics of male infertility. Curr Urol Rep. 2016;17(10):70. doi:10.1007/s11934-016-0627-x
137. Vijayalakshmi J, Venkatchalam P, Paul SF, Usha Rani G, Kumarasamy P, Kannan J. Chromosomal anomalies in patients with azoospermia and oligoasthenoteratozoospermia. Int J Hum Genet. 2011;11(2):117–121. doi:10.1080/09723757.2011.11886133
138. Vog P, Edelmann A, Kirsch S, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet. 1996;5(7):933–943. doi:10.1093/hmg/5.7.933
139. Hopps C, Mielnik A, Goldstein M, Palermo G, Rosenwaks Z, Schlegel P. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18(8):1660–1665. doi:10.1093/humrep/deg348
140. Foresta C, Moro E, Ferlin A. Y chromosome microdeletions and alterations of spermatogenesis. Endocr Rev. 2001;22(2):226–239. doi:10.1210/edrv.22.2.0425
141. Pryor JL, Kent-First M, Muallem A, et al. Microdeletions in the Y chromosome of infertile men. N Engl J Med. 1997;336(8):534–540. doi:10.1056/NEJM199702203360802
142. Choi JM, Chung P, Veeck L, Mielnik A, Palermo GD, Schlegel PN. AZF microdeletions of the Y chromosome and in vitro fertilization outcome. Fertil Steril. 2004;81(2):337–341. doi:10.1016/j.fertnstert.2003.06.030
143. Pan S, Bearelly P, Oates RD. Fertility in men with Klinefelter syndrome and Y chromosome microdeletions: an update. Curr Opin Endocr Metab Res. 2019;6:21–28. doi:10.1016/j.coemr.2019.04.005
144. Blagosklonova O, Fellmann F, Clavequin M-C, Roux C, Bresson J-L. AZFa deletions in Sertoli cell-only syndrome: a retrospective study. Mol Hum Reprod. 2000;6(9):795–799. doi:10.1093/molehr/6.9.795
145. de Kretser DM. Male infertility. Lancet. 1997;349(9054):787–790. doi:10.1016/S0140-6736(96)08341-9
146. Jarzabek K, Zbucka M, Pepiński W, et al. Cystic fibrosis as a cause of infertility. Reprod Biol. 2004;4(2):119–129.
147. Munro NC, Currie DC, Lindsay KS, et al. Fertility in men with primary ciliary dyskinesia presenting with respiratory infection. Thorax. 1994;49(7):684–687. doi:10.1136/thx.49.7.684
148. Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. doi:10.1126/science.1108190
149. Dada R, Kumar M, Jesudasan R, Fernández JL, Gosálvez J, Agarwal A. Epigenetics and its role in male infertility. J Assist Reprod Genet. 2012;29(3):213–223. doi:10.1007/s10815-012-9715-0
150. Rajender S, Avery K, Agarwal A. Epigenetics, spermatogenesis and male infertility. Mutat Res Rev Mutat Res. 2011;727(3):62–71. doi:10.1016/j.mrrev.2011.04.002
151. Boissonnas CC, Jouannet P, Jammes H. Epigenetic disorders and male subfertility. Fertil Steril. 2013;99(3):624–631. doi:10.1016/j.fertnstert.2013.01.124
152. Guerrero-Bosagna C, Savenkova M, Haque MM, Nilsson E, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of altered Sertoli cell transcriptome and epigenome: molecular etiology of male infertility. PLoS One. 2013;8(3):e59922. doi:10.1371/journal.pone.0059922
153. Khazamipour N, Noruzinia M, Fatehmanesh P, Keyhanee M, Pujol P. MTHFR promoter hypermethylation in testicular biopsies of patients with non-obstructive azoospermia: the role of epigenetics in male infertility. Hum Reprod. 2009;24(9):2361–2364. doi:10.1093/humrep/dep194
154. Aston K. Genetic susceptibility to male infertility: news from genome‐wide association studies. Andrology. 2014;2(3):315–321. doi:10.1111/andr.2014.2.issue-3
155. Carrell DT, Aston KI. The search for SNPs, CNVs, and epigenetic variants associated with the complex disease of male infertility. Syst Biol Reprod Med. 2011;57(1–2):17–26. doi:10.3109/19396368.2010.521615
156. Coffey AJ, Kokocinski F, Calafato MS, et al. The GENCODE exome: sequencing the complete human exome. Eur J Hum Genet. 2011;19(7):827. doi:10.1038/ejhg.2011.28
157. Boxmeer JC, Smit M, Weber RF, et al. Seminal plasma cobalamin significantly correlates with sperm concentration in men undergoing IVF or ICSI procedures. J Androl. 2007;28(4):521–527. doi:10.2164/jandrol.106.001982
158. Collins JA. An international survey of the health economics of IVF and ICSI. Hum Reprod Update. 2002;8(3):265–277. doi:10.1093/humupd/8.3.265
159. Haagen E, Tuil W, Hendriks J, Bruijn R, Braat D, Kremer J. Current internet use and preferences of IVF and ICSI patients. Hum Reprod. 2003;18(10):2073–2078. doi:10.1093/humrep/deg423
160. Kushnir VA, Frattarelli JL. Aneuploidy in abortuses following IVF and ICSI. J Assist Reprod Genet. 2009;26(2–3):93–97. doi:10.1007/s10815-009-9292-z
161. Friedler S, Raziel A, Strassburger D, Schachter M, Soffer Y, Ron-El R. Factors influencing the outcome of ICSI in patients with obstructive and non-obstructive azoospermia: a comparative study. Hum Reprod. 2002;17(12):3114–3121. doi:10.1093/humrep/17.12.3114
162. Wosnitzer M, Goldstein M, Hardy MP. Review of azoospermia. Spermatogenesis. 2014;4(1):e28218. doi:10.4161/spmg.28218
163. Abdel Raheem A, Garaffa G, Rushwan N, et al. Testicular histopathology as a predictor of a positive sperm retrieval in men with non‐obstructive azoospermia. BJU Int. 2013;111(3):492–499. doi:10.1111/j.1464-410X.2012.11203.x
164. Vernaeve V, Bonduelle M, Tournaye H, Camus M, Van Steirteghem A, Devroey P. Pregnancy outcome and neonatal data of children born after ICSI using testicular sperm in obstructive and non‐obstructive azoospermia. Hum Reprod. 2003;18(10):2093–2097. doi:10.1093/humrep/deg403
165. Vloeberghs V, Verheyen G, Haentjens P, Goossens A, Polyzos N, Tournaye H. How successful is TESE-ICSI in couples with non-obstructive azoospermia? Hum Reprod. 2015;30(8):1790–1796. doi:10.1093/humrep/dev139
166. Bohring C, Schroeder-Printzen I, Weidner W, Krause W. Serum levels of inhibin B and follicle-stimulating hormone may predict successful sperm retrieval in men with azoospermia who are undergoing testicular sperm extraction. Fertil Steril. 2002;78(6):1195–1198. doi:10.1016/S0015-0282(02)04259-0
167. Ballescá JL, Balasch J, Calafell JM, et al. Serum inhibin B determination is predictive of successful testicular sperm extraction in men with non-obstructive azoospermia. Hum Reprod. 2000;15(8):1734–1738. doi:10.1093/humrep/15.8.1734
168. Franco G, Scarselli F, Casciani V, et al. A novel stepwise micro-TESE approach in non obstructive azoospermia. BMC Urol. 2016;16(1):20. doi:10.1186/s12894-016-0138-6
169. Alrabeeah K, Wachter A, Phillips S, Cohen B, Al‐Hathal N, Zini A. Sperm retrieval outcomes with microdissection testicular sperm extraction (micro‐TESE) in men with cryptozoospermia. Andrology. 2015;3(3):462–466. doi:10.1111/andr.12000
170. Deruyver Y, Vanderschueren D, Van der Aa F. Outcome of microdissection TESE compared with conventional TESE in non‐obstructive azoospermia: a systematic review. Andrology. 2014;2(1):20–24. doi:10.1111/andr.2013.2.issue-1
171. Haliloglu AH, Tangal S, Gulpinar O, Onal K, Pabuçcu R. Should repeated TESE be performed following a failed TESE in men with Klinefelter syndrome? Andrology. 2014;2(1):42–44. doi:10.1111/andr.2013.2.issue-1
172. Kalsi J, Thum M-Y, Muneer A, Abdullah H, Minhas S. In the era of micro‐dissection sperm retrieval (m‐TESE) is an isolated testicular biopsy necessary in the management of men with non‐obstructive azoospermia? BJU Int. 2012;109(3):418–424. doi:10.1111/bju.2012.109.issue-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
