Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Some inferences from in vivo experiments with metal and metal oxide nanoparticles: the pulmonary phagocytosis response, subchronic systemic toxicity and genotoxicity, regulatory proposals, searching for bioprotectors (a self-overview)
Authors Katsnelson B, Privalova L, Sutunkova M, Gurvich V, Loginova N, Minigalieva I, Kireyeva E, Shur V, Shishkina E, Beikin Y, Makeyev O, Valamina I
Received 13 January 2015
Accepted for publication 15 February 2015
Published 16 April 2015 Volume 2015:10(1) Pages 3013—3029
DOI https://doi.org/10.2147/IJN.S80843
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Boris A Katsnelson,1 Larisa I Privalova,1 Marina P Sutunkova,1 Vladimir B Gurvich,1 Nadezhda V Loginova,1 Ilzira A Minigalieva,1 Ekaterina P Kireyeva,1 Vladimir Y Shur,2 Ekaterina V Shishkina,2 Ya B Beikin,3 Oleg H Makeyev,4 Irene E Valamina4
1The Medical Research Center for Prophylaxis and Health Protection in Industrial Workers, Ekaterinburg, Russia; 2The Institute of Natural Sciences, The Ural Federal University, Ekaterinburg, Russia; 3The City Clinical Diagnostics Centre, Ekaterinburg, Russia; 4The Ural State Medical University, Ekaterinburg, Russia
Abstract: The purpose of this paper is to overview and summarize previously published results of our experiments on white rats exposed to either a single intratracheal instillation or repeated intraperitoneal injections of silver, gold, iron oxide, copper oxide, nickel oxide, and manganese oxide nanoparticles (NPs) in stable water suspensions without any chemical additives. Based on these results and some corroborating data of other researchers we maintain that these NPs are much more noxious on both cellular and systemic levels as compared with their 1 µm or even submicron counterparts. However, within the nanometer range the dependence of systemic toxicity on particle size is intricate and non-unique due to complex and often contra-directional relationships between the intrinsic biological aggressiveness of the specific NPs, on the one hand, and complex mechanisms that control their biokinetics, on the other. Our data testify to the high activity of the pulmonary phagocytosis of NPs deposited in airways. This fact suggests that safe levels of exposure to airborne NPs are possible in principle. However, there are no reliable foundations for establishing different permissible exposure levels for particles of different size within the nanometric range. For workroom air, such permissible exposure levels of metallic NP can be proposed at this stage, even if tentatively, based on a sufficiently conservative approach of decreasing approximately tenfold the exposure limits officially established for respective micro-scale industrial aerosols. It was shown that against the background of adequately composed combinations of some bioactive agents (comprising pectin, multivitamin-multimineral preparations, some amino acids, and omega-3 polyunsaturated fatty acid) the systemic toxicity and even genotoxicity of metallic NPs could be markedly attenuated. Therefore we believe that, along with decreasing NP-exposures, enhancing organisms’ resistance to their adverse action with the help of such bioprotectors can prove an efficient auxiliary tool of health risk management in occupations connected with them.
Keywords: nanoparticles, metals, metal oxides, toxicity
Introduction
Nanoparticles (NPs) of metals and of their oxides are of special interest in the light of health risks’ assessment and management because, along with engineered NPs, there exists usually a substantial fraction of nanoscale (“ultrafine”) particles of the same substances within the particle size distribution of condensation aerosols generated by arc-welding and metallurgical technologies. However in such aerosols chemically similar micrometer particles (MPs), including submicron ones having dimensions >100 nm, are usually present as well.
Thus, for metallic NPs more urgently than for many others, some theoretical problems of nanotoxicology in everyday practice needs solving:
- Are NPs recognized by organisms’ main defenses worse than or just as well as respective MPs are?
- Are they much more toxic as compared with MPs on both cellular and systemic levels?
- Is there a definite dependence of defense and adverse responses to NPs on their dimensions within the conventional nanometer range and/or on their chemical nature?
- The last but not the least, is it possible to protect health of people dealing with either engineered or “spontaneous” NPs a) by establishing low but still practicable permissible exposure levels (PELs) and b) by enhancing organisms’ resistance to NPs toxicity (genotoxicity included)?
During 2009–2014 our experimental work was focused on trying to find answers to these important questions.1–10
Although in the vast nanotoxicological literature of the last decade studies concerned with the assessment of metal or metal-oxide NP toxicity are not at the top of the list, such publications are nevertheless quite numerous. To illustrate this, we may refer to several works devoted to NPs of the metals that were the subject-matter of our own studies considered below: iron oxide,11–20 silver,21–45 gold,24,45–58 copper and copper oxide,43,59–68 nickel oxide,65,69–73 and manganese oxides.74–76 A comprehensive critical review of relevant publications is out of this paper’s scope. It should be stressed, however, that the prevailing majority of published researches assessed the cytotoxicity and genotoxicity of NPs on stable cell lines and only rarely on laboratory animals. No doubt, in vitro experiments feature a number of well-known advantages, in particular, relating to analysis of primary mechanisms of toxicity. At the same time, any extrapolation of the results of these experiments to the organism level is associated with a number of uncertainties and assumptions. Moreover, some important aspects (in particular, toxicokinetics, dose-systemic response relationships, the functioning and efficiency of protective mechanisms) can generally be addressed only through experiments on mammals’ whole organism.
Materials and methods
Here we are giving but a brief overview of methodology used in our experiments which were described in detail in original articles.1–10
We experimented with NPs of iron oxide Fe3O4 (magnetite) having mean diameters 10 nm, 50 nm or 1 mcm, gold (4 nm or 50 nm), silver (4 nm, 49 nm or 1.1 mcm), copper oxide (20 nm or 340 nm), nickel oxide NiO (30 nm), and manganese oxide Mn3O4 (32 nm). Keeping in mind the above mentioned theoretical premises of our research, we used purposefully prepared and accurately characterized NPs of pure metals or their oxides suspended in deionized water rather than commercial nanomaterials. Only iron oxide magnetite NPs were synthesized chemically1–3 while in all other cases we used a technique of laser ablation of a 99.99% pure metal target in water followed by laser fragmentation for preventing particle aggregation. The main advantages of this technique are as follows: a) it provides nano-suspensions with a sufficiently narrow particle size distribution (examples are shown in Figures 1 and 2) and b) these suspensions are highly stable as a rule, maintaining their characteristics without any noticeable particle aggregation over periods sufficient for carrying out experiments described below. In no experiment did we add any chemical stabilizer to the suspension, and only in experiments with magnetite and NiO NPs did we have to sonicate the suspensions just before instillations or injections.
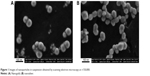  | Figure 1 Images of nanoparticles in suspension obtained by scanning electron microscopy at ×150,000. |
All experiments were carried out on outbred white female rats from our own breeding colony with an initial body weight of 150 to 220 g, with a minimum of 12 animals in different exposed and control groups. Rats were housed in conventional conditions, breathed unfiltered air, and were fed standard balanced food. The experiments were planned and implemented in accordance with the “International Guiding Principles for Biomedical Research Involving Animals” developed by the Council for International Organizations of Medical Sciences (1985) and approved by the Committee on Ethics of the Ekaterinburg Medical Research Center for Prophylaxis and Health Protection in Industrial Workers.
A single intratracheal (IT) instillation of 1 mL of NP or MP water suspension (or of the same deionized water without any particles to controls) served as an experimental model for the response of the low airways to particle deposition. It is well-known that important qualitative and quantitative patterns of the response of the pulmonary free cell population (in particular, its dependence on the cytotoxicity of deposited particles) observed in inhalation exposures to dust particles are principally the same in the case of their IT administration.77 At the same time, IT model provided cellular material for studying the phagocytizing activity of pulmonary macrophages and polymorphonuclear leukocytes, as well as intracellular localization of NPs engulfed by them and ultrastructural damage caused to the cell by those NPs. The results thus obtained are comparable to the data obtained by other researchers in experiments on cell cultures but give a valuable addition to the latter because in vivo interaction between cells and particles occurs in a microenvironment which is not reproducible by artificial cell culture media.
A cell population of bronchoalveolar lavage fluid (BALF) obtained 24 hours after IT instillation to rats of NP or MP suspensions was studied with optical microscopy, transmission electron microscopy (TEM), and semi-contact atomic force microscopy (sc-AFM).
Subchronic toxicity was modeled by means of repeated intraperitoneal (IP) injections of the same particles during 5–7 weeks. Using IP injections for modeling systemic intoxication, which in real conditions can be induced by long-term inhalation exposure of workers needs some justification. It is well-known that “NPs deposit with high efficiency in the entire respiratory tract, from the head airways to the alveoli, due to diffusion”.78 For instance, the widely used Human Respiratory Tract Model of the International Commission of Radiological Protection79,80 predicts 100% total deposition of 0.001 μm (ie, 1 nm) and ~90% for 0.01 μm (ie, 10 nm) particles for a normal adult mouth breathing male human subject. However there are many anatomical, functional, and aerodynamic differences between humans and rodents which make one assume interspecies distinctions in regional particle deposition and thus in the kinetics of their elimination from the airways to the gastrointestinal tract and/or absorption. No wonder that the authors of a comprehensive review of nanotoxicological assessment techniques81 maintain that:
Rodents, the commonly used species for toxicology testing, are obligatory nose breathers and, therefore, not representative models for human respiratory inhalation exposure.
In other words, NP inhalation by laboratory rodents is not as ideal a model of real human exposure as it is often deemed to be.
The IP model permits one to circumvent these interspecies differences of inhaled NP deposition and is adequate enough when one wants to look into body distribution and elimination of NPs, and for organisms’ reactions to NPs after they have penetrated into the blood from a primary deposit. Like any model (always a necessary simplification of a complicated system deliberately omitting some sub-systems and some material or informational flows and feedbacks), it has both drawbacks and virtues. Among the latter, one should take into consideration that dosing by injection is much more accurate and reliable as compared with the more “natural” experimental methods. This reason is crucial for experiments of comparative design like ours. IP modeling of subchronic intoxications is well-known and recognized in general experimental toxicology. Moreover, it was used just in experimental nano-toxicological studies also by other researchers.82,83
In our experiments, systemic toxic effects of particles under comparison were characterized by a lot of biochemical and functional tests, typically comprising:
- weighing of the body and inner organs;
- estimation of the central nervous system ability for the temporal summation of sub-threshold impulses (a variant of withdrawal reflex and its facilitation by repeated electrical stimulations in intact, conscious rat);84
- recording of the number of head-dips into the holes of a hole-board, which is frequently used for studying behavioral effects of toxicants and drugs;85,86
- 24 hours collection of urine for measuring its density, urine output, coproporphyrin, delta-aminolevulinic acid (δ-ALA), and creatinine contents;
- sampling of capillary blood from a notch on the tail for examining the hemogram, hemoglobin content, and for cytochemical determination of SDH activity in lymphocytes (by the reduction of nitrotetrazolium violet to formasan);
- collecting blood by exsanguination from rats killed by decapitation for measuring concentrations of reduced glutathione, total serum protein, albumin, globulin, bilirubin, ceruloplasmin, malondialdehyde, ALP, ALT, AST, CAT, GGT, SH-groups, and creatinine.
The metal content of the liver, spleen, kidneys, and brain was determined with the help of atomic emission spectrometry and, for iron oxide particles, also by the electron paramagnetic resonance method.
Thin sections of different organs were prepared for histological examination by hematoxylin and eosin stain and, where necessary, periodic acid Schiff, Nissl and Perl’s stains. We used a planimetric ocular grid for morphometry of spleen and liver and an image recognition programmed system for that of kidneys and brain.
In the subchronic experiments with NPs and MPs of Ag, Au, and CuO the genomic DNA fragmentation was assessed in cells of several tissues using the random amplified polymorphic DNA test.
Both systemic and genotoxicity of nAg and of nCuO were assessed also against the background of oral administration of bio-protective complexes (BPC) comprising pectin, multivitamin–multimineral preparations, some amino acids, and omega-3 polyunsaturated fatty acid.7,8 Premedication with a similar BPC (without pectin) was administered to rats instilled afterward intratracheally with NiO+Mn3O4 NPs.10
Main results and discussion
We propose to summarize and discuss our results in light of the questions suggested in the Introduction.
Are NPs recognized and dealt with by the organism worse than, or just as well as or even better than respective microparticles are?
Practical implications of this seemingly theoretical question hardly needs explanation. Indeed, if physiological defenses against NPs are of a low effectiveness one should assume that non-harmful exposures to NPs would be hardly possible, in principle. In other words, no safe exposure levels for NPs in the environment should be established.
This problem is not contrived by us. In the beginning of the “nanotoxicological era”, it was maintained by some authors (for instance Donaldson et al and Oberdörster et al)87,88 that the physiological protective mechanisms enabling animals and humans to exist in an atmosphere unavoidably polluted with suspended particles of a wide range of sizes and chemical compositions have, for whatever reasons, little effectiveness in relation to NPs. It was thought, specifically, that NPs deposited in the pulmonary region are not efficiently phagocytized by alveolar macrophages (AM) due either to their inability to recognize the smallest particles or to the failure of the latter to generate a chemotactic signal at the site of their deposition. For instance, 10 years ago a highly reputable group of experts88 maintained that “very small particles […] may not be detected by the normal phagocytic defenses”. Even as recently as in 2012 the author of a comprehensive review89 did not present this problem as solved:
Macrophages engulf microbes and apoptotic debris, but the question is: are NPs recognized by phagocytes or do such particles fly under the radar and escape immune recognition?
We started off our own research into this field1,2 with some criticism of the research data seemingly corroborating those views and with skepticism based on evolutionary considerations. We stressed that terrestrial vertebrates have had to inhale ultrafine nanoscale particles (such as volcanic ash, airborne particles of dispersed seawater, forest fire smoke, sulfates generated in the atmosphere as a result of sulfur dioxide oxidation) for as long as they have inhaled fine microparticles. We reminded that both main mechanisms of pulmonary clearance (engulfing of particles by phagocytes and mucociliary transport) had been present already in amphibians,90 that is even before the morphological structuring of the lungs was complete. We maintained that it would be difficult to understand why these ancient defenses, highly efficient in relation to MPs, should have been selected and fixed by the evolution had they been ineffective in relation to NPs against which defense is even more necessary since they are presumably more noxious (see below).
From the very first experiment, however, our data suggested that organism are not defenseless when confronted with metallic NPs’ exposures. In particular, we have found that just the pulmonary phagocytosis response to deposition of NPs is quite potent. In several experiments it was found that, given equal mass doses and identical metal composition, NPs induced intensive recruitment of phagocytes increasing cell count in the BALF to a greater degree than MPs did. One example illustrating this is given in Table 1. Moreover, this recruitment and especially an increase in neutrophil leukocytes (NL) to AM ratio in the BALF cell count is the more pronounced, the smaller is the particle within the nanoscale range as illustrated by Figure 3.
Such NLs’ recruitment toward the lower airways in response to the deposition of particles is quite often described as “inflammation” and, thus, as a pathological phenomenon rather than a pulmonary defense mechanism. This concept is common to nanotoxicological studies as well.73,91–95 We maintain, however, that it can be misleading. Beyond any doubt, enhanced recruitment of NLs onto the free surface of lower airways is typical of acute and, to a lesser degree, chronic inflammatory processes induced by microbial or chemical agents. However, a certain number of NLs is always present in the BALF of healthy animals, at least when they are constantly inhaling unfiltered air. They are hardly always living with chronic inflammation of the respiratory system. Meantime, it is long ago that we96–100 found fairly strong reasons for considering the NL recruitment response to be an important mechanism of partial compensation for the damage caused by cytotoxic micrometric particles to AMs, the main effector of the pulmonary clearance. A mathematical multi-compartmental model of pulmonary region clearance which comprised just this compensatory mechanism simulated very well the pulmonary retention of dusts of varying degrees of cytotoxicity (quartzite rock, titanium dioxide, standard quartz DQ12) under long-term inhalation exposure as well as a decrease in this retention under the effect of such potent protectors of the macrophage against particle cytotoxicity as glutamate.
In the same way, data obtained in our experiments with metallic NPs demonstrate that in this case too the enhanced NL recruitment, be it a manifestation of the inflammation or a normal physiological pulmonary particle clearance mechanism, takes an important part in this cleaning process. It should be stressed that both types of recruited phagocytes (that is not only AMs but NLs as well) engulf NPs much more avidly compared with MPs of the same metal, and the smaller the diameter of particles, the more active their phagocytosis by these cells. We illustrate these relationships with an example of magnetite particles (Figure 4).
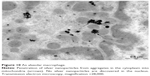
sc-AFM reveals multiple “pits” on the surfaces of both AMs and NLs, and the greater the particles, the greater the dimensions of these pits (examples are shown in Figures 5 and 6). At the same time, the smaller the particle, the higher the surface concentration of these pits (Figure 7). We consider them to be not mere “footprints” of particles passively penetrating into cells through the cell membrane and leaving a hole but a fixed moment of its invagination at the first stage of active endocytosis. Indeed, although the mean diameter of these pits correlates with that of IT instilled NPs, the former is not equal to but usually greater than the latter. For instance, under exposure to 49 nm silver particles and 50 nm gold particles the average diameter of pits was 75.2±0.3 nm and 77.6±1.5 nm, respectively.7
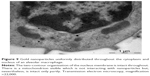
The TEM images (Figure 8) also testify to the physiological endocytosis of NPs as opposed to their direct penetration through cell membrane by diffusion, which, above any doubts, is also possible.4–6
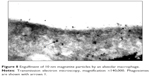  | Figure 8 Engulfment of 10 nm magnetite particles by an alveolar macrophage. |
When NPs are of given dimensions, the pulmonary phagocytosis response strongly depends on their chemical nature. For instance, as can be seen from Table 2, the total cell, NL and AM counts, and the macrophage count of the BALF increased under the impact of silver NPs more than it did under the impact of virtually equidimensional gold NPs. A similar dependence of NL recruitment on the chemical nature of NPs was shown in a comparative experiment with nano-suspensions of NiO and Mn3O4.10
Judging by the sc-AFM images, nanosilver was engulfed by phagocytic cells twice more avidly than nanogold since the average number of surface “pits” per square micrometer was 18.0 and 9.0, respectively.7 Under TEM it was evident that intracellular distribution of the internalized silver and gold NPs was also different, the most significant being a more pronounced affinity of nAg for mitochondria and a lesser ability to penetrate into cell nuclei as compared with nAu (Figures 9 and 10).7
In another experiment we compared responses to IT instillation of CuO NPs (20 nm) and submicron particles (340 nm) and again found that NPs evoked more significant recruitment of NLs than even these fine MPs did (9.41±2.01×106 and 3.64±0.90×106 respectively, the control value being 0.05±0.01×106). The respective NL/AM ratio values were 4.76±1.39, 1.39±0.16, and 0.06±0.01. sc-AFM again revealed pits on the cell surface (Figure 6) with average diameters 25±1 nm under exposure to NPs and 290±14 nm under exposure to MPs, the average surface density of these pits being 32.6 and 1.9 per μm2, respectively. A hypothetical explanation of positive rank correlations between NL/AM ratio and phagocytosis activity as judged by sc-AFM will be discussed in the next section.
To sum up the data discussed in this sub-section, it seems evident that:
- the pulmonary phagocytosis response to metallic NPs’ deposition (namely, phagocytes recruitment toward the free surface of lower airways and phagocytic activity of these cells) is more pronounced than to the deposition of respective MPs,
- this response depends on both NP dimensions and their chemical characteristics.
Most importantly, we found that pulmonary tissue was indeed liberated from deposited NPs quicker than from their micrometric counterparts, the smaller the NPs, the quicker the process.1,3 We believe that this is due not only to quicker dissolution but also to more active phagocytosis of the smallest NPs. However, both physicochemical and physiological mechanisms of NPs pulmonary clearance, while attenuating the harmful impact of NPs on lungs, serve as prerequisites for the toxic action of NPs on other organs either through the gastrointestinal tract (whereto they are translocated with the normal mucociliary transport) or directly through blood (as a result of their dissolution/absorption or of penetration/diffusion). Thus we should discuss the further two crucial questions posed in the Introduction.
Are metallic NPs really much more toxic as compared with microparticles of the same chemical nature on both cellular and systemic level? If they are, is there a definite dependence of organisms’ adverse responses to NP exposure on their dimensions within the conventional nanometer range and/or on their chemical nature?
The paradigm of a considerably higher biological aggressiveness of NPs as compared with particles of the same substance in the micrometric range first emerged as a theoretically sound perception.87,88,101 Very soon some experimental facts corroborating this perception were obtained by different researchers, but this corroboration was during a period neither unanimous nor absolutely reliable due to some drawbacks and lack of comparability between different experimental designs. Thus, even as recently as 5 years ago very reputable authors would justifiably state in the oldest specialized nanotoxicological journal that “this common perception of greater NP toxicity is based on a limited number of studies”,95 and still less was known about the comparative toxicity of chemically identical particles having different sizes within the conventional nanometric range.
However, quickly the situation has changed substantially. Today’s body of facts allows one to say without qualification that, given identical exposure pathways and similar chemistry, the toxic effects of metal and metal oxide NPs are much higher than those of their micrometer counterparts, even of minimal (including submicron) sizes of the latter, while for a given size the toxicity of NPs depends on their chemical nature and related properties, including solubility. Along with the latter property, quite often causing “the Trojan horse effect” (intracellular release of toxic metal ions by engulfed NPs), another now widely recognized major mechanism of NP cytotoxicity and, especially, genotoxicity is the generation of ROS.102
The toxic impact of particles on lung phagocytes is of a special significance not only as an important unfavorable factor influencing pulmonary clearance but also as an in vivo assessment of comparative particle cytotoxicity in a broader sense of the term. As it was mentioned above, recruitment of new echelons of NLs (beginning from enhanced differentiation of stem cells) prevailing over that of macrophages is a mechanism of compensation, even if partial, for the breakdown of AMs damaged by cytotoxic particles. It was found that this recruitment is controlled by the mass of macrophage breakdown products (MBP) and especially by their lipid fraction.96–100 Therefore, the more cytotoxic for AMs are particles deposited in the lungs (or the higher the dose of MBPs obtained by aseptic freezing-thawing or ultrasonic destruction of non-activated peritoneal macrophages and then instilled intratracheally), the higher is a count ratio of NLs to AMs in the BALF. Therefore this ratio (NL/AM) can be used as an indirect but highly informative comparative in vivo index for the cytotoxic action of any low-soluble particle, and it was demonstrated that ranking of dusts by this index was well correlated with the ranking of their cytotoxicity based on the Trypan blue exclusion test for cell viability in vitro. All these earlier revealed facts permitted us to use the BALF NL/AM ratio as an in vivo estimate of comparative cytotoxicity for NPs as well.
Using this index, we showed that the investigated NPs were far more cytotoxic as compared with even the smallest MPs of the same metal (as examples, see Table 1 for silver NPs versus MPs as well as the values given in the text above for copper oxide NPs versus submicron particles). Moreover, within the nanoscale range, the smaller a particle, the more cytotoxic it was (see Figure 3 for iron oxide particles with different dimensions). Given a virtually equal nanoscale size, the cytotoxicity of particles can be quite different depending on their chemical nature. Thus we found, judging by NL/FM ratio, that nano-Ag was far more cytotoxic as compared with nano-Au (see the NL/AM column in Table 2) and that nano-NiO was more cytotoxic as compared with nano-Mn3O4.10 There are differences also in ultrastructural damage to the macrophage caused by different nanometals. For instance, nanosilver was found more prone to damage mitochondrial membranes and cristae than nanogold.7
Different solubility of different nanometals is one of probable explanations of their different cytotoxicity (in particular, silver NPs were proved more soluble as compared with equidimensional gold ones) but hardly the only one. Based on a review of extensive literature, Fröhlich102 believes that, commonly for many metals and their oxides, the release of free ions is a second important mechanism of NP cytotoxicity (the first one being the generation of ROS as a result of various chemical reactions).
When comparing the BALF cell absolute counts and quantity of “pits” on the surface of these cells revealed by the sc-AFM with the NL/AM ratio values, we can see that the more cytotoxic the NPs are (because of either their smaller dimensions or their chemical nature, or both) the more active the pulmonary phagocytosis response is and the more avidly these NPs are engulfed by phagocytes. This phenomenon can be easily explained, as MBPs stimulate not only the recruitment but also the phagocytic activity of viable macrophages judging by the results of an in vitro test with 1 μm polystyrene beadles.103 It stands to reason, however, that such compensation for AMs’ breakdown cannot be complete and, in the final count, the especially high cytotoxicity of NPs can be regarded as their very dangerous characteristic increasing along with decrease in their diameter.
The relationship between particle dimensions and their toxicity is not so unequivocal on the organs and organism levels. For instance, judging by our results,1,3 the subchronic toxicity of magnetite NPs was higher as compared with that of 1 μm particles, but within the nano-scale range the relationship under consideration proved inverse for some effects. Such seemingly paradoxical dependence of adverse effects of NPs on their diameter is quite characteristic of target organs rich in reticuloendothelial system and thus capable of actively accumulating NPs from blood – such as liver and spleen.
This fact should be attributed to differences in NP toxicokinetics. The latter is controlled, first of all, by their more or less easy penetration through biological barriers into the bloodstream from sites of their primary deposition to be then captured by reticuloendothelial system cells of this or that organ. It may be assumed that this mechanism of particle translocation has to be more effective for smaller NPs. However, the smaller the particle, the quicker it dissolves from these secondary depots due to its immense specific surface. Besides, the smallest NPs are presumably more cytotoxic for any cells, resident macrophages included, and so cause their destruction (with eventual liberation of NPs back to bloodstream) more effectively. The balance between these oppositely acting mechanisms of NP retention in different tissues depends on many variables. The result can be a greater load of an organ with larger NPs than with smaller ones (but, at the same time, much greater for both as compared with microparticles) as illustrated for magnetite NPs and MPs in Figure 11. It is no wonder that some morphometric indices for the toxic damage to the liver were more prominent in rats exposed to 50 nm particles than to 10 nm particles of magnetite, while NPs of both sizes proved more hepatotoxic as compared with microparticles (Table 3).
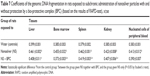
Above any doubt, the systemic toxicity of equidimensional NPs depends on the toxic properties of the metal as such and can differ very much between different metals. For instance, nanosilver is more toxic than nanogold when tested in parallel, and specifically, nanosilver is more genotoxic in vivo compared with nanogold judging by the results of the random amplified polymorphic DNA test performed on cells of different tissues of rats under subchronic intoxication (Table 4).7 The fact that these differences in the genotoxicity of nanometals compared are less pronounced in the kidneys, can, most probably, be explained by the fact that silver NPs, which, judging by their considerably higher solubility in a model biomedium in vitro as compared with gold NPs,7 release substantially higher concentrations of ions into the blood as well in vivo, and, therefore, possess higher nephrotoxicity. We believe that marked toxic damage of kidney epithelial cells can mask the metal’s genotoxic effect. This hypothesis found confirmation in an experiment with copper oxide NPs,8 which are even more soluble in vivo and thus even more nephrotoxic compared with silver NPs. In that experiment, the kidneys proved to be the only organ in which the DNA fragmentation coefficient was not elevated in response to subchronic intoxication by copper oxide NPs in comparison with controls. Meanwhile, in a parallel group, in which the animals were exposed to the same CuO NPs but against the background of bioprotectors (see “Is it possible to protect the health of people exposed occupationally to metallic NPs in the workroom air by enhancing organism’s resistance to their adverse action?” section), the morphometric data revealed a considerably lower death rate of tubular epithelial cells, while it was just against this background that a significant genotoxic effect of nano-CuO became quite manifested.
Is it possible to protect the health of people exposed occupationally to metallic NPs in the workroom air by establishing very low but still practicable PELs?
Although the problem of nanomaterials’ health risk assessment and management is discussed widely and has been comprehensively reviewed by many authors,104,105 a general methodology of safe exposure standard setting in this field is still lacking, and there are but few examples of establishing or recommending reference exposure levels for specific engineered NPs in workroom air. Thus in most cases, in the field of nano-safety both risk assessments and risk management scenarios still have to be implemented with the qualification that exposure limits for individual nano-materials are not available.106 Given this serious uncertainty, the precautionary approach is that of choice in the worker’s health protection in nanotechnology.107
Irrespective of whether toxicologists of this or that country believe in a no-threshold character of the dose-effect relationship for any or, at least, for genotoxic and carcinogenic harmful substances or stick to the paradigm of the existence of thresholds for any harmful effects, there are little doubts as to the practical need for, and the possibility of establishing presumably safe exposure levels. In a growing number of countries such levels are set as law enforceable standards, in particular, for the workplace air quality. Whatever such standards are called (maximal allowable concentrations, PELs, reference exposure levels, workplace exposure limit, occupational exposure limits, etc), establishing them is justified by assuming, explicitly or by default, that the evolutionarily acquired protective mechanisms and the compensation abilities of the organism enable humans to exist under some low level of harmful exposure without any appreciable impairment of health and any perceptible rise over the background risks of stochastic harmful effects.
The experimental results as discussed above permitted us to maintain that the concept of quasi-defenselessness of the organism against NPs had to be critically re-evaluated. On the other hand, however, they provided evidence supporting the prevailing presumption that particulates, even ones that are relatively innocuous in the micrometer range (like iron oxides), may be markedly toxic in the nano-state while within the conventional nanometer range the dependence of toxicity on particle size proved intricate and non-unique. Therefore we believe that: a) establishing permissible or reference values for controlling and assessing health risks due to NPs exposures is possible in principle; b) such levels should be significantly lower as compared with actual exposure limits established (or recommended) for chemically identical MPs; and c) there are no reliable foundations as yet for differentiating such standards for particles of different nano-size.
As to a well-known example of such approach, we may refer to the recommended exposure limit (REL) 0.3 mg/m3 proposed by the US National Institute for Occupational Safety and Health (NIOSH) for the ultrafine (including engineered nanoscale) TiO2 particles that is eight times lower than REL 2.4 mg/m3 for the fine TiO2.108 It is true that the NIOSH maintains that “similarly for other particulate materials, if the toxicity varies by particle size (at the same mass dose), distinct exposure limits for distinct particle size fractions might be a reasonable approach for protecting exposed workers”. Nevertheless, one and the same REL is proposed for all the TiO2 nano-scale fractions while just the TiO2 commercial nano-powders are being manufactured and marketed for a variety of uses in a rather wide range of primary particle diameters (for instance, ~10 nm, 10–30, or ≤50 nm). Thus we may say that the NIOSH REL proposal is in good accordance with, though established independently of, the above postulates (b and c) of ours.
A similar but even more conservative approach to establishing exposure standards for engineered nanomaterials was proposed in 2010 by the Australian Government agency “Safe Work Australia”: for nanocrystals, quantum dots, ceramic oxides, and metals the suggested benchmark exposure level should be 0.066 WEL (workplace exposure limit for bulk material), that is by diminishing the latter by a factor of 15.
In accordance with this regulatory practice, we also believe that for workroom air concentrations of metallic NPs, PELs at this historical stage can and should be based, even if tentatively, on a sufficiently conservative rule of decreasing approximately tenfold the exposure limits previously established for respective micro-scale industrial aerosols. Acting along this line, we propose tentative PELs for NPs of iron oxide (0.4 mg/m3), copper oxide (0.05 mg/m3), silver (0.1 mg/m3), and gold (0.2 mg/m3). As an example, we will discuss here in more detail the case of Fe3O4 NPs as presented by us.4
To begin with, workroom exposure standards are established in different countries for only one of the iron oxides – namely Fe2O3. It may be assumed, however, that there is no significant difference in toxicity between different iron oxides. For example, the Joint Expert Committee on Food Additives gives one and the same acceptable daily intake value for Fe3O4 (the so-called black iron [II, III] oxide) and for anhydrous or hydrated Fe2O3 (respectively, red and yellow iron [III] oxides). So, as a starting point for calculating a tentative PEL for nano Fe3O4 one might assume by inference the actual standards for Fe2O3 dust and/or fumes which are:
- USA Occupational Safety and Health Administration PEL – time-weighted average (TWA) 10 mg/m3 (as Fe);
- USA NIOSH REL – TWA 5 mg/m3 (as Fe);
- Canadian Occupational Exposure Limit – TWA 5 mg/m3 for respirable dust (in Alberta) or 10 mg/m3 for total dust (in British Columbia);
- Russian Maximum Allowable Concentration (MAC) – work shift average 6 mg/m3.
Note that the Russian MAC established for whole Fe2O3 substance is equivalent to ~2 mg/m3 if re-calculated for Fe, and thus it is actually the most conservative of all these standards. Taking into consideration the precautionary principle, we proposed to start just from this MAC and, moreover, to diminish it not eight but 15 times (in accordance with the above-mentioned Australian suggestion as the most conservative, too). Thus we obtained the value 6×0.066≈0.4 mg/m3 (as TWA). This value is close enough to the above-mentioned NIOSH REL for ultrafine TiO2 (0.3 mg/m3).
There is no doubt that a more sophisticated study of the same nanomaterials’ adverse effects (their possible carcinogenicity included) and of dose-response relationships, as well as further discussion of a general methodology for substantiating reference exposure levels for nanomaterials will be necessary to make such safety standards more reliable. Nevertheless, we believe that, for the time being, it is wiser to use the standards obtained with the above-discussed approach (tentative but still not spun out of thin air and obligatorily based on results of actual comparative toxicological experiments) than to be passively waiting for better ones.
Is it possible to protect the health of people exposed occupationally to metallic NPs in the workroom air by enhancing organisms’ resistance to their adverse action?
However low the levels of exposure to NPs were established as permissible, the especially high toxicity of this class of occupational hazards makes it worthwhile to search for possibilities to render exposed people less sensitive to their effects with the help of a combination of specific and unspecific agents which are innocuous themselves in preventively effective doses. The general concept of such “biological prophylaxis” of different occupational and environmental intoxications, its theoretical premises and general principles along with numerous examples of their realization have been published by us over several decades repeatedly, including, in review articles.109,110 Now we began to use this experience for trying to protect the organism against the impacts of nanometals.
In particular, our experiments have demonstrated that both systemic toxicity and in vivo genotoxicity of silver7 and of copper oxide8 NPs were markedly attenuated against the background of oral administration of multi-component BPC. These BPCs comprised pectin, multivitamin–multimineral preparations, some amino acids, and omega-3 polyunsaturated fatty acid (for the detailed BPCs’ composition and doses see the above references).7,8 In both cases the protective action was demonstrated in respect to many adverse effects of subchronic toxicity. Some illustrations are given in Tables 5–7, and in Figures 12 and 13.
We found as well10 that in rats that were being given glutamate, glycine, acetyl cysteine, iodide, and a Se-containing multivitamin preparation orally during 4 weeks before a single IT instillation of NiO+Mn3O4 NPs (0.25 mg each), the latter evoked a significantly lower NL recruitment with lower NL/AM ratio than in rats so exposed without any pretreatment.
Conclusion
The investigated metallic NPs are much more noxious on both cellular and systemic levels as compared with their fine micrometric or even submicron counterparts. However, within the conventional nanometer range the dependence of systemic toxicity on particle size is intricate and non-unique due to complex and often contra-directional relationships between the intrinsic biological aggressiveness of specific NPs (in particular, their cytotoxicity), on the one hand, and complex mechanisms that control their biokinetics, on the other.
Our data testify to a high activity of at least one of the key physiological defense mechanisms (pulmonary phagocytosis of deposited particles) against NPs deposited in airways. This fact suggests that safe low levels of exposure to airborne NPs are possible in principle. For the time being, however, there seems to be no reliable foundations for establishing different PELs for particles of different sizes within the established nanometric range. For workroom air concentrations of metallic NPs, PELs can be proposed at this stage, even if tentatively, based on a sufficiently conservative approach of decreasing by approximately an order of magnitude the exposure limits established for respective micro-scale industrial aerosols. Acting along this line, we propose tentative PELs for NPs of iron oxide (0.4 mg/m3), copper oxide (0.05 mg/m3), silver (0.1 mg/m3), and gold (0.2 mg/m3). However, to substantiate more reliable standards of presumably safe occupational exposures to metallic NPs, a wide international program of long-term inhalation experiments with such low-level concentrations and with a broad spectrum of toxicity, genotoxicity, and carcinogenicity indices has to be designed and implemented.
It has been shown that against the background of adequately composed combinations of some bioactive agents used in innocuous doses the toxicity and even genotoxicity of metallic NPs could be markedly attenuated. Therefore we believe that, along with decreasing exposures to NPs, enhancing organisms’ resistance to their adverse action with the help of such bioprotectors can prove an efficient auxiliary tool of health risk management in related occupations and strongly recommend to further develop this vector of nanotoxicological research.
Acknowledgments
We appreciate a substantial contribution to particular experimental studies summarized in this paper made by Olga S Yeremenko, Anastasia E Tyurnina, Roman V Kozin, Ekaterina Y Meshtcheryakova, Artem V Korotkov, Eugene A Shuman, Anastasia E Zvereva, Svetlana V Kostykova, Marina S Vasilyeva, Larisa A Vlasova, Svetlana V Pichugova, Larisa G Tulakina, Mark Y Khodos, Alisa N Kozitsina, Vladimir A Vazhenin, Aleksandr P Potapov, and Maria V Morozova.
Disclosure
The authors declare no conflict of interest.
References
Katsnelson BA, Privalova LI, Degtyareva TD, et al. Experimental estimates of the toxicity of iron oxide Fe3O4 (magnetite) nanoparticles. Central European Journal of Occupational and Environmental Medicine. 2010;16:47–63. | ||
Katsnelson BA, Privalova LI, Kuzmin SV, et al. Some peculiarities of pulmonary clearance mechanisms in rats after intratracheal instillation of magnetite (Fe3O4) suspensions with different particle sizes in the nanometer and micrometer ranges: Are we defenseless against nanoparticles? Int J Occup Environ Health. 2010;16(4):508–524. | ||
Katsnelson BA, Degtyareva TD, Minigalieva II, et al. Sub-chronic systemic toxicity and bio-accumulation of Fe3O4 nano- and microparticles following repeated intraperitoneal administration to rats. Int J Toxicol. 2011;30(1):60–67. | ||
Katsnelson BA, Privalova LI, Kuzmin SV, et al. An approach to tentative reference levels setting for nanoparticles in the workroom air based on comparing their toxicity with that of their micrometric counterparts: A case study of iron oxide Fe3O4. ISRN Nanotechnol. 2012;2012:12. | ||
Katsnelson BA, Privalova LI, Sutunkova MP, et al. Uptake of some metallic nanoparticles by, and their impact on pulmonary macrophages in vivo as viewed by optical, atomic force, and transmission electron microscopy. J Nanomed Nanotechnol. 2012;3:1–8. | ||
Katsnelson BA, Privalova LI, Sutunkova MP, et al. Interaction of iron oxide Fe3O4 nanoparticles and alveolar macrophages in vivo. Bull Exp Biol Med. 2012;152:627–629. | ||
Katsnelson BA, Privalova LI, Gurvich VB, et al. Comparative in vivo assessment of some adverse bio-effects of equidimensional gold and silver nanoparticles and the attenuation of nanosilver’s effects with a complex of innocuous bioprotectors. Int J Mol Sci. 2013;14(2):2449–2483. | ||
Privalova LI, Katsnelson BA, Loginova NV, et al. Subchronic Toxicity of Copper Oxide Nanoparticles and Its Attenuation with the Help of a Combination of Bioprotectors. Int J Mol Sci. 2014;15(7):12379–12406. | ||
Privalova LI, Katsnelson BA, Loginova NV, et al. Some Characteristics of Free Cell Population in the Airways of Rats after Intratracheal Instillation of Copper-Containing Nano-Scale Particles. Int J Mol Sci. 2014;15(11):21538–21553. | ||
Katsnelson BA, Minigalieva IA, Privalova LI, et al. Lower airways response in rats to a single or combined intratracheal instillation of manganese and nickel nanoparticles and its attenuation with a bio-protective pre-treatment. Toksicol Vestnik. 2014;6:8–14. | ||
Zhu MT, Feng WY, Wang B, et al. Comparative study of pulmonary responses to nano- and submicron ferric oxide in rats. Toxicology. 2008;247(2–3):102–111. | ||
Mahmoudi M, Simchi A, Milani AS, Stroeve P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci. 2009;336(2):510–518. | ||
Mahmoudi M, Laurent S, Shokrgozar MA, Hosseinkhani M. Toxicity Evaluations of Superparamagnetic Iron Oxide Nanoparticles: Cell “Vision” versus Physicochemical Properties of Nanoparticles. ACS Nano. 2011;5(9):7263–7276. | ||
Naqvi S, Samim M, Abdin MZ, et al. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomedicine. 2010;5:983–989. | ||
Wu X, Tan Y, Mao H, Zhang M. Toxic effects of iron oxide nanoparticles on human umbilical vein endothelial cells. Int J Nanomedicine. 2010;5:385–399. | ||
Singh N, Jenkins GJ, Asadi R, Doak SH. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010;1:5358. | ||
Soenen SJ, De Cuyper M, De Smedt SC, Braeckmans K. Investigating the toxic effects of iron oxide nanoparticles. Methods Enzymol. 2012;509:195–224. | ||
Liu G, Gao J, Ai H, Chen X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small. 2013;9(9–10):1533–1545. | ||
Markides H, Rotherham M, El Haj AJ. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J Nanomater. 2012;2012:614094. | ||
Barhoumi L, Dewez D. Toxicity of Superparamagnetic Iron Oxide Nanoparticles on Green Alga Chlorella vulgaris. Bio Med Research International. 2013;2013:647974. | ||
Ahamed M, Karns M, Goodson M, et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharmacol. 2008;233(3):404–410. | ||
Arora S, Jain J, Rajwade JM, Paknikar KM. Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. Toxicol Appl Pharmacol. 2009;236(3):310–318. | ||
Trickler WJ, Lantz SM, Murdock RC, et al. Silver nanoparticle induced blood–brain barrier inflammation and increased permeability in primary rat brain micro vessel endothelial cells. Toxicol Sci. 2010;118(1): 160–170. | ||
Li T, Albee B, Alemayehu M, et al. Comparative toxicity study of Ag, Au, Ag-Au bimetallic nanoparticles on Daphnia magna. Anal Bioanal Chem. 2010;398(2):689–700. | ||
Park EJ, Bae E, Yi Y, et al. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nano-particles. Environ Toxicol Pharmacol. 2010;30(2):162–168. | ||
Park MV, Neigh AM, Vermeulen JP, et al. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011;32(36):9810–9817. | ||
Choi JE, Kim S, Ahn JH, et al. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol. 2010;100(2):151–159. | ||
Kim YS, Song MY, Park JD, et al. Subchronic oral toxicity of silver nanoparticles. Particle and Fibre Toxicology. 2010;7:20. | ||
Ahmadi F, Kordestany AH. Investigation on silver retention in different organs and oxidative stress enzymes in male broiler fed diet supplemented with powder of nano silver. Am-Euras J Toxicol Sci. 2011;3(1):28–35. | ||
Stebounova LV, Adamcakova-Dodd A, Kim JS. Nanosilver induces minimal lung toxicity or inflammation in a subacute murine inhalation model. Particle and Fibre Toxicology. 2011;8(1):5. | ||
Srivastava M, Singh S, Self WT. Exposure to silver nanoparticles inhibits selenoprotein synthesis and the activity of thioredoxin reductase. Environ Health Perspect. 2011;120:56–61. | ||
Hackenberg S, Scherzed A, Kessler M, et al. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cell. Toxicol Lett. 2011;201(1):27–33. | ||
Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85(7):743–750. | ||
Kim HR, Kim MJ, Lee SY, Oh SM, Chung KH. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cells. Mutat Res. 2011;726(2):129–135. | ||
Li Y, Chen DH, Yan J, Chen Y, et al. Genotoxicity of silver nanoparticles evaluated using the Ames test and in vitro micronucleus assay. Mutat Res. 2012;745(1–2):4–10. | ||
Tavares P, Balbino F, Martins de Oliveira H, et al. Evaluation of genotoxic effect of silver nanoparticles (Ag-NPs) in vitro and in vivo. J Nanopart Res. 2012;14:791. | ||
Asare N, Instanes C, Sandberg WJ, et al. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cell. Toxicology. 2012;291(1–3): 65–72. | ||
Flower NA, Brabu B, Revathy M, et al. Characterization of synthesized silver nanoparticles and assessment of its genotoxicity potentials using the alkaline comet assay. Mutat Res. 2012;742(1–2):61–65. | ||
Karlsson H, Gliga AR, Kohonen P, Wallbergb P, Fadeel B. Genotoxicity and epigenetic effects of silver nanoparticles. Toxicol Lett. 2012;211:(Supplement) S40. | ||
Lim DH, Jang J, Kim S, Kang T, Lee K, Choi IH. The effects of sub-lethal concentrations of silver nanoparticles on inflammatory and stress in human macrophages using cDNA microarray analysis. Biomaterials. 2012;33(18):4690–4699. | ||
Beer C, Foldbjerg R, Hayashi Y, Sutherland DS, Autrup H. Toxicity of silver nanoparticles – Nanoparticle or silver ion? Toxicol Lett. 2012;208(3): 286–292. | ||
Cronholm P, Karlsson HL, Hedberg J, et al. Intracellular uptake and toxicity of Ag and CuO nanoparticles: A comparison between nanoparticles and their corresponding metal ions. Small. 2013;8(7):970–982. | ||
Gomes T, Araújo O, Pereira R, Almeida AC, Cravo A, Bebianno MJ. Genotoxicity of copper oxide and silver nanoparticles in the mussel Mytilus galloprovincialis. Mar Environ Res. 2013;84:51–59. | ||
Ahamed M, AlSalhi MS, Siddiqui MK. Silver nanoparticles applications and human health. Clin Chim Acta. 2010;411(23–24):1841–1184. | ||
Singh S, D’Britto V, Prabhune AA, Ramana CV, Dhawan A, Prasad BL. Cytotoxic and genotoxic assessment of glycolipid-reduced and -capped gold and silver nanoparticles. New J Chem. 2010;34:294–301. | ||
Bakri SJ, Pulido JS, Mukerjee P, Marler RJ, Mukhopadhyay D. Absence of histologic retinal toxicity of intravitreal nanogold in a rabbit model. Retina. 2008;28(1):147–149. | ||
Pan Y, Leifert A, Ruau D, et al. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009;5(18):2067–2076. | ||
Chen YS, Hung YC, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4(8):858–864. | ||
Balasurbamanian SK, Jittiwat J, Manikandan J, Ong CN, Yu LE, Ong WY. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials. 2010;31(8):2034–2042. | ||
Zhang Q, Hitchins VM, Schrand AM, Hussain SM, Goering PL. Uptake of gold nanoparticles in murine macrophage cells without cytotoxicity or production of pro-inflammatory mediators. Nanotoxicology. 2010;5(3):284–295. | ||
Li JJ, Lo SL, Ng CT, et al. Genomic instability of gold nanoparticle treated human lung fibroblast cells. Biomaterials. 2011;32(23):5515–5523. | ||
Trickler WJ, Lantz SM, Murdock RC, et al. Brain microvessel endothelial cells responses to gold microparticles: In vitro pro-inflammatory mediators and permeability. Nanotoxicology. 2011;5(4):479–492. | ||
Glazer ES, Zhu C, Hamir AN, Borne A, Thompson CS, Curley SA. Biodistribution and acute toxicity of naked gold nanoparticles in a rabbit hepatic tumor model. Nanotoxicology. 2011;5(4):459–468. | ||
Mustafa T, Watanabe F, Monroe W, et al. Impact of gold nanoparticle concentration on their cellular uptake by MC3T3-E1 mouse osteoblastic cells as analyzed by transmission electron microscopy. J Nanomed Nanotechnol. 2011;2:1–8. | ||
Rudolf R, Friedrich B, Stopic S, Anzel I, Tomic S, Colic M. Cytotoxicity of gold nanoparticles prepared by ultrasonic spray pyrolysis. J Biomater Appl. 2012;26(5):595–612. | ||
Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem Soc Rev. 2012;41(6):2256–2282. | ||
Choi SY, Jeong S, Jang SH, et al. In vitro toxicity of serum protein-adsorbed citrate-reduced gold nanoparticles in human lung adenocarcinoma cells. Toxicology in Vitro. 2012;26(2):229–237. | ||
Shulz M, Ma-Hock L, Brill S, et al. Investigation on the genotoxicity of different sizes of gold nanoparticles administered to the lungs of rats. Mutat Res. 2012;745(1–2):51–57. | ||
Akhtar MJ, Kumar S, Alhadlaq HA, Alrokayan SA, Abu-Salah KM, Ahamed M. Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. Toxicol Ind Health. Epub 2013 Dec 5. | ||
Chen Z, Meng H, Xing G, et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006;163(2):109–120. | ||
Karlsson HL, Cronholm P, Gustafsson J, Möller L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21(9): 1726–1732. | ||
Studer AM, Limbach LK, van Duc L, et al. Nanoparticle cytotoxicity depends on intracellular solubility: comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol Lett. 2010; 197(3):169–174. | ||
Bondarenko O, Ivask A, Käkinen A, Kahru A. Sub-toxic effects of CuO nanoparticles on bacteria: Kinetics, role of Cu ions and possible mechanisms of action. Environ Pollut. 2012;169:81–89. | ||
Pang C, Selck H, Misra SK, et al. Effects of sediment-associated copper to the deposit-feeding snail, Potamopyrgus antipodarum: A comparison of Cu added in aqueous form or as nano- and micro-CuO particles. Aquat Toxicol. 2012;106–107:114–122. | ||
Magaye R, Zhao J, Bowman L, Ding M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp Ther Med. 2012;4(4):551–561. | ||
Alarifi S, Ali D, Verma A, Alakhtani S, Ali BA. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int J Toxicol. 2013;32(4):296–307. | ||
Xu J, Li Z, Xu P, Xiao L, Yang Z. Nanosized copper oxide induces apoptosis through oxidative stress in podocytes. Arch Toxicol. 2013;87(6):1067–1073. | ||
Cuillel M, Chevallet M, Charbonnier P, et al. Interference of CuO nanoparticles with metal homeostasis in hepatocytes under sub-toxic conditions. Nanoscale. 2014;6(3):1707–1715. | ||
Zhang Q, Yukinori K, Sato K, Nakakuki K, Koyahama N, Donaldson K. Differences in the extent of inflammation caused by intratracheal exposure to three ultrafine metals: Role of free radicals. J Toxicol Environ Health A. 1998;53(6):423–438. | ||
Zhang Q, Yukinori K, Zhu X, et al. Comparative toxicity of standard nickel and ultrafine nickel after intratracheal instillation. J Occup Health. 2003;45(1):23–30. | ||
Magaye R, Zhao J. Recent progress in studies of metallic nickel and nickel-based nanoparticles’ genotoxicity and carcinogenicity. Environmental Toxicology and Pharmacology. 2012;34(3):644–650. | ||
MorimotoY, Hirohashi M, Ogami A, et al. Pulmonary toxicity following an intratracheal instillation of nickel oxide nanoparticle agglomerates. Journal of Occupational Health. 2011;53(4):293–295. | ||
Capasso L, Camatini M, Gualtieri M. Nickel oxide nanoparticles induce inflammation and genotoxic effect in lung epithelial cells. Toxicol Lett. 2014;226(1):28–34. | ||
Hussain SM, Javorina AK, Schrand AM, Duhart HM, Ali SF, Schlager JJ. The Interaction of Manganese Nanoparticles with PC-12 Cells Induces Dopamine Depletion. Toxicol Sci. 2006;92(2):456–463. | ||
Singh SP, Kumari M, Kumari SI, Rahman MF, Mahboob M, Grover P. Toxicity assessment of manganese oxide micro and nanoparticles in Wistar rats after 28days of repeated oral exposure. J Appl Toxicol. 2013;33(10):1165–1179. | ||
Bellusci M, La Barbera A, Padella F, et al. Biodistribution and acute toxicity of a nanofluid containing manganese iron oxide nanoparticles produced by a mechanochemical process. Int J of Nanomedicine. 2014;9:1919–1929. | ||
Katsnelson BA, Konysheva LK, Privalova LY, Morosova KI. Development of a multicompartmental model of the kinetics of quartz dust in the pulmonary region of the lung during chronic inhalation exposure of rats. Br J Ind Med. 1992;49(3):172–181. | ||
Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Particle and Fibre Toxicology. 2010;7:2. | ||
International Commission on Radiological Protection [homepage on the Internet]. ICRP Publication 66: Human respiratory tract model for radiological protection. A report of a Task Group of the International Commission on Radiological Protection; 1994. Available from: http://www.icrp.org/publication.asp?id=ICRP Publication 66. Accessed February 27, 2015. | ||
Kreyling WG, Geiser M. Dosimetry of inhaled nanoparticles. In: Marijnissen JC, Gradon L. Nanoparticles in Medicine and Environment, Inhalation and Health Effects. Springer Science and Business Media; 2009:145–173. | ||
Fröhlich E, Salar-Behzadi S. Toxicological assessment of inhaled nanoparticles: Role of in vivo, ex vivo, in vitro, and in silico studies. Int J Mol Sci. 2014;15(3):4795–4822. | ||
Sadauskas E, Wallin H, Stolenberg M, et al. Kupffer cells are central in the removal of nanoparticles from the organism. Part Fibre Toxicol. 2007;4:10–16. | ||
Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393(4):649–655. | ||
Rylova ML. Methods of Investigating Long-Term Effects of Noxious Environmental Agents in Animal Experiments. Leningrad: Meditsina; 1964. | ||
Adeyemi OO, Yemitan OK, Taiwo AE. Neurosedative and muscle-relaxant activities of ethyl acetate extract of Baphianitida nitida AFZEL. J Ethnopharmacol. 2006;106(3):312–316. | ||
Fernandez SP, Wasowski C, Loscalzo LM, et al. Central nervous system depressant action of flavonoid glycoside. Eur J Pharmacol. 2006;539(3):168–176. | ||
Donaldson K, Stone V, Tran CK, Kreyling W, Borm PJ. Nanotoxicology (editorial). Occup Environ Med. 2004;61:727–728. | ||
Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studied of ultrafine particles. Environ Health Perspect. 2005;113(7):823–839. | ||
Fadeel B. Clear and present danger? Engineered nanoparticles and the immune system. Swiss Med Wkly. 2012;142:w13609. | ||
Kilburn KH. Alveolar clearance of particles. A bullfrog lung model. Arch Environ Health. 1969;18(4):556–563. | ||
Renwick LC, Brown D, Clouter K, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61(5):442–447. | ||
Stoeger T, Reinhard C, Takenaka S, et al. Instillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in mice. Environ Health Perspect. 2006;114(3):328–333. | ||
Grassian VH, O’Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ Health Perspect. 2007;115(3):397–402. | ||
Sager TM, Porter DW, Robinson VA, Lindsley WG, Schwegler-Berry VA, Castranova V. Improved method to disperse nanoparticles in vitro and in vivo investigation of toxicity. Nanotoxicology. 2007;1(2):118–129. | ||
Warheit DB, Reed KL, Sayes CM. A role for surface reactivity in TiO2 and quartz-related nanoparticle pulmonary toxicity. Nanotoxicology. 2009;3(3):181–187. | ||
Privalova LI, Katsnelson BA, Osipenko AB, Yushkov BH, Babushkina LG. Response of a phagocyte cell system to products of macrophage breakdown as a probable mechanism of alveolar phagocytosis adaptation to deposition of particles of different cytotoxicity. Environ Health Perspect. 1980;35:205–218. | ||
Privalova LI, Katsnelson BA, Yelnichnykh LN. Some peculiarities of the pulmonary phagocytotic response, dust kinetics, and silicosis development during long term exposure of rats to high quartz levels. Br J Ind Med. 1987;44(4):228–235. | ||
Katsnelson BA, Privalova LI. Recruitment of phagocytizing cells into the respiratory tract as a response to the cytotoxic action of deposited particles. Environ Health Perspect. 1984;55:313–325. | ||
Katsnelson BA, Konyscheva LK, Sharapova Nye, Privalova LI. Prediction of the comparative intensity of pneumoconiotic changes caused by chronic inhalation exposure to dusts of different cytotoxicity by means of a mathematical model. Occup Environ Med. 1994;51(3):173–180. | ||
Katsnelson BA, Konysheva LK, Privalova LY, Sharapova NY. Quartz dust retention in rat lungs under chronic exposure simulated by a multicompartmental model: Further evidence of the key role of the cytotoxicity of quartz particles. Inhalation Toxicology. 1997;9(8):703–715. | ||
Bastus NG, Casals E, Socorro VC, Puntes V. Reactivity of engineered inorganic nanoparticles and carbon nanostructures in biological media. Nanotoxicology. 2008;2(3):99–112. | ||
Fröhlich E. Cellular targets and mechanisms in the cytotoxic action of non-biodegradable engineered nanoparticles. Curr Drug Metab. 2013;14(9):976–988. | ||
Privalova LI, Katsnelson BA, Sharapova NY, Kislitsina NS. On the relationship between activation and the breakdown of macrophages in pathogenesis of silicosis. Med Lav. 1995;86(6):511–521. | ||
Yokel RA, MacPhail RC. Engineered nanomaterials: exposures, hazards, and risk prevention. J Occup Med Toxicol. 2011;6:7. | ||
Murashov V, Shulte P, Geraci C, Howard J. Regulatory approaches to worker protection in nanotechnology industry in the USA and European Union. Ind Health. 2011;49(3):280–296. | ||
Grosco A, Petri-Fink A, Magrez A, Rediker M, Meyer T. Management of nanomaterials safety in research environment. Part Fibre Toxicol. 2010;7:40. | ||
van Broekhuizen P. Dealing with uncertainties in the nanotech workplace practice: making the precautionary approach operational. J Biomed Nanotechnol. 2011;7(1):15–17. | ||
Centers for Disease Control and Prevention [homepage on the Internet]. Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide. CDC and NIOSH. US Department of Health and Human Services; 2011. Available from: http://www.cdc.gov/niosh/docs/2011-160/. Accessed February 27, 2015. | ||
Katsnelson BA, Kuzmin SV, Degtyareva TD, Privalova LI, Soloboyeva JI. “Biological prophylaxis” – One of the ways to proceed from the analytical environmental epidemiology to the population health protection. Cent Eur J Occup Environ Med. 2008;14:41–42. | ||
Katsnelson BA, Privalova LI, Gurvich VB, et al. Enhancing Population’s Resistance to Toxic Exposures as an Auxilliary Tool of Decreasing Environmental and Occupational Health Risks (a Self-Overview). Journal of Environmental Protection. 2014;5:1435–1449. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.