Back to Journals » Drug Design, Development and Therapy » Volume 13
Solutol®HS15+pluronicF127 and Solutol®HS15+pluronicL61 mixed micelle systems for oral delivery of genistein
Authors Ding P, Chen Y, Cao G , Shen H, Ju J, Li W
Received 14 January 2019
Accepted for publication 10 May 2019
Published 7 June 2019 Volume 2019:13 Pages 1947—1956
DOI https://doi.org/10.2147/DDDT.S201453
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Pinggang Ding,1,2,* Yuxuan Chen,3,* Guangshang Cao,4 Hongxue Shen,1,2 Jianming Ju,1,2 Weiguang Li,5
1Department of Pharmaceutical Analysis and Metabolomics, Hospital of Integrated Traditional Chinese and Western Medicine Affiliated to Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 2Department of Pharmaceutical Analysis and Metabolomics, Jiangsu Province Academy of Traditional Chinese Medicine, Nanjing, People’s Republic of China; 3School of Holistic Integrative Medicine, Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 4Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, People’s Republic of China; 5Department of Marine Pharmacy, China Pharmaceutical University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Purpose: We aimed to prepare two oral drug delivery systems consisting of polyoxyl 15 hydroxystearate (HS15) with pluronicF127 (F127) and HS15 with pluronicL61 (L61) to overcome the challenges of genistein’s poor oral bioavailability. This provides a good strategy for enhancing the potential value of genistein.
Methods: We designed two binary mixed micelle systems employing the organic solvent evaporation method using surfactants (HS15, L61, and F127). Formulations (GEN-F and GEN-L) were characterized by transmission electron microscopy. Drug content analysis, including entrapment efficiency (EE%), drug loading (DL%), and the cumulative amount of genistein released from the micelles, was performed using HPLC. The permeability of optimum formulation was measured in Caco-2 cell monolayers, and the oral bioavailability was evaluated in SD rats.
Results: The solutions of GEN-F and GEN-L were observed to be transparent and colorless. GEN-F had a lower EE% of 80.79±0.55% and a DL% of 1.69±0.24% compared to GEN-L, which had an EE% 83.40±1.36% and a DL% 2.26±0.18%. TEM results showed that the morphology of GEN-F and GEN-L was homogeneous and resembled a spherical shape. The dilution and storage conditions had no significant effect on particle size and EE%. Genistein demonstrated a sustained release behavior when encapsulated in micelles. Pharmacokinetics study showed that the relative oral bioavailability of GEN-F and GEN-L increased by 2.23 and 3.46 fold while also enhancing the permeability of genistein across a Caco-2 cell monolayer compared to that of raw genistein.
Conclusion: GEN-F and GEN-L as a drug delivery system provide an effective strategy for enhancing and further realizing the potential value of GEN.
Keywords: genistein, micelles, polyoxyl 15 hydroxystearate, pluronicF127, pluronicL61, oral bioavailability
Introduction
Genistein (GEN) is a biologically active isoflavone found in legume.1 Besides its simple structure, GEN has attracted much attention worldwide owing to its wide spectrum of biological effects.2 Studies have shown that GEN has anti-diabetic,3 anti-tumor,4 and estrogen-like effects.5 However, its impact on diabetes, β-cell proliferation, glucose-stimulated insulin secretion, and protection against apoptosis is independent of its function as an estrogen receptor agonist, antioxidant, and tyrosine kinase inhibitor.6 The effects of GEN are structure-specific and not common to all flavonoids. It is worth mentioning that GEN may induce early mammary gland differentiation, resulting in less active epidermal growth factor signaling in adulthood, which in turn suppresses the development of mammary cancer.7 Besides its effects on breast cancer, GEN acts as a chemotherapeutic agent against different types of cancer, mainly by altering apoptosis, cell cycle, angiogenesis, and inhibiting metastasis. This makes it a vital molecule for cancer chemoprevention.4 Since low water solubility may be the main factor responsible for the poor oral bioavailability of GEN,8 there is a necessity and demand for designing novel drug delivery systems (DDSs) which can increase the oral bioavailability of GEN.
Oral administration is the preferred route of drug delivery owing to it being painless, convenient, and cost-effective.9–12 However, to date, more than 4,000 natural phenolic drugs such as GEN are poorly soluble in water and are rapidly degraded and metabolized in the human body before attaining efficacy.13 Therefore, improving the oral bioavailability of drugs is vital but is limited by their formulation. Currently, oral absorption technology of poorly soluble drugs has been reported in numerous studies, including those that employ solid dispersions,14,15 liposomes,16–19 micelles,20–24 and nanoparticles.25–29 Among these, mixed micelles have attracted much attention as a nano-sized drug carrier in DDSs. Mixed micelles increase the solubilizing ability and stability of small molecule micelles owing to their core–shell structure.30 This structure consists of the drugs being physically incorporated into the micelles’ hydrophobic inner cores by means of hydrophobic interactions while retaining the basic characteristics of polymer micelles.31 In recent years, more studies have focused on the binary micelle system that has helped circumvent the insoluble drug solubilization problem through facilitating and enhancing drug absorption by the body.32
In this study, we designed two binary micelle systems using HS15+F127 and HS15+L61 to overcome the limitations of poor solubility and low oral bioavailability of GEN. HS-15, a non-ionic surfactant consisting of 70% polyglycol mono and diesters of 12-hydroxystearic acid and 30% free polyethylene glycol, was found to be notably effective in enhancing the stability and solubility of insoluble drugs.33 Furthermore, HS-15 can alter plasma binding, enhance adsorption, and induce significant effects on the pharmacokinetics.34 Pluronic, also known as poloxamer, is an amphiphilic, triblock copolymer that consists of a middle hydrophobic polyoxypropylene chain and two hydrophilic polyoxyethylene chains.35 This could form micelles in an oil-in-water emulsion and has been approved by the Food and Drug Administration (FDA) for use as a pharmaceutical ingredient.36
F127 has a Hydrophile–Lipophile Balance (HLB) of 22 and is a relatively hydrophilic pluronic that has been widely explored for drug delivery owing to its ability to solubilize hydrophobic solutes and form micellar structures.37 L61 is relatively hydrophobic with an HLB value of only 3. Attributed to its self-assembly to a single polyether micelle with poor stability, low drug loading,38–40 L61 is commonly used in the preparation of mixed micelles with other materials.41 Therefore, we hypothesized that the combination of pluronic and HS15 will complement each other to form binary mixed micelles. Attempts to explore the combination of the relatively hydrophilic F127 and Solutol HS15 or the relatively hydrophobic L61 and Solutol HS15 are more effective in improving the oral bioavailability of GEN.
In the present study, two novel mixed micelle systems (Figure 1) were prepared by employing an organic solvent evaporation method using surfactants (HS15, L61, and F127), which can enhance the aqueous solubility, permeability, and oral bioavailability of GEN. While one was prepared with HS15 and F127 (GEN-F), the other was prepared with HS15 and L61 (GEN-L). The DDS was characterized by transmission electron microscopy (TEM) and the Malvern Zetasizer Nano System. To evaluate the DDS, we estimated the drug content, storage, dilution stability, and drug release in-vitro. The absorption and efflux characteristics of GEN-loaded micelles (GEN-M) were evaluated in Caco-2 cell monolayers. Finally, oral bioavailability was assessed in vivo through pharmacokinetic studies.
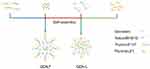 | Figure 1 Structural representation of GEN-F and GEN-L. |
Materials and methods
Materials
Genistein standards, genistein (purity >98%), and daidzein standards (purity >98%) were purchased from Meilun Biological Technology Co., Ltd (Dalian, China). HS15 was purchased from BASF Ltd. (Shanghai, China) and Pluronic F127 and L61 were purchased from Sigma–Aldrich (St. Louis, MO, USA). Caco-2 cell lines were purchased from Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Thermo Fisher Scientific (Bridgewater, NJ, USA). Milli-Q water (Millipore, Bedford, MA, USA) was used throughout the study. Chromatographic grade methanol and acetonitrile (Tedia Company Inc., Fairfield, CT, USA) were used for high-performance liquid phase analysis. All other reagents were of analytical grade.
Animals
The procedures involving animals and their care were conducted in conformity with the ARRIVE guidelines of Laboratory Animal Care (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2012). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Jiangsu Provincial Academy of Chinese Medicine (AEWC-20180711-36) and were performed in accordance with the guidelines of the Laboratory Animal Research Institute for Experimental Animals of Jiangsu Provincial Academy of Chinese Medicine. All efforts were made to minimize animal suffering and to reduce the number of animals used. Male Sprague–Dawley rats (200±20 g) were purchased from the SLAC Lab Animal Center of Shanghai (Shanghai, China). All rats were provided distilled water ad libitum. Animals were housed under a temperature of 25±0.5°C and relative humidity of 45±5% for 2 weeks in the Animal Research Center of Jiangsu Provincial Academy of Chinese Medicine with free access to food and water. Prior to the experiment, animals were fasted for 12 hrs and were only provided water.
Preparation of GEN-loaded micelles
GEN-loaded micelles (GEN-M) were prepared using the organic solvent evaporation method.42 In brief, HS15 and F127 or L61 were combined at different ratios with a defined amount of GEN and dissolved in ethanol at 50°C with constant stirring until a transparent solution was obtained. The solution was transferred to a round-bottomed flask, and ethanol was removed using a rotary evaporator under reduced pressure at 50°C (IKA®RV10, Staufen, Germany) until a film was formed completely in the flask. Deionized water was then added to the flask with shaking until a transparent solution was formed. Unincorporated GEN was removed by filtration through a 0.22-μm microporous membrane. After prescription optimization screening, the final ratio of HS15 to F127 was 35:20 (mg/mL), and the final ratio of HS15 to L61 was 49:6 (mg/mL).
Characterization of GEN-M
Particle size and zeta potential analysis
The mean particle size and zeta potential of GEN-F and GEN-L were determined by dynamic light scattering using a Malvern Zetasizer Nano System (ZEN 3600, Worcestershire, UK). Polydispersity index (PDI) was determined for assessing the particle size distribution. Samples were equilibrated at 25°C for 2 mins before analysis. Some GEN-F and GEN-L droplets were dropped onto a copper grid, and the grid was observed by TEM (JEM-2100; JEOL, Tokyo, Japan) post infrared drying for 5 mins.28
Drug content analysis
To evaluate the entrapment efficiency (EE%) and drug loading (DL%) of GEN-M, the drug concentration of GEN in mixed micelles was measured by high-performance liquid chromatography (HPLC). This consisted of a quaternary pump (Waters 2695 separation module, Waters 2489 UV/Vis detector) and a reversed-phase C18 column (250 mm×4.6 mm×5 μm) which was maintained at 35°C. Absorbance was measured at 260 nm. The mobile phase comprised a mixture of methanol and water (60:40, v/v), and the flow rate was 1.0 mL/min. The injection volume was 10 µL.
The EE% and DL% were determined by microporous membrane filtration method. The micelle solution was suitably diluted with methanol, and the structure of micelles was completely destroyed by ultrasonication. EE% and DL% were calculated using the following equations,24 and all samples were analyzed three times.
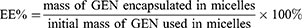
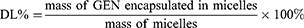
Dilution and storage stability
In storage stability studies, the optimized samples GEN-F (the concentration of GEN was 1.2 mg/mL) and GEN-L (the concentration of GEN was 1.6 mg/mL) were stored at 4°C for 1, 3, 7, 10, and 15 days. The average size, zeta potential, and the EE% of the micelle systems were then quantified at each of these time points to evaluate the storage stability of GEN-M. The dilution stability of GEN-M was studied by diluting in Milli-Q water (1–250 folds) at room temperature (25°C). The change of particle size and EE% after each dilution fold was measured as previously mentioned.
In vitro release studies
The in vitro release behavior of GEN-M was studied using the dialysis method.43 In short, 1 mL of the experimental GEN-M and the control GEN suspension were introduced into dialysis membrane bags (molecular weight cut off of 3500 g/mol; Green Bird Inc., Shanghai, China). The dialysis bags were immersed into fresh 200 mL PBS (pH 1.2 or pH 6.8) containing 0.5% (w/v) Tween 80 (Sinopharm Chemical Reagent Co., Ltd) at 37°C along with constant stirring at 140 rpm. At fixed time intervals (30 mins and 1, 2, 4, 6, 8, 10, 14, and 24 hrs), 1-mL aliquots of the dissolution medium were withdrawn and replaced with an equal volume of fresh release medium. The concentrations of GEN released in the supernatant were evaluated by HPLC analytical methods similar to the chromatographic conditions given earlier. All assays were performed in triplicate.
Caco-2 cell culture and transport experiments
Caco-2 cell lines were used to evaluate the absorption and efflux characteristics of GEN-M.44 Caco-2 cells were grown in DMEM (Sigma–Aldrich) at 37°C in a 5% CO2 atmosphere and supplemented with 10% fetal bovine serum (FBS), 1% nonessential amino acids (NEAA), 1% L-glutamine, 100 μg•mL−1 penicillin, and 100 U•mL−1 streptomycin. Caco-2 cell suspensions were grown in a Transwell culture system (Corning Incorporated, Corning, NY, USA) at a cell density of 0.1×106 cells/cm2. The cells were well developed after 21 d of seeding. Their transepithelial electrical resistance (TEER) values were greater than 600 Ω·cm−2 as measured by Millicell-ERS (Millipore, Bedford, MA, USA) before and after the transport experiments; this is indicative of the integrity of Caco-2 cell monolayers. At the beginning of the experiment, the cell monolayers were washed three times with preheated (37°C) blank Hank’s balanced salt solution (HBSS). To test for transport from the apical (AP) to basolateral (BL) side, 0.5 mL of GEN solution (dissolved in DMSO, 20 μg/mL) or GEN-M (the concentration of GEN was 20 μg/mL) was added to the AP (supply pool) while 1.5 mL HBSS (pH 7.4) was added to the BL (receiving pool). To test for transport in the opposite direction from the BL to AP side, 1.5 mL GEN or GEN-M was added to the BL surface (supply pool) and 0.5 mL HBSS was added to the AP surface (receiving pool). Aliquots of 200 μL from the receiving pool were taken at 120-min intervals. The experiment was repeated in triplicate and the apparent permeability coefficients (Papp) were calculated using the following equation:
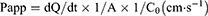
where dQ/dt (µmol·L−1·s−1) is the transport rate, A (cm2) is the surface area of the transport film, and C0 (µmol·L−1·cm−3) is the initial concentration of GEN in the donor.
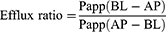
where Papp(AP-BL) is the absorption permeability and Papp(BL-AP) is the secretory permeability.
Pharmacokinetic studies in vivo
In pharmacokinetic study, SD rats were randomly divided into three groups (n=6) and fasted overnight prior to the experiment. In short, GEN-F, GEN-L, and free GEN (suspended in 0.5% CMC-Na) were orally administered at a dose of 60 mg•kg−1. Blood samples (500 μL) were collected from the orbital vein at predetermined times (10, 20, 30, and 45 mins and 1.0, 2.0, 4.0, 6.0, 8.0, 10.0, and 12.0 hrs) and immediately centrifuged to obtain the supernatant plasma. About 100 μL of plasma with 10 μL of the internal standard solution (Daidzein, 10 μg/mL) was vortexed for 15 s. After adding 500 μL ethyl acetate, they were vortexed for 3 mins to precipitate the plasma proteins and centrifuged at 13,620 g for 5 mins at 4°C. The supernatant was then transferred to a clean vial and allowed to evaporate till dry in a nitrogen atmosphere. The dried residue was rinsed with 100 μL mobile phase and centrifuged at 14,000 rpm for 5 mins. After centrifugation, 20 μL of the sample solution was injected into the HPLC system for analysis as per previously described chromatographic conditions.
Statistical analysis
All values are presented as the mean±standard deviation (SD). All data were processed using Phoenix WinNonlin 6.3 (Pharsight Corporation, USA) to construct pharmacokinetic profiles. The statistical significance of the results was analyzed using the statistical package for the social sciences software version 19.0 (SPSS Inc., USA). Data were analyzed using the two-tailed Student's t-test and one-way ANOVA to evaluate differences between the groups, and values of p<0.01 and p<0.05 indicated significant differences compared with controls.
Results
Characterization of GEN-M
The physicochemical characteristics of GEN-F and GEN-L were evaluated (Table 1). Their mean particle size diameters were determined to be 19.79 and 14.24 nm with acceptable PDIs of 0.076 and 0.190, respectively (Figure 2). Zeta potential is illustrated in Figure 2. TEM showed that GEN-F and GEN-L were homogeneous and spherical (Figure 3).
 | Table 1 Characteristics of GEN-F and GEN-L |
 | Figure 2 Particle size and zeta potential of GEN-F (A and C) and GEN-L (B and D). |
 | Figure 3 TEM micrographs and image of GEN-F (A) and GEN-L (B), Scale bar =50 nm. |
Using HPLC analyses, EE% and DL% of GEN-F was determined as 89.79±0.55% and 1.69±0.24%, respectively. As for GEN-L, the EE% and DL% were confirmed to be 83.40±1.36% and 2.26±0.18%, respectively (Table 1). Interestingly, most of the GEN was entrapped in the mixed micelle system.
The dilution stability of GEN-F and GEN-L was investigated and their particle sizes and EE% showed no significant change, suggesting that both formulations (Figure 4) had a stabilizing effect against dilution. No turbidity and layer separations were observed in the GEN-F and GEN-L at day 15. Using the average size, zeta potential, and EE%, stability was evaluated, which showed no significant change after 15 days of storage (Table 2).
 | Table 2 Storage stability of GEN-F and GEN-L |
 | Figure 4 The dilution stability of GEN-F and GEN-L. |
In vitro release
The dialysis bag method was used to estimate drug release. The cumulative release of the drugs at set time intervals is shown in Figure 5. The cumulative release of free GEN was always greater than that of GEN-F and GEN-L; however, GEN-L was slightly larger than GEN-F This release was monitored under conditions of different dialysis media with varied pH to mimic the stomach (pH 1.2) and intestinal environments (pH 6.8). In the first 4 hrs, the cumulative release from GEN-F and GEN-L was only about 10% at both pH conditions, whereas free GEN showed an elevated release of 43.90% and 69.50% at pH 1.2 and pH 6.8, respectively. These results show that free GEN displays a pronounced burst release behavior, while GEN-F and GEN-L show a sustained release even when observed at 24 h.
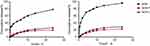 | Figure 5 Cumulative release of GEN-F, GEN-L, and GEN in vitro release study with phosphate-buffered saline pH 1.2 (A) and pH 6.8 (B). Data are presented as mean±SD (n=3). |
Transport experiment across Caco-2 cell monolayers
The Caco-2 cell culture model is widely accepted as a viable model for human intestinal absorption. In this study, the transport of GEN-F, GEN-L, and GEN from both AP-to-BL and BL-to-AP directions was investigated. As shown in Table 3, for AP-BL transport, the PappAB value of GEN was 5.28±0.49×10−6 cm/s. GEN-F and GEN-L show significantly increased absorptive power of GEN (7.50±0.52×10−6 and 8.23±0.35×10−6 cm/s, respectively). In addition, the BL-AP transport was performed to investigate the P-gp efflux inhabitation of GEN-F and GEN-L. The PappAB value for BL-AP transport of GEN-F, GEN-L, and GEN was 8.46±0.43×10−6, 7.59±0.56×10−6, and 7.97±0.36×10−6 cm/s. GEN-F and GEN-L reduced the efflux ratio of GEN from 1.51 to 1.13 and 1.08, respectively. These results indicated that GEN-F and GEN-L could increase the absorption of GEN.
 | Table 3 Permeability and efflux ratio of GEN-F, GEN-L, and GEN in Caco-2 cell model |
Pharmacokinetic studies
The main pharmacokinetic parameters are summarized in Table 4. The mean plasma concentration–time curves of GEN in rats after oral administration of 60 mg/kg GEN and GEN-M (n=6) are shown in Figure 6. The average Cmax value of GEN-F and GEN-L was 2.401±0.480 and 3.563±1.121 µg/mL, whereas that of free GEN was 0.445±0.140 µg/mL. This finding indicates that GEN-F and GEN-L could extensively increase oral absorption compared to free GEN. The average area under the curve from time 0 to 8 h (AUC0-8) of GEN-F, GEN-L, and GEN was 10.821±1.538, 14.929±5.575, and 3.351±1.182 mg•L−1•h−1. This shows a 2.75- and 4.68-fold increase in relative bioavailability. These observations differed from the results of in-vitro release experiments. In conclusion, the GEN-F and GEN-L groups showed a markedly higher relative bioavailability than that of the free GEN group (P<0.01).
 | Table 4 Pharmacokinetic parameters of GEN-F, GEN-L and GEN |
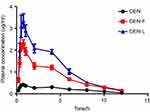 | Figure 6 The plasma drug concentration–time curve in rats after oral administration of 60 mg/kg of GEN and GEN-M. Data are presented as mean±SD (n=6). |
Discussion
Among the several types of nano-sized carrier systems, including nano-spheres, antibodies, and water-soluble synthetic polymers, previous studies of mixed micelle DDSs have proven to be advantageous in applications involving hydrophobic low-molecular-weight drugs. This is owing to the mixed micelle system’s large drug-loading capacity and its ability to interact with each other in the given carrier system.21 Further, the core–shell micelle structure also forms a protective barrier that could prevent drug escape from the core.
The oral administration of drugs is limited by their serious gastrointestinal side effects, instability at intestinal pH, and poor absorption.45 Aiming to solve these problems, we chose GEN as a model drug and developed two novel mixed micelles GEN-F and GEN-L that make the GEN to ‘dissolve’ in a hydrophobic core. GEN molecules can be encapsulated into nanoparticles surrounded by a hydrophobic barrier, thus avoiding direct contact with the hydrophilic rim environment. This eases the intestinal hydrolytic degradation and gastrointestinal irritation.
In this experiment, two different pH dialysis media were used that simulated environmental conditions of the stomach and intestine in order to understand the release of GEN and its dosage form in the gastrointestinal tract. The experimental data suggest that the entrapment of GEN in micelles could facilitate a delayed drug release in the carrier. This is attributed to the structural stability of the micelles that makes it difficult for the drug to escape easily from the core, thereby resulting in sustained release of the drug.46 Additionally, the prolonged drug retention increases the possibility of its absorption by the gastrointestinal tract.
The Caco-2 cell culture model is a viable model of human intestinal absorption, as recognized by the FDA,47 and was selected to investigate GEN absorption in this study. It has previously been used widely as an in-vitro screening model for oral absorption of small molecule drugs. Based on our experiments, GEN-F and GEN-L could reduce drug efflux in the Caco-2 cell monolayer, indicating that the two mixed micelles prepared from HS15+F127 and HS15+L61 could enhance the absorption of GEN.
Mixed micelles have inherent advantages in enhancing oral absorption.37 In-vivo bioavailability testing further confirmed that GEN-L and GEN-L increased the plasma concentration of GEN and improved its oral bioavailability. A high bioavailability indicates that the drug could be absorbed into the blood, resulting in good curative effect. The higher bioavailability of GEN-F and GEN-L after oral administration may be attributed to the following reasons: (1) Pluronics and HS15 result in a formulation with a very fine nanoparticle size that could improve absorption and permeation of GEN; (2) The surface properties of HS15 further make it easier for GEN to transverse the membrane of cells, thereby enhancing the uptake of GEN by cells to a certain degree; (3) The combination of Pluronics and solutol® HS15 increases the water solubility, stability, and sustained release of GEN, which facilitates intestinal absorption. Together, these benefits make GEN-loaded binary mixed micelle systems (GEN-F, GEN-L) a candidate with a potential commercially viable formulation.
Conclusion
Two oral drug delivery systems based on HS15+F127 and HS15+L61 binary mixed micelles were developed to markedly enhance the solubility and oral absorption of GEN. In this study, the organic solvent evaporation method was adopted to prepare two binary mixed micelle systems. These systems possessed a high drug-loading and entrapment efficiency while demonstrating a sustained release behavior. The GEN-F and GEN-L systems significantly enhanced solubility and permeability across the Caco-2 cell monolayer, resulting in increased bioavailability of GEN. Thus, we conclude that GEN-F and GEN-L are effective drug delivery systems that provide an effective strategy for developing the potential value of GEN.
Acknowledgments
This work was supported by Science and Technology Projects of Jiangsu Province State Administration of Traditional Chinese Medicine (NO. FY201507).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nabavi SF, Daglia M, Tundis R, et al. Genistein: A boon for mitigating ischemic stroke. Curr Top Med Chem. 2015;15:1714–1721.
2. Ganai AA, Farooqi H. Bioactivity of genistein: A review of in vitro and in vivo studies. Biomed Pharmacother. 2015;76:30–38. doi:10.1016/j.biopha.2015.10.026
3. Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: mechanisms underlying its effects on pancreatic β-cell function. Food Funct. 2013;4:200–212. doi:10.1039/c2fo30199g
4. Spagnuolo C, Russo GL, Orhan IE, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6:408–419. doi:10.3945/an.114.008052
5. Mukund V, Mukund D, Sharma V, Mannarapu M, Alam A. Genistein: its role in metabolic diseases and cancer. Crit Rev Oncol Hematol. 2017;119:13–22. doi:10.1016/j.critrevonc.2017.09.004
6. Yousefi H, Karimi P, Alihemmati A, Alipour MR, Habibi P, Ahmadiasl N. Therapeutic potential of genistein in ovariectomy-induced pancreatic injury in diabetic rats: the regulation of MAPK pathway and apoptosis. Iran J Basic Med Sci. 2017;20:1009–1015. doi:10.22038/IJBMS.2017.9269
7. van Duursen MB, Nijmeijer SM, de Morree ES, de Jong PC, van den Berg M. Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology. 2011;289:67–73. doi:10.1016/j.tox.2011.07.005
8. Tang J, Xu N, Ji H, Liu H, Wang Z, Wu L. Eudragit nanoparticles containing genistein: formulation, development, and bioavailability assessment. Int J Nanomedicine. 2011;6:2429–2435. doi:10.2147/IJN.S24185
9. Du W, Fan Y, Zheng N, et al. Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials. 2013;34:794–806. doi:10.1016/j.biomaterials.2012.10.003
10. Agrawal U, Sharma R, Gupta M, Vyas SP. Is nanotechnology a boon for oral drug delivery? Drug Discov Today. 2014;19:1530–1546. doi:10.1016/j.drudis.2014.04.011
11. Alqahtani MS, Islam MS, Podaralla S, et al. Food protein based core-shell nanocarriers for oral drug delivery: effect of shell composition on in vitro and in vivo functional performance of zein nanocarriers. Mol Pharm. 2017;14:757–769. doi:10.1021/acs.molpharmaceut.6b01017
12. Chen A, Shi Y, Yan Z, et al. Dosage form developments of nanosuspension drug delivery system for oral administration route. Curr Pharm Des. 2015;21:4355–4365.
13. Leonarduzzi G, Testa G, Sottero B, Gamba P, Poli G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Curr Med Chem. 2010;17(1):74–95.
14. Wang W, Cui C, Li M, Zhang Z, Lv H. Study of a novel disintegrable oleanolic acid-polyvinylpolypyrrolidone solid dispersion. Drug Dev Ind Pharm. 2017;43:1178–1185. doi:10.1080/03639045.2017.1301950
15. Zhang Z, Chen Y, Deng J, Jia X, Zhou J, Lv H. Solid dispersion of berberine-phospholipid complex/TPGS 1000/SiO(2): preparation, characterization and in vivo studies. Int J Pharm. 2014;465:306–316. doi:10.1016/j.ijpharm.2014.01.023
16. Jain S, Kumar D, Swarnakar NK, Thanki K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials. 2012;33:6758–6768. doi:10.1016/j.biomaterials.2012.05.026
17. Vural I, Sarisozen C, Olmez SS. Chitosan coated furosemide liposomes for improved bioavailability. J Biomed Nanotechnol. 2011;7:426–430.
18. Deng J, Zhang Z, Liu C, Yin L, Zhou J, Lv H. The studies of N-Octyl-N-Arginine-Chitosan coated liposome as an oral delivery system of cyclosporine a. J Pharm Pharmacol. 2015;67:1363–1370. doi:10.1111/jphp.12448
19. Chen H, Wu J, Sun M, et al. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. J Liposome Res. 2012;22:100–109. doi:10.3109/08982104.2011.621127
20. Yu F, He C, Waddad AY, et al. N-octyl-N-arginine-chitosan (OACS) micelles for gambogic acid oral delivery: preparation, characterization and its study on in situ intestinal perfusion. Drug Dev Ind Pharm. 2014;40:774–782. doi:10.3109/03639045.2013.786723
21. Zhang H, Yang X, Zhao L, Jiao Y, Liu J, Zhai G. In vitro and in vivo study of Baicalin-loaded mixed micelles for oral delivery. Drug Deliv. 2016;23:1933–1939. doi:10.3109/10717544.2015.1008705
22. Yang X, Fan R, Wang W, Wang J, Le Y. Design and synthesis of pH-sensitive polymeric micelles for oral delivery of poorly water-soluble drugs. J Biomater Sci Polym Ed. 2016;27:1341–1353. doi:10.1080/09205063.2016.1200248
23. Hou J, Sun E, Sun C, et al. Improved oral bioavailability and anticancer efficacy on breast cancer of paclitaxel via Novel Soluplus((R))-Solutol((R)) HS15 binary mixed micelles system. Int J Pharm. 2016;512:186–193. doi:10.1016/j.ijpharm.2016.08.045
24. Li X, Hou X, Ding W, et al. Sirolimus-loaded polymeric micelles with honokiol for oral delivery. J Pharm Pharmacol. 2015;67:1663–1672. doi:10.1111/jphp.12482
25. Kim JH, Baek JS, Park JK, et al. Development of houttuynia cordata extract-loaded solid lipid nanoparticles for oral delivery: high drug loading efficiency and controlled release. Molecules. 2017;22:E2215
26. Zhang YL, Zhang ZH, Jiang TY, et al. Cell uptake of paclitaxel solid lipid nanoparticles modified by cell-penetrating peptides in A549 cells. Pharmazie. 2013;68:47–53.
27. Liu Y, Liu J, Liang J, et al. Mucosal transfer of wheat germ agglutinin modified lipid-polymer hybrid nanoparticles for oral delivery of oridonin. Nanomedicine-Uk. 2017;13:2219–2229. doi:10.1016/j.nano.2017.05.003
28. Zhang ZH, Wang XP, Ayman WY, Munyendo WL, Lv HX, Zhou JP. Studies on lactoferrin nanoparticles of gambogic acid for oral delivery. Drug Deliv. 2013;20:86–93. doi:10.3109/10717544.2013.766781
29. Ma Y, He H, Xia F, et al. In vivo fate of lipid-silybin conjugate nanoparticles: implications on enhanced oral bioavailability. Nanomedicine-Uk. 2017;13:2643–2654. doi:10.1016/j.nano.2017.07.014
30. Chen G, Jaskula-Sztul R, Harrison A, et al. KE108-conjugated unimolecular micelles loaded with a novel HDAC inhibitor thailandepsin-A for targeted neuroendocrine cancer therapy. Biomaterials. 2016;97:22–33. doi:10.1016/j.biomaterials.2016.04.029
31. Lo CL, Huang CK, Lin KM, Hsiue GH. Mixed micelles formed from graft and diblock copolymers for application in intracellular drug delivery. Biomaterials. 2007;28:1225–1235. doi:10.1016/j.biomaterials.2006.09.050
32. Messina PV, Besada-Porto JM, Gonzalez-Diaz H, Ruso JM. Self-assembled binary nanoscale systems: multioutput model with LFER-covariance perturbation theory and an experimental-computational study of NaGDC-DDAB micelles. Langmuir. 2015;31:12009–12018. doi:10.1021/acs.langmuir.5b03074
33. Shaji J, Varkey D. Meloxicam-loaded Phospholipid/solutol(R) HS15 based mixed nanomicelles: preparation, characterization, and in vitro antioxidant activity. Pharm Nanotechnol. 2016;4:167–190. doi:10.2174/2211738504666160720162323
34. Seo SW, Han HK, Chun MK, Choi HK. Preparation and pharmacokinetic evaluation of curcumin solid dispersion using Solutol(R) HS15 as a carrier. Int J Pharm. 2012;424:18–25. doi:10.1016/j.ijpharm.2011.12.051
35. Nguyen-Kim V, Prevost S, Seidel K, et al. Solubilization of active ingredients of different polarity in pluronic(R) micellar solutions - Correlations between solubilizate polarity and solubilization site. J Colloid Interface Sci. 2016;477:94–102. doi:10.1016/j.jcis.2016.05.017
36. Alakhova DY, Kabanov AV. Pluronics and MDR reversal: an update. Mol Pharm. 2014;11:2566–2578. doi:10.1021/mp500298q
37. Zhang Z, Cui C, Wei F, Lv H. Improved solubility and oral bioavailability of apigenin via Soluplus/Pluronic F127 binary mixed micelles system. Drug Dev Ind Pharm. 2017;43:1276–1282. doi:10.1080/03639045.2017.1313857
38. Zhirnov AE, Demina TV, Krylova OO, Grozdova ID, Melik-Nubarov NS. Lipid composition determines interaction of liposome membranes with Pluronic L61. Biochim Biophys Acta. 2005;1720:73–83. doi:10.1016/j.bbamem.2005.11.010
39. Venne A, Li S, Mandeville R, Kabanov A, Alakhov V. Hypersensitizing effect of pluronic L61 on cytotoxic activity, transport, and subcellular distribution of doxorubicin in multiple drug-resistant cells. Cancer Res. 1996;56:3626–3629.
40. Hong W, Shi H, Qiao M, et al. PH-sensitive micelles for the intracellular co-delivery of curcumin and pluronic L61 unimers for synergistic reversal effect of multidrug resistance. Sci Rep. 2017;7:42465. doi:10.1038/srep42465
41. Liu Y, Fu S, Lin L, et al. Redox-sensitive pluronic F127-tocopherol micelles: synthesis, characterization, and cytotoxicity evaluation. Int J Nanomedicine. 2017;12:2635–2644. doi:10.2147/IJN.S122746
42. Harada Y, Yamamoto T, Sakai M, et al. Effects of organic solvents on drug incorporation into polymeric carriers and morphological analyses of drug-incorporated polymeric micelles. Int J Pharm. 2011;404:271–280. doi:10.1016/j.ijpharm.2010.11.016
43. Zhou Z, D’Emanuele A, Attwood D. Solubility enhancement of paclitaxel using a linear-dendritic block copolymer. Int J Pharm. 2013;452:173–179. doi:10.1016/j.ijpharm.2013.04.075
44. Zhang Z, Lv H, Jia X, et al. Influence of vitamin E tocopherol polyethylene glycol succinate 1000 on intestinal absorption of icariside II. Pharmazie. 2012;67:59–62.
45. Wang T, Shen L, Zhang Z, et al. A novel core-shell lipid nanoparticle for improving oral administration of water soluble chemotherapeutic agents: inhibited intestinal hydrolysis and enhanced lymphatic absorption. Drug Deliv. 2017;24:1565–1573. doi:10.1080/10717544.2017.1386730
46. Shiraishi K, Sanada Y, Mochizuki S, et al. Determination of polymeric micelles’ structural characteristics, and effect of the characteristics on pharmacokinetic behaviors. J Control Release. 2015;203:77–84. doi:10.1016/j.jconrel.2015.02.017
47. Awortwe C, Fasinu PS, Rosenkranz B. Application of Caco-2 cell line in herb-drug interaction studies: current approaches and challenges. J Pharm Pharm Sci. 2014;17:1–19.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
