Back to Journals » International Journal of Nanomedicine » Volume 18
Solid-Phase Polymerization Using Anion-Exchange Resin Can Almost Completely Crosslink Hemoglobin to Prepare Hemoglobin-Based Oxygen Carriers
Authors Hui W, Mu W, Zhao C, Xue D, Zhong Z, Fang Y, Gao M, Li X, Gao S, Liu K, Yan K
Received 17 January 2023
Accepted for publication 24 March 2023
Published 5 April 2023 Volume 2023:18 Pages 1777—1791
DOI https://doi.org/10.2147/IJN.S403739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Wenli Hui, Wenhua Mu, Cong Zhao, Dan Xue, Zihua Zhong, Yani Fang, Ming Gao, Xiao Li, Shihao Gao, Kaiyue Liu, Kunping Yan
College of Life Science, Northwest University, Xi’an City, Shaanxi Province, 710069, People’s Republic of China
Correspondence: Kunping Yan, Email [email protected]
Introduction: A limitation of hemoglobin-based oxygen carriers (HBOCs) as oxygen therapeutics is unpolymerized hemoglobin, which induces vasoconstriction leading to hypertension. The removal of unpolymerized hemoglobin from polymerized hemoglobin (PolyHb) is complex, expensive, and time-consuming.
Methods: Herein, we developed a method to completely polymerize hemoglobin almost without unpolymerized hemoglobin. Hemoglobin was adsorbed on the anion-exchange resin Q Sepharose Fast Flow or DEAE Sepharose Fast Flow, and acetal, a crosslinker prepared from glutaraldehyde and ethylene glycol, was employed to polymerize the hemoglobin. The polymerization conditions, including reaction time, pH, resin type, and molar ratios of glutaraldehyde to ethylene glycol and hemoglobin to acetal, were optimized. The blood pressure and blood gas of mice injected with PolyHb were monitored as well.
Results: The optimal polymerization condition of PolyHb was when the molar ratio of glutaraldehyde to ethylene glycol was 1:20, and the molar ratio of 10 mg/mL hemoglobin adsorbed on anion-exchange resin to glutaraldehyde was 1:300 for 60 min. Under optimized reactive conditions, hemoglobin was almost completely polymerized, with < 1% hemoglobin remaining unpolymerized, and the molecular weight of PolyHb was more centrally distributed. Furthermore, hypertension was not induced in mice by PolyHb, and there were also no pathological changes observed in arterial oxygen, blood gas, electrolytes, and some metabolic indicators.
Conclusion: The findings of this study indicate that the use of solid-phase polymerization and acetal is a highly effective and innovative approach to HBOCs, resulting in the almost completely polymerized hemoglobin. These results offer promising implications for the development of new methods for preparing HBOCs.
Keywords: polymerized hemoglobin, glutaraldehyde, acetal, solid-phase adsorption, hemoglobin-based oxygen carriers, HBOCs
Graphical Abstract:
Introduction
Owing to the oxygen-carrying and -releasing capacities of cell-free hemoglobin, it has been widely used as hemoglobin-based oxygen carriers (HBOCs) or red blood cell substitutes. These carriers/substitutes can prolong or save the lives of patients in cases of acute blood loss and hemorrhagic shock. However, hemoglobin has a tendency to easily dissociate in solution into dimeric hemoglobin having a small molecular radius, which can leak from the renal tubules, blocking them and inducing renal toxicity. To avoid renal toxicity, during the preparation of HBOCs, the molecular weight or radius of hemoglobin is usually increased via polymerization or modification. Presently, there exist four primary types of HBOCs:1–3 crosslinked polymerized hemoglobin (PolyHb), conjugated hemoglobin, crosslinked tetrameric hemoglobin, and recombinant human hemoglobin. Among them, various products developed via and efficiency of these methods. Biopure’s product Hemopure® has been approved for clinical use and marketed in South Africa and Russia where an expanded indication is being studied.4
Although polymerization or conjugation has numerous advantages, their major limitation is that they cannot crosslink or modify all hemoglobin. In PolyHb, such as Hemolink® (Hemosol, Toronto, Canada), the Hemosol product prepared using raffinose constitutes 34–42%5 unpolymerized hemoglobin. Furthermore, Hemopure® (Biopure, Cambridge, MA, USA), a product designed using glutaraldehyde as a crosslinking agent, contains less than 40% unpolymerized hemoglobin.6 Moreover, PolyHeme® (Northfield Laboratories Inc., Evanston, IL, USA), prepared using pyridoxal phosphate and glutaraldehyde as a crosslinker, contains 15% free hemoglobin.7 Finally, conjugated hemoglobin also contains higher than 7% free hemoglobin.8 These hemoglobin molecules exhibit strong vasoactivity,9–12 causing vasoconstriction, poor tissue perfusion, and poor prognosis, thereby indicating the importance of removing unpolymerized and unconjugated hemoglobin from HBOCs.
Studies have reported that large doses of intravenous infusion can trigger hypertension when the amount of unpolymerized hemoglobin is <5%. However, vasoconstriction was not observed when the proportion of unpolymerized hemoglobin was ~2%.13,14 Currently, tangential flow ultrafiltration (TFF)15–18 constitutes the most commonly used technique for the large-scale removal of uncrosslinked or unmodified hemoglobin. However, this technique usually requires a long time to remove unpolymerized or unmodified hemoglobin and has a long interception cycle, which further affects the characteristics of PolyHb, such as increasing its viscosity, protein precipitation, and filtration pressure, hampering its performance. In addition, removing unpolymerized or unmodified hemoglobin causes considerable decrease in recovery rates, resulting in high preparation costs.
Herein, we developed a hemoglobin polymerization method using anion-exchange resin. First, the hemoglobin was adsorbed on the anion-exchange resin, and then acetal (2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane), the reaction product of glutaraldehyde (GA) and ethylene glycol (EC),19 was used as a crosslinking agent to prepare PolyHb. The results showed that hemoglobin was comprehensively polymerized. Using this solid-phase adsorption (SPA) method with acetal, we were able to almost completely crosslink hemoglobin for the first time, thus laying a foundation for technological innovation in the preparation of HBOCs.
Materials and Methods
Materials
Porcine deoxyhemoglobin was provided by Xi’an Blood Oxygen Biotechnology Co., Ltd. (Xi’an, China) and 0.22-μm V-Tech®Syringe Filter was purchased from FineTech (Taiwan, China). Q Sepharose® Fast Flow (Q FF) and DEAE Sepharose® Fast Flow (DEAE FF) were obtained from Xi’an Health Bio-tech Co., Ltd. (Xi’an, China). Glutaraldehyde (25% v/v, grade II), ethylene glycol, and dimethylamine borane (DMAB) were procured from Sigma Chemical Co. (St. Louis, MO, USA). 6% HES 130/0.4 (Voluven®) was purchased from (FRESENIUS KABI, Beijing, China). High-purity nitrogen and compressed air were purchased from Xi’an Tianhai Gas Co., Ltd. (Xi’an, China).
Resin Treatment
GA at a concentration of 0.15 M was added to the resin at a volume ratio of 1:1 and mixed for 2 h on a rotary shaker (Haimen Kylin-Bell Lab Instruments Co., Ltd., Xi’an, China). Then, 10% DMAB at a molar ratio of 12.5:1 was added to quench the reaction. The mixture was centrifuged at 2500 × g for 5 min. The supernatant was removed and the resin was washed thrice with normal saline and used for adsorbing the hemoglobin.
Preparation of GA Acetals
GA and EC were mixed at a molar ratio of 1:12 under the action of 0.1 M hydrochloric acid to adjust the pH to 4. After stirring for 40 min, the reaction product 2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane was obtained. Before its use in polymerization reactions, 1 M NaOH was added to adjust the pH to 8.
Polymerization of Hemoglobin Adsorbed on the Resin
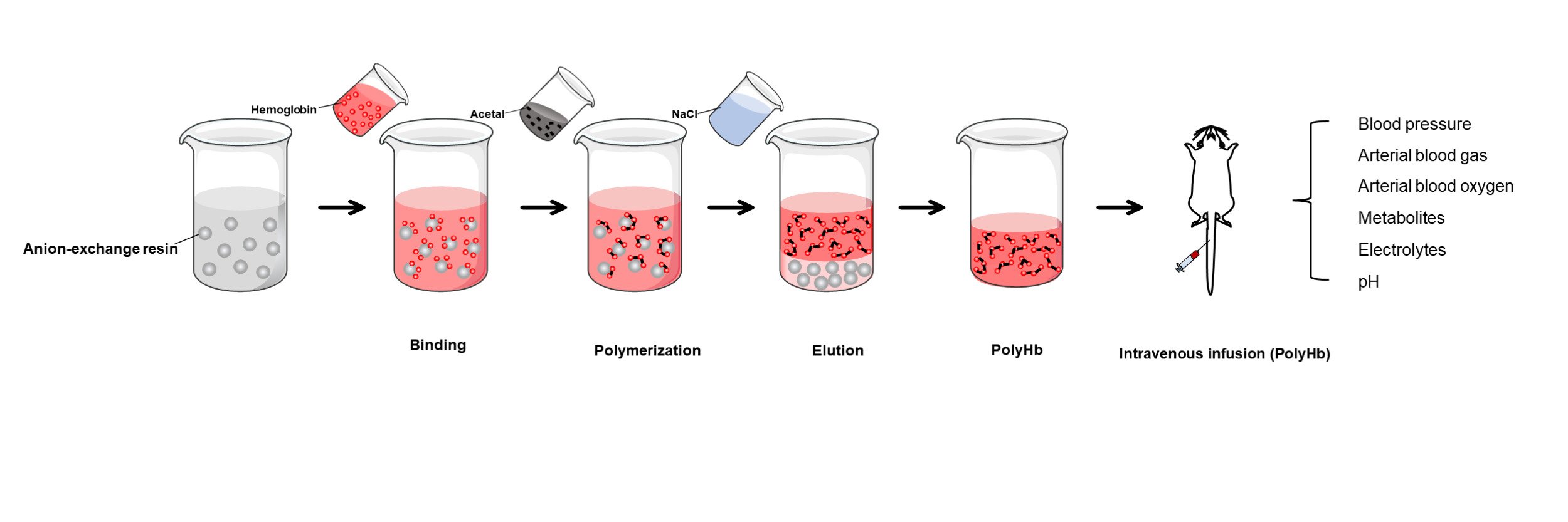
A schematic diagram of the solid-phase polymerization of hemoglobin is presented in Figure 1. Briefly, the pretreated Q FF was equilibrated using 20 mM phosphate buffer solution (pH 8), following which excess hemoglobin was added to the resin for full adsorption. The excess unadsorbed hemoglobin was washed out using 20 mM phosphate buffer solution. Next, 2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane was added to the resin for polymerization for 1 h at a molar ratio of Hb to GA of 1:270, after which 10% DMAB was added to quench the reaction and reduce the schiff’s base for 1 h at a molar ratio of DMAB to GA of 12.5:1. The unabsorbed materials in the polymerization system were washed out using 20 mM phosphate buffer (pH 8) thrice. Finally, PolyHb adsorbed on the Q FF was released through 1 M NaCl; after being left to stand for 10 min, the upper supernatant was collected as the PolyHb solution. The PolyHb solution was processed by using a 10 kDa hollow fiber column, with physiological saline as the counterbalance solution. The solution was then concentrated to 10 g/dL, pH adjusted between 7.4 and 7.8, sterilized using a 0.22 μm filter, and finally stored at 2–8°C. Q FF was regenerated using 1.2 M NaCl.
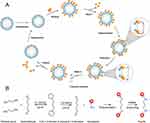 |
Figure 1 Schematic diagram of PolyHb preparation via solid-phase adsorption (A). Chemical process of PolyHb preparation (B). |
Characterization of PolyHb
The molecular weight distribution profile of PolyHb was determined via AKTA Explore system (Amersham Biosciences, Piscataway, USA) at 415 nm using a size-exclusion chromatography (SEC) column (SuperdexTM 200, 10×300 mm; GE Healthcare, USA). The contents of unpolymerized hemoglobin were integrated using UNICORN 5.1 software. The flow rate was 0.5 mL/min, mobile phase comprised 50 mM phosphate buffer and 150 mM NaCl at pH 7.4, and particle size and its distribution were determined using a multi-angle laser scattering instrument (MALS; Wyatt Technology Co., Santa Barbara, CA, USA) at 25°C. A HiLoadTM 16/600 Superdex 200 prep-grade column (16 × 600 mm; GE Healthcare, USA) was used to accurately measure the contents of unpolymerized hemoglobin. The oxygen affinity (P50) of PolyHb was determined via Hemox (TCS-Scientific, USA) at 37°C., colloid osmotic pressure tester was detected using OSMOMAT®O5O (Viesco, Germany) at 25°C, Viscosity was measured by NDJ-8S Rotary viscometer (TIANJIN CHANGJI TEST INSTRUMENT TECHNOLOGY CO., Ltd, China) at 37°C, The hydro-dynamic sizes were characterized by a Zetasizer Nano-ZS (Malvern, UK) at 25°C. The pH of the solution can be determined by first removing proteins using a 30kDa ultrafiltration centrifuge tube. The resulting filtrate can then be collected and the pH measured using a pH meter (SevenDirect SD20 LowVol Kit, METTLER TOLEDO, USA) and the concentrations of hemoglobin and its methemoglobin have been determined using the HiCN method.20,21
Animal Experiment
Animal Care Efficacy
Adult male ICR (weighing 23 ± 2 g) mice (n = 6) 6–8 weeks of age were purchased from Chengdu Dossy Experimental Animals Co. The mice were raised via standard laboratory methods for 2 days before the experiment. For postoperative analgesia, the mice were provided with water supplemented with acetaminophen or codeine (0.5 and 0.05 mg/mL, respectively) for 48 h and fasted for 12 h before the experiment. The animals were weighed before infusion.
Intravenous Infusion
The mice were anesthetized via intraperitoneal injection of 1% sodium pentobarbital (40 mg/kg). All catheters were rinsed with heparinized saline (50 U/mL) before use and discharged of bubbles to ensure good air tightness of the device. Following satisfactory anesthesia, the mice were fixed in a supine position and their limbs were fixed on a wax plate with pins. Subsequently, the left groin area was disinfected with betadine. The skin of the inguinal area was incised, opened, and the femoral arteries, veins, and nerves were exposed under sterile conditions. Forceps were inserted to widen the arterial diameter, and an I.V. Catheter (0.6 × 16 mm; AnderMed, China) filled with heparin NS was carefully inserted into the femoral artery. Then, the intravenous infusion needle (0.55 × 18 mm; AnderMed, China) was inserted into a lateral caudal vein and advanced into the vein. The femoral artery intubation was connected with MP 150 Data Acquisition System (BIOPAC, Goleta, CA) to continuously monitor blood pressure until the mice woke up. PolyHb was filtered using a 0.22-μm filter and returned to the approximate 25°C for transfusion. On stabilization of the blood pressure, PolyHb or 6% HES 130/0.4 (Voluven®) were injected separately through the tail vein using an infusion pump (Halma, UK) at a rate of 0.01 mL/min, and the maximum load was 15% of the blood volume of mice (70 mL/Kg).
Blood Pressure Monitoring
A top-load animal model was selected to investigate the response of blood pressure to PolyHb in mice.22,23 Briefly, a femoral artery I.V. Catheter was connected to MP150 data acquisition system and the blood pressure changes of mice were monitored using RM6240E multi-channel physiological signal acquisition and processing system (Lixian Instrument Scientific Co., Chengdu, China). The basal values of the mice were recorded following blood pressure stabilization for ~1 h. The blood pressure of mice was monitored continuously from the beginning of transfusion to 90 min after the completion of blood exchange. For systolic blood pressure (SBP) and mean arterial pressure (MAP), a data acquisition system was used for continuous monitoring at a frequency of 20 Hz. An automatic blood gas analyzer (GEM Premier 4000, Instrumentation Laboratory Co. MA, USA) was used to measure the blood pressure, blood gas levels, blood oxygen levels, electrolyte levels, and lactic acid levels of animals.
Statistical Analysis
Data are shown as mean ± standard error from at least five independent experiments. All statistical analyses involved one-way analysis of variance to analyze differences between the mean values. p < 0.05 was considered significant.
Results
Pretreatment of Q FF
As there might be some residual untreated amino sites in the resin, the crosslinker could crosslink hemoglobin at this site during polymerization, thereby reducing the adsorption capacity (Figure 2). The adsorption capacity of untreated resin decreased gradually with increasing duration of use. Therefore, to avoid decreased adsorption capacity, excess GA was used to completely react with these sites. During polymerization, GA was gradually released from 2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane and crosslinked the hemoglobin using Schiff’s base, and the reaction was subsequently quenched using DMAB. The unstable -C=N- double bond of Schiff’s base was reduced to a stable -C–N- single bond. Despite the adsorption capacity of the resin exhibiting a certain degree of reduction following treatment, the adsorbed amount remained stable after repeated use.
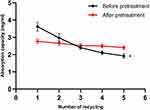 |
Figure 2 Adsorption capacity of hemoglobin on the Q Sepharose Fast Flow (Q FF) before and after pretreatment. *P < 0.05. |
Effects of Solid-Phase Polymerization Conditions on Hemoglobin Crosslinking
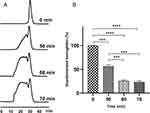
Time
Before polymerization, the retention time of hemoglobin was 30.46 min in the SEC chromatogram. On addition of 2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolaneto the hemoglobin solution, GA was gradually released, and hemoglobin was gradually polymerized (Figure 3A). After ~1 h of the reaction, the average molecular weight gradually increased and the reaction rate gradually decreased. The chromatograms of the molecular weight distribution of PolyHb did not change between 60 and 70 min, indicating that the polymerization reaction stopped at that time, and the content of unpolymerized hemoglobin remained at ~25.4% (Figure 3B).
The chromatogram of PolyHb indicated that its molecular weight distribution was from 64 to 1300 kDa (the column exclusion limit was 1300 kDa). PolyHb having an excessive molecular weight is undesirable because it might cause immunogenicity, hemodynamics changes, disordered microcirculation, or other adverse toxicities.24 The optimized polymerization reaction time was thus set at 60 min to ensure no marked increase in molecular weight and low levels of unpolymerized hemoglobin.
Molar Ratio of GA to EC
The yield of 2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane depended on the ratio of mGA: mEC. When EC was added at a ratio mGA: mEC of 1:12, it did not completely react with GA to produce 2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane, and some GA remained. More GA resulted in a fast polymerization rate and a wide molecular weight distribution of PolyHb (Figure 4A). Then, with increasing concentrations of EC, the formation of 2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane increased while that of GA decreased. Furthermore, during polymerization, more GA was released from 2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane, and the amount of unpolymerized hemoglobin gradually decreased, with its molecular weight distribution becoming narrower (Figure 4B). The optimized polymerization molar ratio of GA:EC was 1:20, while the proportion of unpolymerized hemoglobin remained at 9%.
pH
The adsorption of hemoglobin on the resin, GA release from 2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane, and GA reactive activity were affected by pH, thereby affecting the efficiency of hemoglobin polymerization. As shown in Figure 5A, the profile of PolyHb was narrower, and the proportion of unpolymerized hemoglobin was lower at pH 8.0. Specifically, the proportion of unpolymerized hemoglobin was 4.53% (Figure 5B), which was significantly lower than its proportion at pH 7 and 9.
Molar Ratio of Hb to GA
The molecular weight of PolyHb gradually increased with increasing concentration of GA, while the other reaction conditions remained unchanged. The chromatograms of the molecular weight distribution of PolyHb did not change between the ratios of 1:300 (mHb: mGA) and 1:330 (mHb: mGA), indicating that the concentration of GA was sufficient to completely react with hemoglobin at a ratio of 1:300 (mHb: mGA) (Figure 6A). In addition, the proportion of unpolymerized hemoglobin was <1% under these conditions (Figure 6B). The optimized polymerization reaction ratio of mHb: mGA was 1:300.
Selection of Resins
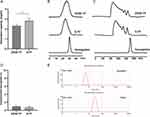
The weak and strong anion-exchange resins DEAE FF and Q FF, respectively, were selected to compare the yield of unpolymerized hemoglobin. It was found that Q FF exhibited a 12% higher adsorption capacity than DEAE FF (Figure 7A), and the molecular weight distribution of the polymerized products on Q FF was slightly more centralized than that of the products on DEAE FF (Figure 7B).
To determine the proportions of unpolymerized hemoglobin prepared on the two different resins more precisely, a longer HiLoadTM16/600 Superdex 200 prep-grade column was used to increase the resolution between the polymerized and unpolymerized hemoglobin. The proportions of unpolymerized hemoglobin were <1% in all cases for the two different resins at a retention time of 178.66 min (Figure 7C and D), indicating that hemoglobin can be almost completely polymerized on DEAE FF and Q FF. Furthermore, the particle sizes of PolyHb and hemoglobin were measured; the mean particle sizes of hemoglobin and PolyHb were 5.83 and 25.69 nm (size distribution by intensity), respectively (Figure 7E). Furthermore, there was almost without PolyHb in the central site of particle size of the hemoglobin, further indicating that there was barely any unpolymerized hemoglobin in the PolyHb.
Characterization of Hemoglobin and PolyHb
The physical and chemical characteristics of hemoglobin and polymerized hemoglobin have been summarized in Table 1. PolyHb exhibits a moderate oxygen affinity (P50 = 27.66 ± 2 mmHg), close to that of healthy human red blood cells (25–28 mmHg). The methemoglobin content in PolyHb is 3.5%, which is within the acceptable range of the body.25 The colloidal osmotic pressure (COP) of PolyHb was 18 mmHg.26 The viscosity of PolyHb is 1.3 cP.
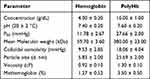 |
Table 1 Characteristics of Hemoglobin and PolyHb |
Response of PolyHb to Blood Pressure in Mice
PolyHb without unpolymerized hemoglobin was infused into mice to investigate the mechanism of influence of unpolymerized hemoglobin of HBOCs on hemodynamics. The animals were first anesthetized and then perform relevant surgical procedures; then, their blood pressure was measured, waiting for the anesthesia to wear off and the animal to wake up, this process lasts for about 1.5–2 hours. The blood pressure gradually increases by about 10mmHg and then stabilizes. There were no noteworthy fluctuations in blood pressure, and no significant changes were observed. Once the blood pressure stabilizes for 30 minutes, the sample will be infused, and the resulting blood pressure data will be presented in Figure 8. The results indicate that the baseline levels of SBP and MAP were 110 ± 7 and 97 ± 5 mmHg, respectively. After maintaining a stable baseline for 30 min, the PolyHb was infused and SBP was observed to transiently increase to 117 ± 18 mmHg during the administration. Then, SBP recovered to the baseline and was maintained at that level till 120 min. In the Voluven® group, SBP increased and continued to increase until the end of infusion. MAP remained stable during and after the PolyHb infusion, suggesting that PolyHb did not cause vasoconstriction or hypertension.
Artery Blood Analysis of PolyHb in Mice
After PolyHb was infused into the veins of mice, there was a significant increase in the total hemoglobin (tHb) level. While PolyHb has some expansion ability, it did not significantly affect the hematocrit (Hct) of red blood cells. However, when Voluven® was infused, its strong expansion ability caused the protein to be diluted, resulting in a significant decrease in both Hct and tHb levels. This also caused a significant decrease in oxygen pressure and carbon dioxide pressure. The PolyHb group had little effect on oxygen pressure but showed a short-term effect of increasing carbon dioxide pressure, which quickly returned to normal levels. The infusion of PolyHb and Voluven® had no significant effect on other arterial blood oxygen indicators and electrolyte ion levels (Table 2).
 |
Table 2 Arterial Blood Analysis in Mice after Infusion of PolyHb or Voluven® |
In terms of metabolism, the infusion of PolyHb had little effect on glucose and lactate metabolism, but infusion of Voluven® rapidly reduced glucose levels and caused rapid accumulation of lactate in the short term, indicating that Voluven® affected glucose aerobic metabolism, resulting in a large amount of glucose being converted to lactate. However, after about one hour, glucose levels can be restored to normal.
Discussion
The molar ratio of GA to EC plays a crucial role in hemoglobin polymerization. The aqueous solution of GA always forms single-hydrated GA and polymeric forms, which are heterogeneous. Reactions with free amine groups occur rapidly when they are present in this solution. Under conditions with acidic catalysts, GA and EC can undergo nucleophilic addition and protonation to produce corresponding acetals (2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane). Their properties are similar to those of ether, which is very stable.27,28 Acetals are hydrolyzed under weak acidic conditions and reduced to the original aldehyde. In this manner, the homogenous distribution of GA in the solution can be achieved. The addition of EC in advance for reaction with GA and then reacting with hemoglobin as a crosslinker during (2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane) synthesis is performed to protect the formyl group, thereby controlling the polymerization reaction.
Another essential factor affecting the yield of unpolymerized hemoglobin is the choice of resin. In contrast to an anion-exchange resin, hemoglobin adsorbed onto a cation-exchange resin is easily oxidized to generate methemoglobin, which loses the ability to carry and release oxygen. Through the selection of resins, it was found that the anion-exchange resin Q FF has more adsorption capacity compared with DEAE FF. The densities of hemoglobin adsorbed on Q FF and DEAE FF were slightly different, thereby inducing different degrees of crosslinking. This was probably because the adsorption capacity of DEAE FF for hemoglobin was lower than that of Q FF, resulting in the large gap between the hemoglobin molecules adsorbed on DEAE FF. In addition, the distribution of functional groups differed between the resins. All of these factors could lead to a difference in the molecular weight distribution of PolyHb. Nevertheless, both anion-exchange resins could achieve complete hemoglobin polymerization.
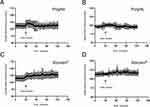
The SPA of hemoglobin and GA polymerization released from (2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane) constitute two key factors for the complete polymerization of hemoglobin and either of them alone cannot fully polymerize hemoglobin. The polymerization of hemoglobin via either GA polymerization released from (2-[3-(1,3-dioxolan-2-yl) propyl]-1,3-dioxolane) or SPA was incomplete (Figure 9A and B), with 28% and 17.28% of hemoglobin still remaining unpolymerized (Figure 9C), respectively.
In addition to the most common method of removing unpolymerized hemoglobin, ie, TFF, many researchers have fixed hemoglobin on concanavalin A-Sepharose29 by flowing GA through a cation column for in situ polymerization. However, following the completion of the polymerization reaction, the product still contained a certain amount of 64 kDa molecules. As another approach, hemoglobin adsorbed onto a cation-exchange resin was easily oxidized to generate methemoglobin, which loses the ability to carry and release oxygen. Therefore, these approaches have not been applied in an industrial setting.
The pre-treated resin Q FF has an adsorption capacity of 2.89 mg/mL for hemoglobin, as shown in Figure 2, the adsorption capacity of this protein is relatively low, so the disadvantage of this method is that the amount of PolyHb produced in a single preparation is relatively small. However, the advantages are that the preparation time is short, the method can be repeated multiple times, and the long process of removing non-polymerized proteins, which may damage the protein due to shear forces, is avoided. Additionally, the recovery rate is relatively high, indicating that this method is feasible.
During the process of preparing PolyHb, it was observed that the concentration of methemoglobin increased to a certain extent, reaching 3.5 ± 0.5, which was primarily caused by the oxidation of oxygen upon exposure to air. To investigate whether the reducing agent DMAB could decrease the level of methemoglobin, different concentrations of DMAB were used to reduce it. The results showed that DMAB had no reducing effect on methemoglobin in PolyHb, and in fact, high concentrations of DMAB could even increase the content of methemoglobin. Nevertheless, the concentration of high-iron hemoglobin in the PolyHb prepared by this method was relatively low, and it would not affect the results of other tests.
The autoxidation rate of hemoglobin is also an important characteristic of PolyHb. We conducted further autoxidation experiments on PolyHb. These experiments were conducted under conditions that simulated the human body, with hemoglobin exposed to air and in contact with oxygen at a temperature of 37°C. After prolonged observations, as demonstrated in Figure 10, we observed that the oxidation rate of PolyHb was initially slightly higher than that of hemoglobin within the first 6 hours. However, at later stages, the oxidation rates of both were almost identical. We also investigated the oxidation of PolyHb after being infused into the animal. As indicated in Table 2, the concentration of high-iron hemoglobin increased only slightly within 70 minutes after its infusion, without any significant rise.
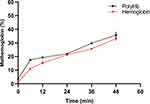 |
Figure 10 Autoxidation rates of PolyHb and hemoglobin at 37°C. |
In clinical applications, super molecular weight PolyHb could have exerted certain side effects,30 such as increased blood viscosity, increased vascular resistance, decreased microcirculatory blood flow, insufficient blood oxygen to tissues, and increased immunogenicity. Although there is no uniformly recognized standard for the optimal molecular weight of HBOC products, a consensus has formed among researchers that the molecular weight of such products should be reduced appropriately. For instance, the molecular weight of the product Hetastarch modified using hydroxyethyl starch was gradually decreased from 670 kDa (Hospira, Inc., Lake Forest, USA) to 200 kDa Pentastarch (Serumwerk Bernburg AG, Bernburg, Germany) and 130 kDa Voluven (Gallandat Huet, Fresenius/Hospira, Germany).31–34 Thus, intermediate molecular weight PolyHb may be a trend in preparing oxygen carriers.
Conclusion
Hemoglobin polymerization using SPA and acetal is a simple, reproducible, and efficient method for which quality control can be readily performed. With this approach, almost all the hemoglobin was polymerized, with a rate of unpolymerized hemoglobin of <1%. Furthermore, the molecular weight distribution of PolyHb was narrow and it did not induce hypertension in mice. These results suggest that solid-phase polymerization using acetal is an innovative method of preparing HBOCs.
Abbreviations
HBOCs, hemoglobin-based oxygen carriers; Q FF, Q Sepharose Fast Flow; DEAE FF, DEAE Sepharose Fast Flow; NO, nitric oxide; TFF, tangential flow ultrafiltration; PolyHb, polymerized hemoglobin; SPA, solid-phase adsorption; DMAB, dimethylamine borane; SBP, Systolic Blood Pressure; MAP, Mean Arterial Pressure; EC, GA, glutaraldehyde, ethylene glycol; Hb, hemoglobin; P50, O2 partial pressure associated with a 50% hemoglobin saturation.; COP, colloidal osmotic pressure; pO2, partial pressure of oxygen; pCO2, partial pressure of carbon dioxide; Hct, Hematocrit; tHb, Total hemoglobin; 02Hb, oxyhemoglobin; MetHb, methemoglobin; sO2, oxygen saturation; HHb, deoxyhemoglobin; COHb, carboxyhemoglobin; Glu, glucose; Lac, lactic acid.
Data Sharing Statement
All available data are included in this article.
Ethics Declarations
All animal experimental procedures were approved by Northwestern University laboratory animal management, welfare ethical review application and the Animal Care Committee of Northwest University. The experiments described in this study were performed in accordance with the guidelines of the National Institutes of Health on the use of experimental animals.
Funding
The work was supported by grants from the National Natural Science Foundation (Grant No. 81973225), and Natural Science Foundation of Shaanxi Province (Grant No. 2020JM-421).
Disclosure
The authors declare no conflict of interest.
References
1. Bialas C, Moser C, Sims CA. Artificial oxygen carriers and red blood cell substitutes: a historic overview and recent developments toward military and clinical relevance. J Trauma Acute Care Surg. 2019;87(1SSuppl 1):S48–S58. doi:10.1097/TA.0000000000002250
2. Winslow RM. Oxygen: the poison is in the dose. Transfusion. 2013;53(2):424–437. doi:10.1111/j.1537-2995.2012.03774.x
3. Lucas A, Ao-Ieong ESY, Williams AT, et al. Increased hemoglobin oxygen affinity with 5-Hydroxymethylfurfural supports cardiac function during severe hypoxia. Front Physiol. 2019;10:1350. doi:10.3389/fphys.2019.01350
4. Lok C. Blood product from cattle wins approval for use in humans. Nature. 2001; 410(6831):855. doi:10.1038/35073775
5. Wicks D, Wong LT, Sandhu R, et al. The intravascular persistence and methemoglobin formation of Hemolink (hemoglobin raffimer) in dogs. Artif Cells Blood Substit Immobil Biotechnol. 2003;31(1):1–17. doi:10.1081/BIO-120018000
6. Light William R, Gawryl Maria S, Laccetti Anthony J, Inventors; BIOPURE CORP, assignee. Stable polymerized hemoglobin and use thereof. US Patent 5895810. 1995.
7. Spahn DR, Kocian R. Artificial O2 carriers: status in 2005. Curr Pharm Des. 2005;11(31):4099–4114. doi:10.2174/138161205774913354
8. Clerc Y, Dubos M, Bihoreau N, et al. Pyridoxylated polymerized hemoglobin solution processing. Interest of a membrane molecular fractionation step. Appl Biochem Biotechnol. 1987;14(3):241–251. doi:10.1007/BF02800311
9. Alayash AI. Hemoglobin-based blood substitutes and the treatment of sickle cell disease: more harm than help? Biomolecules. 2017;7:1. doi:10.3390/biom7010002
10. Alayash AI. Mechanisms of toxicity and modulation of hemoglobin-based oxygen carriers. Shock. 2019;52(1SSuppl 1):41–49. doi:10.1097/SHK.0000000000001044
11. Jahr JS, Akha AS, Holtby RJ. Crosslinked, polymerized, and PEG-conjugated hemoglobin-based oxygen carriers: clinical safety and efficacy of recent and current products. Curr Drug Discov Technol. 2012;9(3):158–165. doi:10.2174/157016312802650742
12. Reynolds PS, Barbee RW, Skaflen MD, et al. Low-volume resuscitation cocktail extends survival after severe hemorrhagic shock. Shock. 2007;28(1):45–52. doi:10.1097/shk.0b013e31802eb779
13. Gould SA, Moore EE, Hoyt DB, et al. The life-sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg. 2002;195(4):445–452. doi:10.1016/S1072-7515(02)01335-2
14. Williams AT, Muller CR, Eaker AM, et al. Polymerized hemoglobin with increased molecular size reduces toxicity in healthy guinea pigs. ACS Appl Bio Mater. 2020;3(5):2976–2985. doi:10.1021/acsabm.0c00039
15. Belcher DA, Cuddington CT, Martindale EL, et al. Controlled polymerization and ultrafiltration increase the consistency of polymerized hemoglobin for use as an oxygen carrier. Bioconjug Chem. 2020;31(3):605–621. doi:10.1021/acs.bioconjchem.9b00766
16. Gu X, Savla C, Palmer AF. Tangential flow filtration facilitated fractionation and PEGylation of low and high-molecular weight polymerized hemoglobins and their biophysical properties. Biotechnol Bioeng. 2022;119(1):176–186. doi:10.1002/bit.27962
17. Palmer AF, Sun G, Harris DR. Tangential flow filtration of hemoglobin. Biotechnol Prog. 2009;25(1):189–199. doi:10.1002/btpr.119
18. Stefanescu A, Chang TM. Use of the labscale tangential flow diafiltrator to remove tetrameric hemoglobin from polyhemoglobin, purify hemolysate, and concentrate polyhemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 2009;37(2):61–68. doi:10.1080/10731190902742455
19. Roamcharern N, Payoungkiattikun W, Anwised P, et al. Physicochemical properties and oxygen affinity of glutaraldehyde polymerized crocodile hemoglobin: the new alternative hemoglobin source for hemoglobin-based oxygen carriers. Artif Cells Nanomed Biotechnol. 2019;47(1):852–861. doi:10.1080/21691401.2019.1579733
20. Grote-Koska D, Klauke R, Kaiser P, et al. Total haemoglobin - a reference measuring system for improvement of standardisation. Clin Chem Lab Med. 2020;58(8):1314–1321. doi:10.1515/cclm-2019-1177
21. Zwart A, van Assendelft OW, Bull BS, et al. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobinocyanide standard. J Clin Pathol. 1996;49(4):271–274. doi:10.1136/jcp.49.4.271
22. Yu B, Shahid M, Egorina EM, et al. Endothelial dysfunction enhances vasoconstriction due to scavenging of nitric oxide by a hemoglobin-based oxygen carrier. Anesthesiology. 2010;112(3):586–594. doi:10.1097/ALN.0b013e3181cd7838
23. Song BK, Nugent WH, Moon-Massat PF, et al. Effects of a hemoglobin-based oxygen carrier (HBOC-201) and derivatives with altered oxygen affinity and viscosity on systemic and microcirculatory variables in a top-load rat model. Microvasc Res. 2014;95:124–130. doi:10.1016/j.mvr.2014.07.005
24. Baek JH, Zhou Y, Harris DR, et al. Down selection of polymerized bovine hemoglobins for use as oxygen releasing therapeutics in a Guinea pig model. Toxicol Sci. 2012;127(2):567–581. doi:10.1093/toxsci/kfs109
25. Silverman TA, Weiskopf RB. Hemoglobin-based oxygen carriers: current status and future directions. Transfusion. 2009;49(11):2495–2515. doi:10.1111/j.1537-2995.2009.02356.x
26. Kure T, Sakai H. Transmembrane difference in colloid osmotic pressure affects the lipid membrane fluidity of liposomes encapsulating a concentrated protein solution. Langmuir. 2017;33(6):1533–1540. doi:10.1021/acs.langmuir.6b04643
27. Gowda SGB, Nakahashi A, Yamane K, et al. Facile chemoselective strategy toward capturing sphingoid bases by a unique glutaraldehyde-functionalized resin. ACS Omega. 2018;3(1):753–759. doi:10.1021/acsomega.7b01440
28. Karaagac E, Besir Y, Kurus M, et al. The effect of bovine serum albumin-glutaraldehyde and polyethylene glycol polymer on neointimal hyperplasia in rabbit carotid artery anastomosis. J Biomater Appl. 2021;36(1):152–164. doi:10.1177/0885328220964913
29. Hu T, Su Z. A solid phase adsorption method for preparation of bovine serum albumin-bovine hemoglobin conjugate. J Biotechnol. 2003;100(3):267–275. doi:10.1016/S0168-1656(02)00246-8
30. Muller CR, Williams AT, Munoz CJ, et al. Safety profile of high molecular weight polymerized hemoglobins. Transfusion. 2021;61(1):212–224. doi:10.1111/trf.16157
31. Estep T, Bucci E, Farmer M, et al. Basic science focus on blood substitutes: a summary of the NHLBI Division of Blood Diseases and Resources Working Group Workshop, March 1, 2006. Transfusion. 2008;48(4):776–782. doi:10.1111/j.1537-2995.2007.01604.x
32. Lehmann GB, Asskali F, Boll M, et al. HES 130/0.42 shows less alteration of pharmacokinetics than HES 200/0.5 when dosed repeatedly. Br J Anaesth. 2007;98(5):635–644. doi:10.1093/bja/aem068
33. Li Y, Yan D, Hao S, et al. Polymerized human placenta hemoglobin improves resuscitative efficacy of hydroxyethyl starch in a rat model of hemorrhagic shock. Artif Cells Nanomed Biotechnol. 2015;43(3):174–179. doi:10.3109/21691401.2015.1024846
34. Wierenga JR, Jandrey KE, Haskins SC, et al. In vitro comparison of the effects of two forms of hydroxyethyl starch solutions on platelet function in dogs. Am J Vet Res. 2007;68(6):605–609. doi:10.2460/ajvr.68.6.605
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.