Back to Journals » International Medical Case Reports Journal » Volume 7
Soil-acquired cutaneous nocardiosis on the forearm of a healthy male contracted in a swamp in rural eastern Virginia
Authors Palmieri JR , Santo A, Johnson S
Received 17 December 2013
Accepted for publication 18 January 2014
Published 7 March 2014 Volume 2014:7 Pages 41—47
DOI https://doi.org/10.2147/IMCRJ.S59315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
James R Palmieri,1 Arben Santo,2 Shawn E Johnson1
1Department of Microbiology, Infectious and Emerging Diseases, 2Department of Pathology, Edward Via College of Osteopathic Medicine, Virginia Campus, Blacksburg, VA, USA
Abstract: A 45-year-old man complained of pain and swelling on his right wrist after receiving a scratch while playing paintball in a swampy area of eastern Virginia. Two weeks later, he noticed a pimple-like lesion developing, which quickly grew in size and then ulcerated. Because of the severity of his condition, the patient was taken to the emergency room where surgical drainage of the abscess was carried out and the pus was sent for culture and sensitivity testing. Enlarged and tender lymph nodes were palpable going up the arm and surrounding the right axillary area. Three days following culture of pus from his lesion, colonies of Nocardia brasiliensis were isolated. He was successfully treated with an extended regimen of trimethoprim-sulfamethoxazole. Because of its low incidence, nocardiosis is usually not considered in the initial diagnosis. The rapidity with which his infection developed from a pimple-like lesion into an extensive ulcerated area, the involvement of his lymphatic system, the extended time needed to successfully treat his infection, and the potential for infection to rapidly disseminate, reinforces the necessity for laboratory identification and immediate treatment of severe pyogenic cutaneous lesions.
Keywords: actinomycetes, cutaneous nocardiosis, immunocompromised, mycetoma, Nocardia brasiliensis, nocardiosis, trimethoprim-sulfamethoxazole
Introduction
Nocardia species are ubiquitous soil saprophytes associated with dust, water, decaying vegetation, and fecal matter.1 Microbiologically, Nocardia is an abundant aerobic, Gram-positive actinomycete bacillus that has the microscopic appearance of branching hyphae and can become localized or disseminated in humans and animals, causing considerable disease.2 The genus Nocardia was named after the French veterinarian, Edmond Isidore Etienne Nocard, who in 1888 isolated a Gram-positive, acid-fast, aerobic bacillus from cattle infected with bovine farcy, causing a purulent lymphadenitis and lymphangitis. After the discovery by Nocard, the disease was linked to Nocardia species, and Nocardia farcinica was identified as the causal agent. Later, researchers found that isolates from cattle with bovine farcy in Africa were Mycobacterium farcinogenes and not Nocardia.3 The taxonomy of Nocardia continues to evolve, with several hundred species and strains described to date.4,5 Infections caused by Nocardia species are uncommon in humans yet challenging to clinicians. The clinical and microbiological spectrum of nocardiosis has changed recently due to the emergence of new categories of immunocompromised and immunosuppressed patients, and improved molecular diagnostic techniques used for identification of Nocardia isolates.5 Approximately 60% of cases of nocardiosis occur in patients with pre-existing immunocompromised or immunosuppressed conditions, with cell-mediated conditions, or with ongoing infections. Immunocompromised patients include those who have received solid organ transplantation and are on immunosuppressive therapy, those with acquired immune deficiency syndrome, and those with malignancies such as non-Hodgkin lymphoma, Hodgkin lymphoma, and leukemia. Other immunocompromised states include alcohol abuse, chronic granulomatous disease, emphysema, pulmonary alveolar proteinosis, asthma, diabetes, sarcoidosis, tuberculosis, and systemic lupus erythematosus.6–8
Increasing numbers of cases of nocardiosis have been reported in the literature over the past two decades, reflecting both increasing numbers of immunocompromised patients and improved methods for laboratory identification of organisms. Because nocardiosis is not a reportable disease in the USA, its actual incidence is unknown.9 It has been estimated that 500–1,000 new cases of nocardiosis infection occur every year in the USA in both immunocompromised and immunocompetent patients. Men are more frequently affected than women, with a male to female ratio of 3:1. This difference may be related to exposure frequency rather than a sex difference in susceptibility. The mean age at diagnosis is in the fourth decade of life, with the majority between 20 and 60 years of age.10,11 Globally, the annual incidence of nocardiosis averages about 0.375 cases per 100,000 persons.2,8,12,13
The lung is most often the site of primary infection following inhalation of the organism, although the skin can also be the site of primary infection through traumatic inoculation.8,14,15
The exact mechanism of pulmonary nocardiosis is probably through direct inhalation of contaminated particles. Most of the cases start as minor respiratory syndromes that self-limit spontaneously. In some patients, the infection spreads from the lung to the brain, skin, and subcutaneous tissues.16,17 Infection with Nocardia may also occur by direct inoculation through the skin, producing cellulitis, lymphangitis, or both. Intravenous drug abuse may provide another route of entry, leading to abscess formation at the injection site. Gastrointestinal colonization has been reported resulting from inhalation of spores and swallowing sputum. There is no definitive evidence of person-to-person transmission of Nocardia infection.6,10,12,18
Case report
A 45-year-old man presented to a local urgent care medical center in Roanoke, Virginia, with complaints of pain and a swelling on his right wrist. The patient reported having scratched his right wrist while playing paintball in eastern Virginia 3 weeks prior. He reported slipping while running through a swamp and received a small cut on the volar aspect of his right wrist. Two weeks later, he noticed a pimple-like lesion developing at the same site on his right wrist. The lesion quickly grew in size and then ulcerated. The patient’s temperature was 36.3°C (97.3°F). On physical examination, a large abscess, 1 inch × 4 inches in diameter was noted on the volar aspect of his right wrist, with erythema spreading along the anterior surface of his distal forearm (Figures 1 and 2). A chain of enlarged and tender lymph nodes was palpable going up the arm and surrounding the right axillary area.
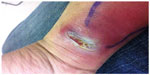  | Figure 1 Clinical photograph showing close-up view of skin abscess on the volar aspect of the patient’s right wrist. |
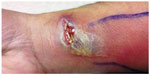  | Figure 2 Photograph of skin abscess surrounded by skin erythema taken 2 days later. |
Because of the severity of his condition, the patient was taken to the emergency room at Carillion Medical Center, where surgical drainage of the abscess was carried out and the pus was sent for culture and sensitivity testing. The emergency room physician suspected the patient had a methicillin-resistant Staphylococcus aureus infection and the patient was prescribed tetracycline, trimethoprim-sulfamethoxazole, and vancomycin. Blood tests revealed a peripheral white blood cell count of 5,500/mm3 (normal 4,000–10,500/mm3) with a differential of neutrophils, lymphocytes, and monocytes of 55.9%, 33.3%, and 6.2%, respectively (normal 42%–75%, 21%–51%, and 2%–13%, respectively). Laboratory studies revealed a hemoglobin of 14.0 g/dL (normal 13–16 g/dL), a hematocrit of 41.2% (normal 37%–49%), and a platelet count of 223,000/mm3 (normal 130,000–400,000/mm3). Urine analysis was negative for blood, leukocytes, protein, and glucose. A comprehensive metabolic panel revealed total protein of 6.6 g/dL (normal 6.0–8.3 g/dL), albumin of 4.6 g/dL (normal 3.2–5.5 g/dL), glucose of 77 mg/dL (normal 70–99 mg/dL), creatinine of 1.03 mg/dL (normal 0.5–1.4 mg/dL), calcium of 9.7 mg/dL (normal 8.5–10.7 mg/dL), total bilirubin of 0.3 mg/dL (normal <1.3 mg/dL), aspartate transaminase of 21 IU/L (normal 10–42 IU/L), and alanine transaminase of 20 IU/L (normal 10–60 IU/L). Three days later, culture of pus yielded colonies of Nocardia brasiliensis. After consultation with the infectious disease service, the tetracycline and vancomycin were stopped and the patient was continued on double-strength oral trimethoprim-sulfamethoxazole tablets (800–160 mg) once every 12 hours for 30 days. The patient reported remarkable improvement in his symptoms by day 5 after initiation of treatment with trimethoprim-sulfamethoxazole. Most of the lymphatic spread had resolved and only some erythema and swelling were seen at the area of the original lesion. A chest X-ray was reported as normal.
Four weeks later, the patient returned for follow-up of his cutaneous nocardiosis infection. The site on his wrist was completely closed and there was no longer any erythema present. The patient retained a residual scar at the infection site (Figure 3). His axillary lymph nodes were still enlarged and tender. The patient continued on double-strength oral trimethoprim-sulfamethoxazole tablets (800–160 mg) every 12 hours for a further 60 days. On follow-up after 2 months, the patient reported that he was doing well.
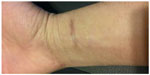  | Figure 3 Photograph of completely healed wrist wound taken 2 months later. |
Discussion
Epidemiology
Because nocardiosis is not a reportable disease in the USA, its frequency is unknown. It has been estimated that 500–1,000 new cases of nocardiosis infection occur every year. Most patients are between 20 and 60 years of age.10 Nocardia asteroides is the most common species associated with human disease,19 while N. brasiliensis is the most common Nocardia species causing cutaneous disease.11 N. brasiliensis has been recovered from the soil in many tropical and subtropical areas but rarely in temperate areas. Traumatic inoculation of N. brasiliensis into the skin is the most typical mode of acquisition of infection due to this organism. The subject of this case report most likely acquired his nocardiosis infection when he fell and scratched his right wrist while participating in a paintball tournament in a swamp area of eastern Virginia. There are reports of nocardiosis acquired from swamps in North and South Carolina, which are ecologically similar to the swamps found along the eastern shore of Virginia.20,21 Nocardia autotrophica is one of the more common species of Nocardia isolated from soil and from swamp environments, but is more often associated with infection in immunocompromised individuals.20–22 Unlike N. autotrophica, N. brasiliensis causes disease predominantly in immunocompetent individuals.
Pathogenesis
At least three basic forms of primary cutaneous disease may be recognized following Nocardia infection, ie, localized superficial cutaneous nocardiosis, lymphocutaneous (sporotrichoid) nocardiosis, and Nocardia-induced mycetoma.7
Cutaneous nocardiosis
Localized superficial cutaneous nocardiosis usually occurs following a local skin trauma such as a thorn, splinter, or puncture wound contaminated by soil in immunocompromised and immunocompetent individuals.23 It is likely that cutaneous inoculation of Nocardia organisms into the skin is relatively common, since they are ubiquitous in the soil. Once the integrity of the skin is breached, an acute inflammatory response develops, resulting in necrosis and abscess formation. Clinically, superficial cutaneous nocardiosis presents as pustules, pyoderma, localized cellulitis, or localized abscess. In most instances, this infectious process is self-limiting. These types of cutaneous infections have the same appearance as diseases caused by other pyogenic bacteria, such as Staphylococcus spp. and Streptococcus spp., except that nocardial infections tend to be more indolent.23–26 Generally, this form is misdiagnosed mostly because culture incubation is not routinely performed. Because cutaneous nocardiosis heals completely in response to antibiotic therapy, it is likely that patients with cutaneous nocardiosis are often treated based on the diagnosis of phlegmon or other similar diseases.7,26
Lymphocutaneous nocardiosis
This is the most common form of primary cutaneous nocardiosis. Organisms invade deep into the skin involving the lymphatic system.27–30 The infection spreads proximally from the painful suppurative nodule located at the site of inoculation along the lymphatic channels to the regional lymph nodes. Several abscesses develop along the line of lymphatic channels, leading to a clinical pattern of nodular lymphangitis. These clinical characteristics resemble sporotrichosis.28,31,32 The clinical course of lymphocutaneous nocardiosis is more acute and highly inflammatory when compared with clincal characteristics for sporotrichosis. Only Nocardia-induced mycetoma has granules, and the absence of granules differentiates lymphocutaneous nocardiosis from Nocardia-induced mycetoma.32–34
Nocardia-induced mycetoma
Mycetoma is a chronic suppurative infection of the subcutaneous tissue. Mycetomas are characterized by formation of hard nodules which over time soften and discharge a viscous, purulent exudate through sinus tracts. The drained pus contains white or yellowish granules which are actually microcolonies of the infecting organism surrounded by masses of inflammatory cells. Mycetomas are almost always painless and there are few or no constitutional symptoms.35,36 Most cases of mycetoma are found in tropical and subtropical regions. This disease has been reported from countries between latitudes 30°N and 15°S. Countries with the highest number of mycetoma cases reported are Mexico, Sudan, and India.37,38 Both bacteria and fungi are able to cause mycetoma. There are two types of mycetoma, depending on the type of microorganism causing the disease. These include actinomycetomas caused by the actinomycete bacteria and eumycetomas caused by fungi such as Madurella mycetomatis. Worldwide, approximately 51% of cases are caused by actinomycetes, and the majority of these are caused by Actinomadura madurae. Nocardia spp. are responsible for only about 5% of all reported cases.37,38
Laboratory diagnosis
The clinical diagnosis of nocardiosis is difficult, because clinical findings are nonspecific and serological diagnosis is often unreliable. The diagnosis of nocardiosis should always be based on isolation of Nocardia organisms by smear and by culture from abscess samples or from skin biopsies. Detection of Gram-positive actinomycete bacilli in Gram-stained smears and isolation of Nocardia spp. on primary and/or selective culture medium such as Sabouraud agar or Thayer-Martin agar with antibiotics are routine for most clinical laboratories. Growth of typical Nocardia colonies is usually seen after 2–7 days.8,14,39–41 Nocardia colonies have a variable appearance depending on the species involved, and colonies may appear circular, convex, smooth, or rough. Colonies usually appear firmly adherent to the agar surface, and colony color may vary from white, to tan, orange, or red. Colonies often present with aerial hyphae that are characteristic of fungal organisms. Colonies also have a characteristic powdery dry surface appearance. On Gram stain, Nocardia appear as long, thin, branching, and finely beaded Gram-positive rods that are usually acid-fast.42 Organisms acquired from direct smears or culture can be identified using Gram staining and acid-fast staining (Kinyoun staining method). Direct smears typically show Gram-positive, beaded, branching filaments that are usually acid-fast. Signs, symptoms, and radiological studies may suggest the diagnosis but are not pathognomonic. Serological diagnosis is unreliable, and serological tests are not readily available commercially.8,14,39,42–44 Evaluation of appropriate specimens by smear and culture remains the principal method of diagnosis. Nocardia spp. can be isolated and cultured from blood.45–47
Treatment
General treatment recommendations for nocardiosis are hindered by a lack of prospective controlled trials. Optimal antimicrobial regimens have not been firmly established for the treatment of nocardiosis. Nocardia species display variability with regard to in vitro antimicrobial susceptibility patterns, so management of Nocardia infections must be individualized.2,48,49 The use of surgery in the management of nocardiosis depends on the site and extent of the infection. In extraneural disease, indications for aspiration, drainage, or excision of abscesses are similar to those for other chronic bacterial infections.10,50,51 Therapeutic aspiration is generally inadequate in patients with thick-walled multiloculated abscesses containing free-flowing pus and in patients with mycetomas. The Clinical and Laboratory Standards Institute has published recommendations for antimicrobial susceptibility testing for Nocardia and other aerobic actinomycetes.52 Clinical experience has shown that successful therapy requires the use of antimicrobial drugs in combination with appropriate surgical drainage. The optimal antimicrobial therapy depends on the severity and localization of the infection, the species of Nocardia, host immune status, potential drug interactions, and toxicity associated with antibiotic use.53 In some circumstances, and especially in cases of relapse after therapy, antimicrobial susceptibility testing is recommended, and it is appropriate for a reference laboratory to confirm test results. Indications for testing include isolation of Nocardia organisms from areas of deep-seated or disseminated infection, lack of response to initial therapy, contraindications to the use of sulfonamides, and infections that are caused by resistant strains such as N. farcinica and Nocardia otitidiscaviarum.14,54,55 Nocardia isolated from clinically significant infections should undergo antimicrobial susceptibility testing to assist in treatment decisions. Sulfonamides, including sulfadiazine and sulfisoxazole, have been the antimicrobials of choice to treat nocardiosis for the past 50 years despite bacteriostatic activity.56 Trimethoprim-sulfamethoxazole is the most commonly used sulfonamide preparation in the USA, although the benefit of the trimethoprim component is unclear. Divided doses of 5–10 mg/kg per day of the trimethoprim component (or 25–50 mg/kg per day of sulfamethoxazole) are recommended to produce sulfonamide serum concentrations between 100 and 150 μg/mL. Adverse reactions to high-dose trimethoprim-sulfamethoxazole therapy are frequent, and include myelosuppression, hepatotoxicity, and renal insufficiency. Trimethoprim-sulfamethoxazole is active against most Nocardia species; however, N. otitidiscaviarum is commonly resistant to trimethoprim-sulfamethoxazole, and strains of N. nova and N. farcinica are occasionally resistant. Alternative antimicrobial agents with activity against Nocardia include amikacin, imipenem, meropenem, ceftriaxone, cefotaxime, minocycline, moxifloxacin, levofloxacin, linezolid, tigecycline, and amoxicillin-clavulanic acid. Imipenem is more active than either meropenem or ertapenem against most Nocardia species.22,57–60 Clinical improvement following treatment is evident within 7–10 days. Parenteral therapy can be changed to an oral regimen; high doses of trimethoprim-sulfamethoxazole may be reduced after 3–6 weeks. Patients with extensive nocardiosis, those with lesions not accessible to surgery, and those who respond slowly may benefit from prolongation of parenteral and oral treatment.22,56,57,59,60 The clinical outcome of therapy depends on the site of infection, the extent of disease, and underlying host factors. Cure rates of almost 100% are found in patients with skin and soft tissue involvement, as compared with 90% in patients with pleuropulmonary disease, 60% in patients with disseminated nocardiosis, and 50% in patients with brain abscesses.57,58,60 Mortality is highest among immunocompromised patients and in those patients with multiple brain abscesses. In summary, most patients have favorable outcomes and show a good response to treatment, in almost all cases involving immunosuppression and when early treatment is given.57 Delay in diagnosis and early suspension of treatment, especially in patients with acquired immune deficiency syndrome, are associated with relapse and failure of treatment.22,53
Conclusion
The severity of the infection in this case report combined with the extended time required for successful treatment of his infection reinforces the need to culture and determine the antimicrobial sensitivity of organisms causing severe cutaneous lesions. This is especially true with cutaneous infections that have the same appearance as abscesses caused by other pyogenic bacteria. Because of its low incidence, nocardiosis is usually not considered in the initial diagnosis. The rapidity with which this patient’s infection developed from a pimple-like lesion to an extensive ulcerated area, the involvement of his lymphatic system, the extended time needed to treat his infection successfully, and the potential for infection to disseminate rapidly, reinforces the necessity for laboratory identification and immediate treatment of severe pyogenic cutaneous lesions.
Disclosure
The authors have no conflicts of interest to report in this work. None of the authors are receiving any financial benefit from the research conducted or from the reporting of this research.
References
Martinez TR, Menendez Villanueva R, Reyes Calzada S, et al. Pulmonary nocardiosis: risk factors and outcomes. Respirology. 2007;12(3):394–400. | |
Muñoz J, Mirelis B, Aragón LM, et al. Clinical and microbiological features of nocardiosis 1997–2003. J Med Microbiol. 2007;56(4):545–550. | |
Hamid ME. Epidemiology, pathology, immunology and diagnosis of bovine farcy: a review. Prev Vet Med. 2012;105(1–2):1–9. | |
Gordon RE, Mihm JM. The type species of the genus Nocardia. J Gen Microbiol. 1962;27(1):1–10. | |
Haas L. Edmond Isidore Etienne Nocard (1850–1903). J Neurol Neurosurg Psychiatry. 2000;69(1):130. | |
McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7(3):357–417. | |
Beaman BL, Beaman LV. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7(2):213–264. | |
Saubolle MA, Sussland D. Nocardiosis review of clinical and laboratory experience. J Clin Microbiol. 2003;41(10):4497–4501. | |
Filice GA. Nocardiosis in persons with human immunodeficiency virus infection, transplant recipients, and large, geographically defined populations. J Lab Clin Med. 2005;145(3):156–162. | |
Corti ME, Villafañe-Fioti ME. Nocardiosis: a review. Int J Infect Dis. 2003;7(4):243–250. | |
Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–407. | |
Schlaberg R, Fisher MA, Hanson KE. Susceptibility profiles of Nocardia isolates based on current taxonomy. Antimicrob Agents Chemother. November 18, 2013. [Epub ahead of print.] | |
Conville P, Witebsky FG, Murray PR, et al. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology Volume 1. 9th ed. Washington, DC, USA: ASM Press; 2007. | |
Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19(2):259–282. | |
Ambrosioni J, Lew D, Garbino J. Nocardiosis: updated clinical review and experience at a tertiary center. Infection. 2010;38(2):89–97. | |
Kontogiorgi M, Opsimoulis P, Kopterides P, et al. Pulmonary nocardiosis in an immunocompetent patient with COPD: the role of defective innate response. Heart Lung. 2013;42(4):247–250. | |
Kajorn I. Pulmonary nocardiosis presenting as lung abscess in an immunocompetent patient. Internet J Microbiol. 2009;8(2):776–780. | |
Sullivan DC, Chapman SW. Bacteria that masquerade as fungi: actinomycosis/Nocardia. Proc Am Thorac Soc. 2010;7(3):216–221. | |
Vanegas S, Franco-Cendejas R, Cicero A, López-Jácome E, Colin C, Hernández M. Nocardia Brasiliensis-Associated femorotibial osteomyelitis. Int J Infect Dis. December 18, 2013. [Epub ahead of print.] | |
Gordon RE, Barnett DA, Handerhan JE, Pang CHN. Nocardia coeliaca, Nocardia autotrophica, and the nocardin strain. Int J Syst Bacteriol. 1974;24(1):54–63. | |
Smego RA, Galli HA. The clinical spectrum of Nocardia brasiliensis infection in the United States. Rev Infect Dis. 1984;6(2):164–180. | |
Bodro M, Paterson DL. Has the time come for routine trimethoprim-sulfamethoxazole prophylaxis in patients taking biologic therapies? Clin Infect Dis. 3013;56(11):1621–1628. | |
Dodiuk-Gad R, Cohen E, Ziv M, et al. Cutaneous nocardiosis: report of two cases and review of the literature. Int J Dermatol. 2010;49(12):1380–1385. | |
Satterwhite TK, Wallace RJ. Primary cutaneous nocardiosis. JAMA. 1979;242(4):333–336. | |
Inamadar AC, Palit A, Peerapur B, Rao SD. Sporotrichoid nocardiosis caused by Nocardia nova in a patient infected with human immunodeficiency virus. Int J Dermatol. 2004;43(11):824–826. | |
Saoji VA, Saoji SV, Gadegone RW, Menghani PR. Primary cutaneous nocardiosis. Indian J Dermatol. 2012;57(5):404–406. | |
Maraki S, Chochlidakis S, Nioti E, Tselentis Y. Primary lymphocutaneous nocardiosis in an immunocompetent patient. Ann Clin Microbiol Antimicrob. 2004;3:24. | |
Fukuda H, Saotome A, Usami N, Urushibata O, Mukai H. Lymphocutaneous type of nocardiosis caused by Nocardia Brasiliensis: a case report and review of primary cutaneous nocardiosis caused by N. brasiliensis reported in Japan. J Dermatol. 2008;35(6):346–353. | |
Bryant E, Davis CL, Kucenic MJ, Mark LA. Lymphocutaneous nocardiosis: a case report and review of the literature. Cutis. 2010; 85(2):73–76. | |
Shelkovitz-Shilo I, Feinstein A, Trau H, Kaplan B, Sofer E, Schewach-Millet M. Lymphocutaneous nocardiosis due to Nocardia asteroides in a patient with intestinal lymphoma. Int J Dermatol. 1992;31(3):178–179. | |
Bosamiya SS, Vaishnani JB, Momin AM. Sporotrichoid nocardiosis with cutaneous dissemination. Indian J Dermatol Venereol Leprol. 2011;77(4):535. | |
Maraki S, Scoulica E, Alpantaki K, Dialynas M, Tselentis Y. Lymphocutaneous nocardiosis due to Nocardia brasiliensis. Diagn Microbiol Infect Dis. 2003;47(1):341–344. | |
Wlodaver CG, Tolomeo T, Benear JB. Primary cutaneous nocardiosis mimicking sporotrichosis. Arch Dermatol. 1988;124(5):659–660. | |
Keystone JS, Kozarsky PE, Freedman DO, Nothdurft HD, Connor BD. Travel medicine. J Travel Med. 2010;17(1):74. | |
Elmaci I, Senday D, Silav G, et al. Nocardial cerebral abscess associated with mycetoma, pneumonia, and membranoproliferative glomerulonephritis. J Clin Microbiol. 2007;45(6):2072–2074. | |
Sharma NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74(6):635–640. | |
Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25(2):195–202. | |
van de Sande WW. Global burden of human mycetoma: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2013;7(11):e2550. | |
Kiska DL, Hicks K, Pettit J. Identification of medically relevant Nocardia species with an abbreviated battery of tests. J Clin Microbiol. 2002;40(4):1346–1351. | |
Roth A, Andrees S, Kroppenstedt RM, Harmsen D, Mauch H. Phylogeny of the genus Nocardia based on reassessed 16S rRNA gene sequences reveals underspeciation and division of strains classified as Nocardia asteroides into three established species and two unnamed taxons. J Clin Microbiol. 2003;41(2):851–856. | |
Read RC. Nocardiosis and actinomycosis. Medicine. 2005;33(5):114–115. | |
Larone DH. Medically Important Fungi: A Guide to Identification. New York, NY, USA: Elsevier; 1987;189. | |
Georg LK. Nocardia species as opportunists and current methods for their identification. In: Prier, JE, Friedman H. Opportunistic Pathogens. Baltimore, MD, USA: University Park Press; 1974. | |
Glupczynski Y, Berhin C, Janssens M, Wauters G. Determination of antimicrobial susceptibility patterns of Nocardia spp. from clinical specimens by Etest. Clin Microbiol Infect. 2006;12(9):905–912. | |
Esteban J, Ramos JM, Fernandez-Guerrero ML, Soriano F. Isolation of Nocardia sp. from blood cultures in a teaching hospital. Scand J Infect Dis. 1994;26(6):693–696. | |
Kontoyiannis DP, Jacobson KL, Whimbey EE, Rolston KV, Raad II. Central venous catheter-associated Nocardia bacteremia: an unusual manifestation of nocardiosis. Clin Infect Dis. 2000;31(2):617–618. | |
Höpler W, Laferl H, Szell M, et al. Blood culture positive Nocardia asteroides infection: a case report. Wien Med Wochenschr. 2013; 163(1–2):37–39. | |
Wellinghausen N, Pietzcker T, Kern WV, Essig A, Marre R. Expanded spectrum of Nocardia species causing clinical nocardiosis detected by molecular methods. Int J Med Microbiol. 2002;292(3):277–282. | |
Von Graevenitz, A. Antimicrobial therapy of infections with aerobic Gram-positive rods. Clin Microbiol Infect. 2001;7(s4):43–46. | |
Sirisena D, Al Swedan L, Jayne D, Chakravarty K. A case of systemic nocardiosis in systemic vasculitis and a review of the literature. Singapore Med J. 2013;54(6):e127–e130. | |
Amatya R, Koirala R, Khanal B, Dhakal SS. Nocardia brasiliensis primary pulmonary nocardiosis with subcutaneous involvement in an immunocompetent patient. Indian J Med Microbiol. 2011;29(1):68–70. | |
Clinical and Laboratory Standards Institute. CLSI M24-A2 – Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard, M24A2E. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2011. | |
Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14(17):2387–2398. | |
Carrasco G, Valdezate S, Garrido N, Villalón P, Medina-Pascual MJ, Sáez-Nieto JA. Identification, typing, and phylogenetic relationships of the main clinical Nocardia species in Spain according to their gyrB and rpoB genes. J Clin Microbiol. 2013;51(11):3602–3608. | |
Nocardia species. In: Long SS, Larry Pickering KM, Prober CG, editors. Principles and Practice of Pediatric Infectious Diseases. Philadelphia, PA, USA: Churchill Livingston Elsevier; 2012. | |
Conville PS, Brown-Elliott BA, Wallace RJ, Witebsky FG et al. Multisite reproducibility of the broth microdilution method for susceptibility testing of Nocardia species. J Clin Microbiol. 2012;50(4):1270–1280. | |
Peabody JW, Seabury JH. Actinomycosis and nocardiosis: a review of basic differences in therapy. Am J Med. 1960;28(1):99–115. | |
Smego J, Raymond A, Moeller MB, Gallis HA. Trimethoprim-sulfamethoxazole therapy for Nocardia infections. Arch Intern Med. 1983;143(4):711–718. | |
Cercenado E, Marín M, Sánchez-Martínez M, Cuevas O, Martínez-Alarcón J, Bouza E. In vitro activities of tigecycline and eight other antimicrobials against different Nocardia species identified by molecular methods. Antimicrob Agents Chemother. 2007;51(3):1102–1104. | |
Grayson ML, Crowe SM, McCarthy JS, et al, editors. Kucers’ the use of Antibiotics. 6th ed, London, UK: Hodder Education/ASM Press; 2010. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
