Back to Journals » International Journal of Women's Health » Volume 12
Sociodemographic and Obstetric Determinants of Antenatal Depression in Jimma Medical Center, Southwest Ethiopia: Facility Based Case–Control Study
Authors Alenko A , Dejene S , Girma S
Received 4 March 2020
Accepted for publication 18 June 2020
Published 27 July 2020 Volume 2020:12 Pages 557—565
DOI https://doi.org/10.2147/IJWH.S252385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Everett Magann
Arefayne Alenko,1 Sisay Dejene,2 Shimelis Girma1
1Department of Psychiatry, Faculty of Medical Science, Institute of Health, Jimma University, Jimma, Ethiopia; 2Department of Health Service Management, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Correspondence: Arefayne Alenko
Department of Psychiatry, Faculty of Medical Science, Institute of Health, Jimma University, PO Box 378, Jimma, Ethiopia
Tel +251967670149
Email [email protected]
Background: Worldwide, 10– 20% of women experience depression during pregnancy. In sub-Saharan countries, depression during pregnancy is estimated to be 15– 57%. Even though there is a high burden of depression during pregnancy, little attention has been given to identify sociodemographic and obstetric determinants in diverse populations like Ethiopia.
Objective: To identify sociodemographic and obstetric determinants of antenatal depression among women attending an antenatal clinic at Jimma Medical Center, southwest Ethiopia.
Patients and Methods: A case–control study was conducted among 246 pregnant mothers (82 cases and 164 controls) attending an antenatal clinic in Jimma Medical Center from June 1 to August 30, 2019. Antenatal depression was assessed using the Beck Depression Inventory-II. Epidata 3.1 and SPSS v24 were used for data entry and analysis, respectively. Adjusted odds ratios (AOR) and 95%CIs were estimated using logistic regression models. Statistical significance was set at P< 0.05.
Results: Married mothers were 67% (AOR=0.33, 95%CI: 0.15– 0.75), housewives were 97% (AOR=0.03, 95%CI: 0.01– 0.14), private workers were 87% (AOR=0.13, 95%CI: 0.04– 0.44), and government employees were 84% (AOR=0.16, 95%CI: 0.05– 0.46), less likely to develop antenatal depression. Multigravida were 88% (AOR=0.12, 95%CI: 0.04– 0.37) less likely to develop antenatal depression. Third trimester pregnancy was four times (AOR=4.04, 95%CI: 1.51– 10.81) more likely to have depression. Mothers who having wanted pregnancy were 83% (AOR=0.17, 95%CI: 0.04– 0.81) less likely to develop antenatal depression compared with mothers having unwanted pregnancy.
Conclusion and Recommendation: Being married, multigravida, having wanted pregnancy and occupation status (housewives, private workers and government employees) can protect mothers from developing antenatal depression. Mothers with third trimester pregnancy were four times more likely to have depression. Designing a screening and intervention strategy for antenatal depression must consider the aforementioned protective and risk factors.
Keywords: antenatal depression, determinants, Ethiopia
Introduction
Depression is a leading cause of global disability and the second leading cause of global disease burden among peoples of reproductive age group, especially women of childbearing age during pregnancy.1 Antenatal depression is a form of clinical depression that affects women during pregnancy.2,3 Approximately, 10–20% of women experience depression during pregnancy.4 In low- and middle-income countries (LAMICs), antenatal depression is more prevalent, particularly among poorer women with gender-based risks and prior psychiatric illness.5 Strong evidence from LAMICs using culturally validated measures shows that antenatal depression is estimated to be 15–57%. Health professionals working in antenatal clinics estimated at least one in four pregnant women experience depression.6,7
Depression in pregnancy has negative consequences and adverse health outcomes for mothers and infants. Disability associated with depression in pregnancy is likely to interfere with many essential functions, such as loss of interest in self-care, child care and feeding, and failure to follow antenatal care.8,9 The risk of suicide or self-harm, alcohol use, and poor weight gain during pregnancy is common in women with depression.1,7,10 It can also lead to increased risk for prolonged labor, low birth weight, and long-lasting or even permanent effects on child development and well-being in later life.1,11 In addition, depression during pregnancy results in increased risk for diarrhea and delayed initiation of breastfeeding.6,7,12
There are sociodemographic and obstetric factors that put pregnant mothers at risk for depression in addition to physiological and hormonal changes during pregnancy. Older age, less education, being single, unemployment, and low income are some of the sociodemographic determinants of antenatal depression.13,16 In addition, social factors that can contribute to antenatal depression are marital conflict,17 intimate partner violence,18,19 and low/lack of social support.20,22 Obstetric factors like unplanned pregnancy and previous pregnancy loss can significantly contribute to antenatal depression.16,23 In addition, gravidity, history of abortion, and obstetric complications are proven risk factors for antenatal depression.24 In addition to sociodemographic and obstetric factors, substance abuse is a proven risk factor for antenatal depression.25,26 Moreover, evidence from a study conducted in southern Ethiopia shows there is significant association between intimate partner violence and antenatal depression.27
In Ethiopia, one in three pregnant mothers suffers from depression.12,28,29 This implies large numbers of pregnant mothers suffer from adverse outcomes of depression in Ethiopia. Since Ethiopia is a multiethnic and multicultural country, there is variation in sociodemographic factors. In addition, there is variation in obstetric factors like planning pregnancy and awareness about pregnancy complications. Moreover, the use of local psychoactive stimulants like “khat” and locally made alcohol containing drinks during pregnancy also varies in Ethiopia. Despite this prevailing difference, little attention has been given to identify sociodemographic and obstetric determinants of antenatal depression in southwest Ethiopia. The previous researches conducted in Ethiopia22,27,31 used cross-sectional study design, and did not exclude pregnant mothers with preexisting chronic illnesses like mental illness, hypertension, diabetes mellitus (DM), and HIV/AIDS. These chronic illnesses are risk factors for depression in addition to pregnancy. So, pregnant mothers with the abovementioned chronic illnesses were excluded from this study. Therefore, this case–control study aimed to identify the sociodemographic and obstetric determinants of antenatal depression among mothers attending an antenatal clinic in Jimma Medical Center, southwest Ethiopia.
Patients and Methods
Study Area and Design
A hospital-based unmatched case–control study design was employed. The study was conducted in Jimma Medical Center (JMC), which is located in Jimma town, Southwest Ethiopia. JMC is one of the oldest teaching and service centers in the country, which serves 15 million people in the catchment area. Currently, it provides maternal and child health services for 160,000 outpatients and 45, 000 inpatients with a total of 600 beds per year. The study was conducted from June 1 to August 30, 2019.
Study Population
Cases
- All selected pregnant women attending the antenatal clinic in Jimma Medical Center during the study period found to have depression based on the BDI-II cut-off point 14.32
- Pregnant mothers who do not have a history of mental illness, hypertension, HIV/AIDS, and diabetes mellitus.
Controls
- All pregnant women who were attending the antenatal clinic in Jimma Medical Center during study period who scored BDI-II cutoff point less than 14.32
- Pregnant mothers who do not have history of mental illness, hypertension, HIV/AIDS, and diabetes mellitus.
A ratio of 1:2 was used to select cases vs controls. Pregnant mothers having a history of mental illness and currently on psychiatric follow-up were excluded from both groups.
Variables of the Study
Outcome Variable
Antenatal depression.
Independent Variables
Age, residence, educational status, marital status, occupational status and household/family size, gravidity, parity, current pregnancy complication, stages of pregnancy, ANC visit category, wanted pregnancy and history of abortion, family history of mental illness, intimate partner violence (IPV), level of social support, khat use risk, and alcohol use risk.
Sample Size Determination and Sampling Techniques
The sample size was computed using the STATCALC application of Epi Info 7. Statistical software with the assumptions of the following parameters:
- Proportion of nondepressed pregnant mothers (controls) with a previous history of abortion were 16%.31
- Proportion of depressed pregnant mothers (cases) with a previous history of abortion was 45.5%.31
- 95% confidence interval and 5% margin of error
- 80% power of the study and case to control ratio of 1:233 to detect an odds ratio of 2.57.31
Thus, the sample size required for the study was 246 (82 cases and 164 controls). To select study participants, all mothers visiting the medical center for antenatal care (ANC) during the data collection period were screened by BDI-II. Following the identification of a case, two respective controls were randomly identified from the same setup. The cases and controls were identified by initial screening of pregnant mothers for depression using the BDI tool. Once cases and controls were identified, further assessment was done to identify determinants using standard questionnaire and record review.
Method of Data Collection and Measurement
Data was collected from all selected pregnant women using semi-structured and pretested interviewer administered questionnaires. Participants’ sociodemographic and obstetric factors were assessed using a semi-structured questionnaire through face-to-face interviews and client document review (ANC registration book and medical record).
Depression Screening
The presence and absence of maternal depression was assessed using the Beck Depression Inventory (BDI-II). The tool was developed in 1996 and has a 21-item questionnaire that measures cognitive, affective, somatic, and vegetative symptoms of depression, with each item on the scale ranging from 0 (not at all) to 4 (very extreme symptoms), for two-week period.32 The tool was translated into 17 different languages and is used worldwide. It was validated in certain population groups with different languages. Furthermore, the tool allows quick assessment of depression and is inexpensive both in clinical and nonclinical setups.34 The BDI-II tool has excellent internal consistency and reliability,35 and in the current study the internal consistency was found to be 0.82. The English version of BDI-II is translated to local languages (Afaan Oromo, and Amharic), and finally back translated to English language by another person who has a good command of English, Afaan Oromo and Amharic to check its consistency. The data was collected by the Afaan Oromo and Amharic version questionnaire.
Substance Use Risk Level
Participant’s substance use risk was assessed using the Alcohol, Smoking and Substance Involvement Screening Test (WHO ASSIST) version 3.1. The test’s total score ranges from 0–31 for tobacco and 0–39 for alcohol and khat (amphetamine-like substance). The total score was categorized as: low-risk score of 0–10, moderate (score 11–26), and high-risk (score ≥27) for alcohol. For tobacco and khat the score is similar and categorized as: low (score 0–3), moderate (score 4–26), and high (score ≥27).36 In the current study, the internal consistency of ASSIST was found to be 0.71.
Level of Social Support
Participants’ level of social support was assessed by the Oslo Social Support Scale (OSS-3). The scale has a three-item questionnaire that is used to assess the level of social support. It has a sum score scale ranging from 3–14 with three broad categories: “poor support” 3–8, “moderate support” 9–11 and “strong support” 12–14.37 In this study, the internal consistency of OSS-3 was found to be 0.83.
Maternal Intimate Partner Violence
Maternal IPV was assessed using the hurt/insult/threaten/scream (HITS)38 scale. HITS is validated and the most commonly used tool to screen IPV among women with a cutoff score of ≥10.38,40 In this study, the internal consistency of OSS-3 was found to be 0.78.
The English version questionnaire was translated to Afaan Oromo and Amharic, and finally translated back to English language by another person who has a good command of English, Afaan Oromo and Amharic to check its consistency. The data was collected by the Afaan Oromo and Amharic version questionnaire. Six trained female psychiatry nurses collected the data. The data collection was supervised by three clinical nurses.
Statistical Analysis
Data was checked for completeness and entered into EpiData version 3.1, and then exported to SPSS version 24.0 (IBM Corporation, Armonk, NY, USA) statistical software for analysis. Model fitness was checked using the Hosmer–Lemeshow goodness of fit statistic (P=0.75), and log likelihood statistics. Multicollinearity was checked by the variance inflation factor (VIF <10) and tolerance. Variables were candidates for multiple logistic regression after checking assumptions like meaningful coding, outliers and large/adequate sample. Descriptive statistics such as mean, frequency and percentage were calculated, and presented using tables. Bivariate logistic regression analysis was done and all independent variables that were associated with a dependent variable with a P-value of less than 0.25 were included in the multivariable logistic regression analysis. A multivariable logistic regression analysis was employed to determine independent determinants. Adjusted odds ratio (AOR) with 95%CI and a P-value of less than 0.05 was used to decide statistically significant associations.
Ethics Approval and Consent to Participate
Ethical approval was obtained from the Institutional Review Board of Jimma University Institute of Health. Participants were fully informed about the purpose and objective of the study. Mothers who agreed to participate gave written consent. Confidentiality was maintained by omitting identifiers from the study tool and privacy was ensured during the interview. The study was conducted in accordance with the Declaration of Helsinki.
Results
Sociodemographic Characteristics of the Study Participants
The response rate of the study was 246 (100%). Eighty-two cases and 164 controls participated in the study. The mean age and standard deviation of the study participants were 25.0 ± 4.63 years for cases and 26.09 ± 5.39 years for controls. The majority of cases (61.0%) and more than half the controls (52.4%) were in the age category of 18–25 years. The majority of cases (84.1%) and controls (88.4%), were living in urban areas. Regarding educational status, more than one-third both of the cases (40.2%) and of the controls (37.8%) attained higher education. More than one-third (42.7%) of the cases and more than half (54.9%) of the controls were married. More than one third of cases (37.9%) and controls (37.8%) were housewives. Regarding family size, 47 (57.3%) of cases were 2 and 75 (45.7%) of controls were ≥4 (Table 1).
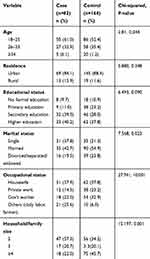 |
Table 1 Sociodemographic Characteristics of the Study Participants Attending Antenatal Clinic in Jimma Medical Center, Southwest Ethiopia (n=246) |
Obstetric Factors
Most cases, 60 (73.2%) were primigravida and most controls, 99 (60.4%) were multigravida. More than one-third both of cases (40.2%) and of controls (44.5%) were primiparous. Regarding current pregnancy complication, the majority both of cases (59.7%) and of controls (62.2%) had no complication. The majority of cases (62.2%) and of controls (44.5%) were third trimester pregnancies. The majority of cases (59.8%) and more than half of the controls (51.8%) were fourth ANC visit category. The majority both of cases (85.4%) and of controls (73.8%) wanted pregnancy (Table 2).
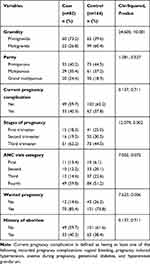 |
Table 2 Obstetric Factors Among Study Participants Attending the Antenatal Clinic in Jimma Medical Center, Southwest Ethiopia (n=246) |
Psychosocial and Substance Use Risk Related Factors
The majority of cases (70.7%) and controls (79.9%) do not have a family history of mental illness. Regarding IPV, 15.9% of cases and 21.1% of controls had experienced IPV. The majority, both of cases (56.1%) and of controls (53%), had strong social support. One-fourth (25.6) of both groups had moderate risk khat use. In addition, 17.1% of the cases and 28.7% of the controls had moderate risk alcohol use (Table 3).
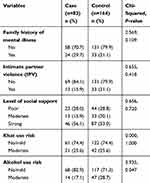 |
Table 3 Psychosocial and Substance Use Related Factors Among Study Participants Attending the Antenatal Clinic in Jimma Medical Center, Southwest Ethiopia (n=246) |
Determinants of Depression Among Mothers Attending Antenatal Clinic
All seven variables with a P-value less than 0.25 in the bivariate logistic regression analysis (marital status, occupational status, household size, gravidity, stages of pregnancy, wanted pregnancy, alcohol use risk level) were included in the final multivariable logistic regression model for analysis. Five variables were found to be independent predictors of depression. These variables are marital status, occupational status, gravidity, stage of pregnancy (gestational age), and wanted pregnancy. Married mothers were 67% less likely hood (AOR=0.33, 95%CI: 0.15–0.75) to develop antenatal depression compared with single and divorced/separated/widowed mothers. In the occupational status category, housewives were 97% (AOR=0.03, 95%CI: 0.01–0.14), private workers were 87% (AOR=0.13, 95%CI: 0.04–0.44) and government employees were 84% (AOR=0.16, 95%CI: 0.05–0.46), less likely to develop antenatal depression compared to their comparators.
Pregnancy-related factors like gravidity, gestational age, and wanted pregnancy were independent determinants of depression among pregnant mothers attending antenatal clinic. Multigravida were 88% (AOR=0.12, 95%CI: 0.04–0.37) less likely to develop antenatal depression compared primigravida. Regarding gestational age, women in their third trimester of pregnancy are four times (AOR=4.04, 95%CI: 1.51–10.81) more likely to develop depression compared to their first and second trimester pregnancy.
Mothers who wanted pregnancy were 83% (AOR=0.17, 95%CI: 0.04–0.81) less likely to develop antenatal depression compared with mothers having unwanted pregnancies. All psychosocial and substance use related factors/variables were not independently associated with antenatal depression in the final logistic regression model (Table 4).
 |
Table 4 Bivariate and Multivariate Logistic Regression Analysis of Factors Associated with Depression Among Pregnant Mothers Attending the Antenatal Clinic in Jimma Medical Center, 2019 (n=246) |
Discussion
Previous studies conducted regarding antenatal depression have focused on the prevalence and associated factors of antenatal depression.12,16,22,23,29 However, this study was conducted using a case–control study design to identify determinants of antenatal depression by comparing pregnant mothers with and without depression. We used the standard Beck Depression Inventory (BDI-II) tool to detect the presence of depression among pregnant women visiting the antenatal clinic. Thus, cases and controls were classified, and they were interviewed by considering a ratio of one to two of cases to controls. In our study, marital status, occupational status, gravidity, gestational age and wanted pregnancy were found to be determinants of depression among pregnant women.
In this study, it was revealed that married pregnant women were 67% less likely to develop antenatal depression compared to pregnant women in other categories (single and divorced/separated/widowed). This finding is in agreement with other studies that found being married was protective from antenatal depression.13,16 This may imply that married women gain support from their husband and share information regarding life stressors related to their pregnancy; thus, decreasing the chance of acquiring depression during pregnancy. This study also found that housewives, private workers, and government employees were 97%, 87%, and 84% less likely to develop antenatal depression than others (farmers, daily laborers, and cleaners), respectively. This findings agrees with the finding of a study conducted in Gondar, Ethiopia.28 This might be due to the fact that stress is associated with occupation or work burden and lack of enough rest. The other possible reason is that in the Ethiopian context, mothers who are involved in farming, daily labor, and cleaning are mostly from low socioeconomic class. Therefore, low socioeconomic status may mediate the observed association.
In addition, ANC clients who had wanted/unplanned pregnancy were 83% less likely to develop antenatal depression compared to clients who had unwanted pregnancies. This is consistent with a study conducted in South Africa,23 rural Ghana,16 and Ethiopia.30,41
This might be an indication of the importance of decision-making before getting pregnant by women, which possibly mitigates predisposition to antenatal depression. Furthermore, mother’s experienced unplanned pregnancies might experience poor relationships with partners, which further hinders social support from relatives and increases the level of marital conflict. Stress related to unplanned role transition, fear of increased financial pressure of a new child, and psychological readiness for parenthood could be factors.42,43
In our study, multigravida mothers were 88% less likely to develop antenatal depression compared to primigravida. This finding showed that women facing first pregnancy are at higher risk of developing depression than women who have more than one pregnancy. This could be related to the lack of experience in parenthood and increased fear related to pregnancy and its outcome.
Finally, we found that gestational age (stage of pregnancy) was another determinant of antenatal depression; ie pregnant women in the third trimester pregnancy were four times more likely to develop antenatal depression compared to women in first and second trimester pregnancies. This may be due to increased body weight and stressful feelings as labor approaches when pregnancy progresses. Third trimester pregnancy is associated with trouble in sleeping, shortness of breath, and false labor contractions.44 These pregnancy-related changes can precipitate depression and resemble vegetative symptoms of depression.
Unlike other studies, the current study did not find any association between history of abortion and current pregnancy complication with antenatal depression. Rather, unwanted pregnancy is significantly associated with antenatal depression. This might be due to the fact that the psychological impact of unwanted pregnancy in the case of single, divorced, separated or widowed mothers/women in the Ethiopian cultural context is more severe than abortion because unwanted pregnancy is considered as a result of sex outside marriage bed. Therefore, mothers having unwanted pregnancies were less likely get support from their relatives and community and in addition, were less likely prepared for a pregnancy-related need and complications. In this study, IPV levels were high in both depressed and nondepressed women. This might be due to the fact that early marriage and being pregnant are risks for IPV,45,46 according to studies conducted in Ethiopia. Therefore, both depressed and nondepressed pregnant mothers are at risk for IPV.
Strength and Limitations of the Study
The major strength of this study is the use of culturally validated tools and relatively strong study design compared to previous studies. However, this study is not without limitations. Since the study was institution-based, its generalizability is limited. The other limitations of this study are inclusion of women with obstetric complication and the BDI tool that could overestimate symptoms of depression through the physical symptoms that normally exist during pregnancy.
Conclusion and Recommendation
Pregnant mothers who were married, multigravida, and having wanted pregnancy were less likely to develop antenatal depression compared to their counterparts. In addition, mothers who were housewives, private workers, and government employees were less likely to have depression compared to daily laborers, farmers, and cleaners. Mothers with third trimester pregnancy were more likely to have depression. Designing a screening and intervention strategy for antenatal depression at the antenatal clinic must consider the aforementioned protective and risk factors. In addition, we recommend further longitudinal studies using standard diagnostic tools.
Data Sharing Statement
The datasets used and analyzed during the current study are included in the manuscript.
Acknowledgments
The authors acknowledge Jimma University medical center for facilitating the study. Study participants and data collectors are also highly acknowledged.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests to disclose.
References
1. WHO. Maternal Mental Health and Child Health and Development in Low and Middle Income Countries. 2008:1–34
2. Michael B, First MD, Maria N, Ward M, Eds. RHIT C-P. DSM-5.
3. Lund C. Maternal Depression. Health And Education Advice & Resource Team; 2016:1–7 p.
4. Shidhaye PR, Giri PA. Maternal depression: a hidden burden in developing countries. Ann Med Heal Sci Res. 2014;4(4):463–465. doi:10.4103/2141-9248.139268
5. Fisher J, Cabral de Mello M, Patel V, Atif Rahman Thach Tran SH a & WH. Prevalence and determinants of common perinatal mental disorders in women in low- and lower- middle-income countries: a systematic review Jane. Bull World Heal Organ Menu. 2012;2011(90):139–149. doi:10.2471/BLT.11.091850
6. Gelaye B, Rondon M, Araya PR, PM A. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. 2016;3(10):973–982. doi:10.1016/S2215-0366(16)30284-X
7. WHO. Global burden of mental disorders. J WHO. 2011;139:1–6.
8. Leung BM, Kaplan BJ. Perinatal depression: prevalence, risks, and the nutrition link—a review of the literature. J Am Diet Assoc. 2009;109(9):1566–1575. doi:10.1016/j.jada.2009.06.368
9. Rahman A, Bunn J, Lovel H, et al. Association between antenatal depression and low birthweight in a developing country. Acta Psychiatr Scand. 2007;115(6):481–486. doi:10.1111/j.1600-0447.2006.00950.x
10. Pajulo M, Savonlahti E, Sourander A, et al. Antenatal depression, substance dependency and social support. J Affect Disord. 2001;65(1):9–17. doi:10.1016/S0165-0327(00)00265-2
11. Pawlby S, Hay DF, Sharp D, et al. Antenatal depression predicts depression in adolescent offspring: prospective longitudinal community-based study. J Affect Disord. 2009;113(3):236–243. doi:10.1016/j.jad.2008.05.018
12. Sahile MA, Mesfin Tafa Segni TA, D B. Prevalence and predictors of antenatal depressive symptoms among women attending Adama Hospital. Int J Nurs Midwifery. 2017;9(5):58–64. doi:10.5897/IJNM2016.0239
13. Farr SL, Bitsko RH, Hayes DK, Dietz PM. Mental health and access to services among US women of reproductive age. YMOB. 2010;203(6):542.
14. Daud S, Anjum A. Prevalence, predictors and determinants of depression in women of the reproductive age group. BMC. 2014;24:1–6.
15. Tabassum FDMSL. Social determinants of depression among reproductive age women residing. J Educ Humanit. 2018;3(1):1–22.
16. Weobong B, Soremekun S, Ten Asbroek AH, et al. Prevalence and determinants of antenatal depression among pregnant women in a predominantly rural population in Ghana: the DON population-based study. J Affect Disord. 2014;165:1–7. doi:10.1016/j.jad.2014.04.009
17. Marks NF. Marital Conflict, Depressive Symptoms, and Functional Impairment. NIH Public Access. 2008;70(2):377–390.
18. Lövestad S, Löve J, Vaez M, Krantz G. Prevalence of intimate partner violence and its association with symptoms of depression; a cross-sectional study based on a female population sample in Sweden. BMC Public Health. 2017;17(335):1–11. doi:10.1186/s12889-017-4222-y
19. Islam J, Broidy L, Baird K, Mazerolle P. Intimate partner violence around the time of pregnancy and postpartum depression: the experience of women of Bangladesh. PLoS One. 2017;12(5):1–24. doi:10.1371/journal.pone.0176211
20. Eastwood J, Ogbo FA, Hendry A, Noble J, Page A, Years E. Impact of antenatal depression on perinatal outcomes in australian women. Early Years Res Group (EYRG). 2017;1–16.
21. Aktas S, Calik KY. Factors affecting depression during pregnancy and the correlation between social support and pregnancy depression. Iran Red Crescent Med J. 2015;17(9):1–9. doi:10.5812/ircmj.16640
22. Gemta WA. Prevalence and factors associated with antenatal depression among women following antenatal care at Shashemane health facilities, South Ethiopia. Ann Glob Heal. 2011;81(1):90. doi:10.1016/j.aogh.2015.02.709
23. Manikkam L, Burns JK, Manikkam L, Burns JK. Antenatal depression and its risk factors: an urban prevalence study in KwaZulu-Natal. South Africa Med. 2012;102(12):940–944. doi:10.7196/SAMJ.6009
24. Ajinkya S, Jadhav PR, Srivastava NN. Depression during pregnancy: prevalence and obstetric risk factors among pregnant women attending a tertiary care hospital in Navi Mumbai. Ind Psychiatry J. 2013;22(1):37. doi:10.4103/0972-6748.123615
25. Vythilingum B, Roos A, Faure SC, Geerts L, Stein DJ. Risk factors for substance use in pregnant women in South Africa. 2012;102(11):851–854.
26. Bowen A, Muhajarine N. Prevalence of antenatal depression in women enrolled in an outreach program in Canada. J Obstetric Gynecol Neonatal Nurs. 2006;35(4):491–498. doi:10.1111/j.1552-6909.2006.00064.x
27. Belay S, Astatkie A, Emmelin M, et al. Intimate partner violence and maternal depression during pregnancy: a community-based cross-sectional study in Ethiopia. PLoS One. 2019;14(7).
28. Ayele TA, Azale T, Alemu K, Abdissa Z. Prevalence and associated factors of antenatal depression among women attending antenatal care service at Gondar University Hospital, Northwest Ethiopia. PLoS One. 2016;11(5):1–12. doi:10.1371/journal.pone.0155125
29. Tilahun B, Mossie TB, Sibhatu AK, Dargie A, Ayele AD. Prevalence of antenatal depressive symptoms and associated factors among pregnant women in Maichew, North Ethiopia: an institution based study. Ethiop J Heal Sci. 2017;27(1):59–66. doi:10.4314/ejhs.v27i1.8
30. Biratu A, Haile D. Prevalence of antenatal depression and associated factors among pregnant women in Addis Ababa, Ethiopia: across-sectional study. Reprod Health. 2015;12:99. doi:10.1186/s12978-015-0092-x
31. Bisetegn TAMG, Muche T. Prevalence and predictors of depression among pregnant women in debretabor Town, Northwest Ethiopia. PLoS One. 2016;11(9):e0161108. doi:10.1371/journal.pone.0161108
32. Beck AT, Steer RA, Brown GK. Beck depression inventory -II manual. Purp. 1996;13(10):38.
33. Lewallen S, Courtright P. Epidemiology in practice: case-control studies. Community Eye Health. 1998;11(28):57.
34. Beck AT, Steer RA, Carbin MG. Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. doi:10.1016/0272-7358(88)90050-5
35. Mignote HG. An analysis of Beck Depression Inventory 2nd Edition (BDI-II). Glob J Endocrinol Metab. 2018;2:3.
36. Humeniuk R, Henry-Edwards S, Ali R, Poznyak V, Monteiro MGV. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) Manual for Use in Primary Care. World Health Organization; 2010.
37. Dalgard S. The Oslo 3-Items Social Support Scale. 2003:3–5.
38. Sherin KM, Sinacore JM, Li XQ, Zitter RE, Shakil A. HITS: a short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30(7):508–512.
39. Katherine M, et al. Clinical utility of an intimate partner violence screening tool for female VHA patients. J Gen Intern Med. 2013;28(10):1288–1293. doi:10.1007/s11606-013-2534-x.
40. Rabin, Rabin RF, Jennings JM, et al. Intimate partner violence screening tools. Am J Prev Med. 2009;36(5):439–445.e4. doi:10.1016/j.amepre.2009.01.024
41. Dibaba Y, Fantahun M, Hindin MJ. The association of unwanted pregnancy and social support with depressive symptoms in pregnancy: evidence from rural Southwestern Ethiopia. BMC Pregnancy Childbirth. 2013;13(1):135. doi:10.1186/1471-2393-13-135
42. Bahk J, Yun S-C, Kim Y-M, Khang Y-H. Impact of unintended pregnancy on maternal mental health: a causal analysis using follow up data of the panel study on Korean children (PSKC). BMC Pregnancy Childbirth. 2015;15(1):85. doi:10.1186/s12884-015-0505-4
43. Elsenbruch S, Benson S, Rücke M, et al. Social support during pregnancy: effects on maternal depressive symptoms, smoking and pregnancy outcome. Human Reprod. 2006;22(3):869–877. doi:10.1093/humrep/del432
44. U.S. Department of Health and Human Services, Office on Women’s Health. Page last updated. April 18, 2019. Available from: https://www.womenshealth.gov/pregnancy/youre-pregnant-now-what/stages-pregnancy.
45. Erulkar A. Early marriage, marital relations and intimate partner violence in Ethiopia. Int Perspect Sex Reprod Health. 2013;39(01):6–13. doi:10.1363/3900613
46. Gebrezgi BH, Badi MB, Cherkose EA, et al. Factors associated with intimate partner physical violence among women attending antenatal care in Shire Endaselassie town, Tigray, northern Ethiopia: a cross-sectional study, July 2015. Reprod Health. 2017;14(1):76. doi:10.1186/s12978-017-0337-y
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
