Back to Journals » Journal of Pain Research » Volume 14
Socio-Demographics, Pain Characteristics, Quality of Life and Treatment Values Before and After Specialized Interdisciplinary Pain Treatment: Results from the Danish Clinical Pain Registry (PainData)
Authors Vaegter HB, Christoffersen LO, Enggaard TP, Holdggard Snr DEM, Lefevre TN, Eltved R, Reisenhus CH, Licht TW, Laustsen MM, Hansson SH, Jensen PF, Larsen TRF, Alpiger S, Mogensen BG, Høybye MT
Received 16 February 2021
Accepted for publication 24 March 2021
Published 4 May 2021 Volume 2021:14 Pages 1215—1230
DOI https://doi.org/10.2147/JPR.S306504
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jonathan Greenberg
Henrik Bjarke Vaegter,1,2 Lars Oxlund Christoffersen,3 Thomas Peter Enggaard,4 Dorte Elise Møller Holdggard Snr,5 Tram Nguyen Lefevre,6 Randi Eltved,7 Christina Høegh Reisenhus,8 Torsten Wentzer Licht,9 Mette Mebus Laustsen,10 Susanne Haase Hansson,11 Per Føge Jensen,12 Thomas Rene Friis Larsen,13 Stephan Alpiger,14 Bibsen Guldhammer Mogensen,15 Mette Terp Høybye16,17
1Pain Research Group, Pain Center, Odense University Hospital, Odense, Denmark; 2Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark; 3Danish Regions, Copenhagen, Denmark; 4Interdisciplinary Pain Center, Sjællands University Hospital Køge, Køge, Denmark; 5Interdisciplinary Pain Center, University Hospital Aalborg, Aalborg, Denmark; 6Interdisciplinary Pain Center Allévia, Aarhus, Denmark; 7Pain Clinic, Regional Hospital Silkeborg, Silkeborg, Denmark; 8Interdisciplinary Pain Center, Lillebaelt Hospital, University Hospital of Southern Denmark, Odense, Denmark; 9Pain Clinic, Friklinikken, Grindsted, Denmark; 10Interdisciplinary Pain Center, Holbæk Hospital, Holbæk, Denmark; 11Interdisciplinary Pain Center, Næstved Hospital, Næstved, Denmark; 12Interdisciplinary Pain Center, Gentofte Hospital, Gentofte, Denmark; 13Interdisciplinary Pain Center, Rigshospitalet, Denmark; 14The Private Pain Clinic, Herlev, Denmark; 15CFR Capio, Skørping, Denmark; 16Interdisciplinary Research Unit, Elective Surgery Center, Silkeborg Regional Hospital, Silkeborg, Denmark; 17Interacting Minds Centre, Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Correspondence: Henrik Bjarke Vaegter
Pain Research Group, Pain Center, Odense University Hospital, Heden 7-9, Indgang 200, Odense, DK-5000, Denmark
Tel +45 65413869
Fax +45 65415064
Email [email protected]
Background and Aims: PainData is an electronic internet-based clinical pain registry established to improve the understanding and treatment of high-impact chronic pain. The primary aim of this paper is to describe socio-demographics, pain characteristics, quality of life, and treatment values at baseline and follow-up in individuals referred to public and private interdisciplinary pain centers in Denmark between 2018 and 2020.
Methods: Self-reported patient-reported outcomes collected through PainData before (n=12,257) and after (n=4,111) treatment across 13 public and private interdisciplinary specialized pain centers in Denmark (87% of all pain centers in Denmark) are described.
Results: Mean duration of pain was 10 years, and one in three patients reported chronic widespread pain. More than 40% reported opioid use, and 50% had tried four or more different treatment modalities prior to referral. More than 60% reported poor sleep, severe fatigue, and memory and/or concentration deficits. Mean scores on pain catastrophizing, fear of movement, and pain-related disability were high, whereas scores on pain acceptance and self-efficacy were low. Physical and mental health were rated as poor and fair, respectively. One in four patients reported being very much improved or much improved after treatment. Items commonly reported after treatment were increased knowledge about pain, emotions and mood (66.5%), being better at accepting life with chronic pain (63.1%), and improved activity pacing (60.6%).
Conclusion: The PainData registry, containing data from a large cohort of individuals, can help to improve the understanding and treatment of high-impact chronic pain, and collaborations with other researchers are welcome.
Keywords: chronic pain, registry, PainData, questionnaires
Introduction
Chronic pain, as recognized by the International Association for the Study of Pain (IASP) as pain that persists past normal healing time,1 is one of the leading causes of disability in the western world,2 and it is associated with high rates of opioid consumption,3 healthcare utilization,4 depression and anxiety,5 as well as exclusion from the workforce.6 Similarly to many other countries, chronic pain in Denmark is a costly health problem, with an estimated annual expenditure for low back pain alone of over $1 billion dollars in 2015.7
The majority of individuals with chronic pain are treated in the primary care sector, for example by their general practitioner, a physiotherapist, or a chiropractor, with a smaller part attending hospital departments (eg, rheumatology or neurology) in secondary care. For individuals who do not benefit from primary or secondary care treatments, clinical guidelines recommend referral to interdisciplinary pain management programs in tertiary care settings.8,9 Based on recent data from the USA, approximately 8% of the population report high-impact chronic pain, defined as persistent pain with substantial restriction of life activities lasting 6 months or more,10 indicating that more than 350,000 Danes above 18 years of age may need treatment in a specialist interdisciplinary pain center.11
Across a range of health conditions, including chronic pain,12,13 large-scale clinical registries are established to provide a data resource for quality assurance and research to inform clinical decision making and health policy.14,15 PainData is a clinical pain registry that aims to improve the understanding and treatment of individuals experiencing high-impact chronic pain. This involves the collection of patient-reported outcome (PRO) data from individuals referred to one of the public or private pain centers that offer specialized interdisciplinary pain treatment in Denmark. The collection of PRO data in the registry has three main aims: 1) to serve as a clinical tool to facilitate the dialogue between the individual and the interdisciplinary team during consultations, 2) to serve as a tool for national benchmarking and quality assurance, and 3) to facilitate national and international research in the field of high-impact chronic pain. PainData was developed and first implemented at the Pain Center at Odense University Hospital (Smertecenter Syd) in 2015. At that time, there was no equivalent national pain registry, and that is still the case. In 2018, the Danish Regions, which is the association and interest organization for the five regions in Denmark, launched a series of projects on value-based healthcare (Værdibaseret Sundhed [VBS]),16 including a project focusing on VBS within interdisciplinary pain treatment. A series of workshops were hosted to progress the outcome evaluation and benchmarking agenda according to what gives the greatest value and effect for the individual patient. In each workshop, there was representation from many different stakeholders, including the Danish Pain Society, the Danish Rheumatology Association, the Association of Chronic Pain Patients, the Fibromyalgia Association, the Association of General Practitioners, the Danish Psychology Association, leaders from public and private pain centers, as well as representatives from different interdisciplinary professions (social workers, physiotherapists, pain physicians, nurses, and psychologists). The major outcomes of the workshops were 1) to explore patient values and goals in relation to interdisciplinary pain treatment, 2) to develop and test a battery of questions to assess the patient’s values and goals, and 3) to agree on a minimum core data set, which would give insights into the patient’s situation while reducing the burden of the patient, and allow for meaningful comparisons between centers. By June 1st 2019, questions related to patients’ values and goals were developed and subsequently implemented in PainData in 10 out of 11 public interdisciplinary pain centers in Denmark, as well as in four out of five private interdisciplinary pain centers in Denmark, along with the Patient-Reported Outcomes Measurement Information System (PROMIS) Scale v1.2 – Global Health. The aims of this paper are to introduce the PainData registry and to summarize the self-reported characteristics of individuals with high-impact chronic pain who had an initial consultation, as well as the characteristics of individuals who completed treatment in a public or private interdisciplinary pain center in Denmark from 2018 to 2020.
Methods
Design
The included data are taken from an observational study of individuals who had their initial consultation or individuals who completed treatment in a public or private interdisciplinary pain center in Denmark between January 1st 2018 and December 31st 2020. Reporting of the study follows the STROBE guidelines (STrengthening the Reporting of OBservational studies in Epidemiology).17
Study Population
Participants are Danish individuals with chronic pain registered in the PainData registry at one of the following interdisciplinary pain centers: 1) Pain Center, University Hospital Odense; 2) Interdisciplinary Pain Center University Hospital Aalborg; 3) Pain Clinic, Regional Hospital Silkeborg; 4) Interdisciplinary Pain Center University Hospital Køge; 5) Interdisciplinary Pain Center Næstved Hospital; 6) Interdisciplinary Pain Center Holbæk Hospital; 7) Interdisciplinary Pain Center Gentofte Hospital; 8) Interdisciplinary Pain Center Rigshospitalet; 9) Pain Clinic, Friklinikken, Grindsted; 10) Interdisciplinary Pain Center, Lillebaelt Hospital; 11) Interdisciplinary Pain Center Allévia; 12) CFR Capio, Skørping; or 13) The Private Pain Clinic, Herlev. Pain centers/clinics 1–10 are public hospitals, and clinics 11–13 are private pain clinics with public funding. All interdisciplinary pain centers using PainData are outpatient hospital settings treating patients with chronic (>6 months) non-cancer pain conditions. Patients referred to the pain centers have previously tried a number of treatments in primary and secondary care settings with an unsatisfactory clinical response. Treatments across the different pain centers can be diverse, but commonly consist of one or more of the following elements: medical treatment with a specialist pain consultant (ie, individual adjustment of analgesics to improve effects and reduce side effects), individual consultations with a pain psychologist, physiotherapist, or social worker with cognitive–behavioral therapy training, participation in group sessions with relaxation therapy, acceptance and commitment therapy, or mindfulness-based stress reduction programs, which have shown moderate effects in this population.
Inclusion and Exclusion Criteria
Individuals with chronic pain,1 who were scheduled for an initial consultation, completed the baseline questionnaire and gave data consent, or individuals who had completed treatment and completed the follow-up questionnaire and gave data consent in one of the involved public or private interdisciplinary pain centers in Denmark between January 1st 2018 and December 31st 2020, were included in the study.
The PainData Registry
The patient part of the PainData registry (see https://paindata-test.rsyd.dk) is designed to capture PRO data at different time points related to the clinical contact in one of the interdisciplinary pain centers. Patients can access the registry 24 hours per day, 7 days per week, via different web browsers. After referral to a pain center but before the initial consultation, all referred patients are invited to answer questions about their clinical pain characteristics and adaptations to pain through a web-based questionnaire system sent via a personal link to the patients’ official inbox, e-Boks (the channel that the Danish State and municipalities use to send official documents to citizens). Questionnaires are completed at home before the first consultation. None of the questions are mandatory. In addition to basic demographics, data include questionnaires for measures of pain intensity, disability, physical and mental health, quality of life, several psychological constructs, and patient values in relation to treatment. During completion of the questionnaires, patients are invited to give consent so that the questionnaire data can be stored in the PainData research database and used for later research. The Danish Data Protection Agency approved the data collection (18/35221). The collected data are protected in accordance with the “Act on the Processing of Personal Data” (Act No. 429 of 31/05/2000) and the “Law on the Status of Patients’ (Act No. 482 of 01/07/1998). Participants’ personal data are protected in accordance with the Personal Data Processing Act and the Health Act. The duration of data retention is in accordance with notification to the Danish Data Inspectorate. The PainData registry runs on servers managed by the governmental administration of the Region of Southern Denmark. In addition to pain-related questionnaires at baseline and after treatment, PRO data are collected 12, 24, 36, 48, and 60 months after completed treatment, if patients consent to being contacted, to establish a unique prospective cohort over time. As several of the centers/clinics that use PainData have not been collecting data for more than 1–2 years, we only report data from baseline and immediately after treatment in this paper.
In the clinician part of the PainData registry, a summary report from each individual patient’s self-reported questionnaire is generated, so that the interdisciplinary team can see the report before seeing the patient during the consultations. All information processing is in real time so that summary data are instantly available to the interdisciplinary team. The registry also has the possibility for clinicians to enter information (eg, pain diagnosis, comorbidities, or planned treatment); however, a consensus between the involved clinics on collection of clinician data has not yet been reached.
The PainData registry was developed by the Pain Center, University Hospital Odense, with the support of the Department of Anaesthesiology and Intensive Care Medicine, University Hospital Odense. The ongoing maintenance costs are divided evenly between the involved pain centers/clinics based on a cooperation agreement. The registry has been built to meet all current Danish health data security standards for data access, storage, back-up, and the tracking of who accesses, inputs, or modifies data. This is achieved via login requirements, passwords, two-factor authentication, firewalls, and logging of access.
Baseline and Follow-Up Questionnaires
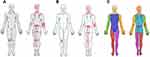
Based on the biopsychosocial model of pain,18 PRO data are collected across several biopsychosocial domains, including pain, disability, work participation, psychological factors, quality of life, contextual factors, and patient values. Wherever possible, the choice of questions and questionnaires was based on recommendations and evidence of their role in chronic pain.19–21 The 35 questions in the minimum core data set include sex, age, height, weight, education, and work situation, as well as items related to manifestations of pain: pain chart (any locations of pain during the previous week [Figure 1A], which are used in other clinical registries in Denmark22), onset date of pain, pain intensity (worst, average, and least in the last 24 hours, using numeric rating scales [NRSs] ranging from 0 [no pain] to 10 [worst imaginable pain],23 and the average during the last 7 days item from the PROMIS-10 Global Health24), use of analgesics (type, brand, dose, frequency), analgesic effect in the last 24 hours, screening for 1) sleep disturbance using the four items adapted from the Karolinska Sleep Questionnaire (KSQ) sleep quality subscale, assessing frequency of sleep disturbance, frequency of early awakenings, frequency of difficulties falling asleep and frequency of night-time awakenings with difficulty returning to sleep,25 2) stress, pain catastrophizing, and fear of movement using three-item screening questions,26 and 3) general health, physical and mental health, social activities, and fatigue using the PROMIS-10 Global Health.24 Items related to patient values in relation to treatment were based on workshops in the value-based healthcare project (see Table 3).
When the treatment course has been completed, patients are invited to complete a follow-up questionnaire, which contain approximately two-thirds of the questions in the baseline questionnaire. Further details about the questionnaires in the additional larger data set used by the 10 public pain centers/clinics are provided in Supplementary Table S1.
Statistical Analysis
Descriptive statistics (mean and standard deviation [SD], median and percentiles) were used to describe socio-demographic status, pain characteristics, psychological distress, quality of life, and responses to value-based healthcare items at baseline and follow-up for women and for men. As the aim of this paper was not to compare PRO measures between men and women but to describe the population in the registry, no sex-related hypotheses were established a priori, and thus no formal statistical tests were performed. For the purpose of the description, the duration of pain was also grouped into less than 1 year, 1–3 years, 3–5 years, 5–10 years, and more than 10 years. The variable on education was grouped into the following categories: “Primary school education”, “Upper secondary education”, “Vocational education and training”, “Short cycle higher education”, “Vocational bachelor education”, and “Master’s program”. Baseline differences between responders and non-responders to the follow-up questionnaire were analyzed with independent t-tests or χ2 tests, where appropriate. Differences in PRO measures at baseline and follow-up for patients who started and completed treatment within the period 2018–2020, who completed both questionnaires and gave data consent, are presented with 95% CI, and effect sizes were calculated as Cohen’s d, with d=0.2 reflecting a small, d=0.5 a medium, and d=0.8 a large effect.27 All statistical tests were two tailed and the level of significance was set at p<0.05.
Results
Baseline Characteristics
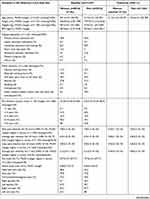
In all, 17,351 patients were registered in PainData, with an initial consultation date in a public or private interdisciplinary pain center in Denmark between January 1st 2018 and December 31st 2020. How many of these patients showed up for their initial consultation is not registered in the database. The baseline questionnaire completion rate was 76.6% (n=13,291) of all registered patients. The consent rate for the use of personal questionnaire data for quality assurance and research use among completers of the baseline questionnaire was 92.3%. In total, 12,257 patients completed the baseline questionnaire and gave data consent. The amount of missing data on individual questions among these patients was relatively low (Table 1). Characteristics of the population at baseline in the 13 interdisciplinary pain centers/clinics are illustrated in Table 1 for women and for men. Percentiles for each variable are illustrated in Table 2 for women and for men. Responses to value-based healthcare questions are illustrated in Table 3.
Socio-Demographics
The mean age of all individuals was 50.0±14.6 years (range 18–99 years) (Table 1). In total, 67.9% of patients in the registry were women. More than 30% of the individuals had vocational education and training as the highest level of education (higher proportion of men) and less than 5% had an educational level equivalent to a master’s program. Almost 30% of individuals were on a pension, and 20% of patients not on a pension were on sick leave.
Pain Characteristics
The mean duration since the pain debut was 10.0 years (range 0.25–84 years). On average, the peak pain intensity level during the last 24 hours corresponded to moderately severe pain (~7.8 out of 10) (Table 1). Of all the individuals, back pain, followed by pain in the thorax/abdomen/genital area within the past week were reported most frequently (Table 1). Based on the body drawing illustrated in Figure 1B, the mean number of painful body areas was 21.6, and the median number of painful body regions was 5 (Figure 1C). Based on the American College of Rheumatology’s definition of chronic widespread pain (CWP) as pain localized in both the right and left sides of the body, both above and below the waist plus in the axial skeleton,28 30.9% of patients had CWP. Almost 90% reported using analgesics, with 43% reporting opioid use. Most patients reported that several different treatment modalities had previously been tried to relieve the pain, with more than 40% reporting having had surgery. The most common non-invasive treatments were exercise, massage, and acupuncture. More than 50% had tried four or more different treatment modalities prior to referral to an interdisciplinary pain center/clinic.
Psychological Distress and Quality of Life
In general, mean scores on pain catastrophizing, fear of movement, and pain-related disability were high, whereas scores on pain acceptance and self-efficacy were low (Table 1). However, mean scores on depression and anxiety were low to moderate. Physical and mental health assessed with the PROMIS Scale v1.2 – Global Health averaged 33.7 and 38.3 (T-scores), respectively, corresponding to a classification of poor and fair physical and mental health, respectively (Table 1). More than 40% reported emotional problems often or always during the last 7 days.
Other Symptoms
More than 80% of all individuals reported disturbed sleep several times per week or more. Sixty percent reported severe or very severe fatigue during the last 7 days, and moderate to severe memory and/or concentration deficits were reported by almost 70% of the individuals.
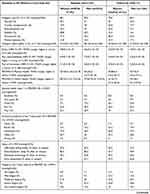
Value-Based Healthcare Items
Since the implementation of the value-based items in 2019, the items that most individuals reported being of value to them in relation to treatment (scores from “to a moderate degree” to “to a very great degree”) were “being able to participate in social activities” (93.9%), “improved activity pacing” (89.6%), and “help to learn pain coping strategies” (83.8%). The items that fewest individuals reported being of value to them (scores from “to a moderate degree” to “to a very great degree”) were “help with work and income” (58.3%), “improved communication about living with chronic pain” (69.3%), and “gain knowledge about pain, emotions and mood” (75.5%) (Table 3).
Characteristics at Follow-Up
In total, 10,452 patients were registered in PainData as having completed treatment in a public or private interdisciplinary pain center in Denmark between January 1st 2018 and December 31st 2020. The follow-up questionnaire completion rate was 41.5% (n=4,341) of all completed patients. The consent rate for use of personal questionnaire data for quality assurance and research use among completers of the follow-up questionnaire was 94.4% (n=4,111). Characteristics of the population at follow-up in the interdisciplinary pain centers/clinics are shown in Table 1 for women and for men, and the responses to the value-based healthcare questions at follow-up are shown in Table 3.
Impression of Change
Individuals at follow-up had improved scores compared with baseline scores for all domains, and one out of four individuals reported being very much improved or much improved after treatment. The proportion of individuals using analgesics at follow-up was reduced, and, specifically, the proportion of opioid users was decreased at follow-up compared with baseline. For the patients who started and completed treatment within the period 2018–2020, who completed both questionnaires and gave data consent, the mean treatment duration was 217±170 days (range 0–1043 days). The within-group changes in outcomes were mostly of small to moderate effect sizes, with the largest effect observed for pain catastrophizing (Table 4).
Value-Based Healthcare Items
The achievements that most individuals reported (scores from “to a moderate degree” to “to a very great degree”) were “gained knowledge about pain, emotions and mood” (66.5%), “had become better at accepting life with chronic pain” (63.1%), and “improved activity pacing” (60.6%).
The achievements that fewest individuals reported (scores from: “to a moderate degree” to “to a very great degree”) were “help with work and income” (24.4%), “helped to sleep better” (38.1%), and “recieved help to learn pain coping strategies” (49.2%) (Table 3).
Comparison of Follow-Up Questionnaire Completers and Non-Completers
Individuals who completed the follow-up questionnaire were not different regarding sex, pain duration, pain intensity, pain-related disability, opioid use, and fear of movement scores compared with non-responders (Table 5). However, individuals who did not complete the follow-up questionnaire were on average 3 years younger, with higher scores on pain catastrophizing, stress, anxiety and depression, and with lower scores on pain acceptance, self-efficacy, and physical and mental health at baseline.
 |
Table 5 Baseline Ccmparison of Completers and Non-Completers of the Follow-Up Questionnaire. |
Discussion
In this paper, we have described the aims and content of the clinical PainData registry as well as the participant recruitment/response rate across public and private interdisciplinary pain centers in Denmark. We have also reported socio-demographics, pain characteristics, quality of life, and treatment values before and after treatment in a large sample of individuals with high-impact chronic pain referred to public and private interdisciplinary pain centers in Denmark. A higher proportion of women compared with men attended specialized interdisciplinary pain centers in Denmark. Most patients had had pain for more than 5 years and one in three patients reported chronic widespread pain. The use of opioids was common and most patients had tried a number of treatments in primary and secondary care settings with an unsatisfactory clinical response. Poor sleep, severe fatigue, and memory and/or concentration deficits were reported by more than 60% of patients. The patient group had high scores on pain catastrophizing, fear of movement, and pain-related disability, whereas scores on pain acceptance and self-efficacy were low. Scores on depression and anxiety were low to moderate, indicating that patients were more distressed than depressed. Physical and mental health were rated as poor and fair, respectively. One in four patients reported being very much improved or much improved after treatment. Items commonly reported after treatment were increased knowledge about pain, emotions and mood (66.5%), better at accepting life with chronic pain (63.1%), and improved activity pacing (60.6%).
The socio-demographics (age, sex, work status) and pain characteristics (duration, patient-reported cause, localization, intensity, use of analgesics, mood and cognitions) of the PainData sample in Denmark are in line with the characteristics of populations with high-impact chronic pain in, for example, Australia and New Zealand12 and Sweden.13 In addition, the improvements reported after interdisciplinary pain rehabilitation in Denmark are of similar magnitude to those reported in the EPOCC registry (Australia and New Zealand)12 and the SQRP registry (Sweden).13 Among the patients referred to specialized interdisciplinary pain treatment in Denmark, the proportion of women was larger than the proportion of men. Some differences in PROs between women and men were noted. Women reported a higher number of painful body areas, whereas men reported higher scores on fear of movement and pain catastrophizing, and lower scores on acceptance and self-perceived general health. In addition, a higher proportion of men reported opioid use and pain related to trauma.
To date, several research projects26,29–33 have used data from the PainData registry, resulting in a number of publications, with more in press or under preparation. These projects have included collaborations between researchers from Denmark, Australia, Germany, and Ireland. The functionality of the registry allows easy implementation of additional questions and questionnaires for future research projects.
Strengths and Limitations
The PainData registry has a number of strengths. One strength is that the population is a consecutive cohort of all patients referred to the public and private interdisciplinary pain centers/clinics in Denmark, which improves its generalizability. Another strength is the recording of each patient’s central person registration number, which facilitates data linkage with other Danish registries. In the context of the Danish healthcare system, population-based registers include those containing data on primary and secondary healthcare utilization, hospital diagnoses, prescription drugs, social conditions, work participation, and public welfare support.14,15
The registry also has a number of weaknesses. First, less than half the patients complete follow-up questionnaires. This may reflect that the registry is not a discrete research project where only people who agree to full participation are included. It may also reflect that the main focus within the interdisciplinary pain centers/clinics so far has been on completion of the baseline questionnaire, as an integrated part of clinical practice and used during the initial consultation with the patient. Several measures to improve follow-up participation are currently being implemented, including providing automated reminders in the official e-Boks to those who had not completed the questionnaire, and a reduction in the number of questions at follow-up to reduce the response burden. Despite this, the follow-up completion rate may also remain a weakness of the registry in the future. Routine data collection as part of clinical practice differs from collecting data to answer a specific research question, as it involves a trade-off between using detailed and validated questionnaires and being able to cover a number of potentially important clinical aspects of chronic pain. For example, in the PainData registry minimum core data set, one-item screening questions to assess fear of movement, pain catastrophizing, and stress are used to reduce the length of the survey and responder burden. Although these screening questions have known concurrent validity in this clinical setting,26 relative to full reference validated standard questionnaires, their test–retest reliability, relative responsiveness, and prognostic value are still unknown. The inclusion of the full validated questionnaires in some of the pain centers will allow further investigation of this in the future. Other limitations may be the use of online questionnaires, as some patients may have limited access to a computer, and some elderly patients may have difficulties in handling a computer. However, according to the Danish Agency of Digitization, almost 93% of the Danish population above 15 years of age uses digital mail, although the proportion of citizens not using digital mail is larger among individuals above 65 years of age,34 and the proportion of citizens not using digital mail among this group of severely affected patients may be even larger.
Conclusion
The PainData registry contains PRO data from a large and comprehensive observational cohort of individuals attending public and private interdisciplinary pain centers in Denmark for the assessment and treatment of high-impact chronic pain. The registry contains detailed baseline and outcomes data on a broad range of biopsychosocial factors, and new data (eg, new questionnaires for clinical or research purposes) can be collected in the future. These data are in line with the characteristics of populations with high-impact chronic pain in other countries and can be linked to the Danish population-based registries. Collaborations with other researchers are welcome.
Disclosure
Mr Torsten Wentzer Licht reports personal fees from Grünenthal, outside the submitted work. The authors report no conflicts of interest in this work.
References
1. Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–1007. doi:10.1097/j.pain.0000000000000160
2. Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986–995.e1. doi:10.1016/j.apmr.2013.10.032
3. Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1–2):172–179. doi:10.1016/j.pain.2006.06.009
4. Eriksen J, Sjøgren P, Ekholm O, Rasmussen NK. Health care utilisation among individuals reporting long-term pain: an epidemiological study based on Danish National Health Surveys. Eur J Pain. 2004;8(6):517–523. doi:10.1016/j.ejpain.2003.12.001
5. Burke AL, Mathias JL, Denson LA. Psychological functioning of people living with chronic pain: a meta-analytic review. Br J Clin Psychol. 2015;54(3):345–360. doi:10.1111/bjc.12078
6. Mathew J, Singh SB, Garis S, Diwan AD. Backing up the stories: the psychological and social costs of chronic low-back pain. Int J Spine Surg. 2013;7:e29–e38. doi:10.1016/j.ijsp.2013.02.001
7. Flachs E, Eriksen L, Koch M, Ryd J, Juel K. The disease burden in Denmark. Sundhedsstyrelsen, editor. Copenhagen: Statens Institut for Folkesundhed, Syddansk Universitet; 2015. Available from: https://www.sst.dk/da/sygdom-og-behandling/~/media/00C6825B11BD46F9B064536C6E7DFBA0.ashx.
8. Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American pain society/American college of physicians clinical practice guideline. Ann Intern Med. 2007;147(7):492–504. doi:10.7326/0003-4819-147-7-200710020-00007
9. Oliveira CB, Maher CG, Pinto RZ, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–2803. doi:10.1007/s00586-018-5673-2
10. Von Korff M, Scher AI, Helmick C, et al. United States national pain strategy for population research: concepts, definitions, and pilot data. J Pain. 2016;17(10):1068–1080. doi:10.1016/j.jpain.2016.06.009
11. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi:10.15585/mmwr.mm6736a2
12. Tardif H, Arnold C, Hayes C, Eagar K. Establishment of the Australasian electronic persistent pain outcomes collaboration. Pain Med. 2017;18(6):1007–1018. doi:10.1093/pm/pnw201
13. Alföldi P, Dragioti E, Wiklund T, Gerdle B. Spreading of pain and insomnia in patients with chronic pain: results from a national quality registry (SQRP). J Rehabil Med. 2017;49(1):63–70. doi:10.2340/16501977-2162
14. Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Pub Health. 2011;39(7 Suppl):12–16. doi:10.1177/1403494811399956
15. Thygesen LC, Ersbøll AK. Danish population-based registers for public health and health-related welfare research: introduction to the supplement. Scand J Pub Health. 2011;39(7 Suppl):8–10. doi:10.1177/1403494811409654
16. Danske-Regioner. Værdibaseret sundhed i Danmark Anbefalinger for vejen frem. Hvad er ambitionen med værdibaseret sundhed? 2018. Available from: https://docplayer.dk/151942518-Vaerdibaseret-sundhed-i-danmark-anbefalinger-for-vejen-frem-hvad-er-ambitionen-med-vaerdibaseret-sundhed.html.
17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi:10.1371/journal.pmed.0040296
18. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133(4):581–624. doi:10.1037/0033-2909.133.4.581
19. Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. doi:10.1016/j.pain.2003.08.001
20. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
21. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
22. Kent P, Kongsted A, Jensen TS, Albert HB, Schiøttz-Christensen B, Manniche C. SpineData - a Danish clinical registry of people with chronic back pain. Clin Epidemiol. 2015;7:369–380. doi:10.2147/CLEP.S83830
23. Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15(Suppl 1):S17–S24. doi:10.1007/s00586-005-1044-x
24. Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi:10.1016/j.jclinepi.2010.04.011
25. Kecklund G, Akerstedt T. The psychometric properties of the Karolinska Sleep Questionnaire. J Sleep Res. 1992;1:113. doi:10.1111/j.1365-2869.1992.tb00005.x
26. Vaegter HB, Handberg G, Kent P. Brief psychological screening questions can be useful for ruling out psychological conditions in patients with chronic pain. Clin J Pain. 2018;34(2):113–121. doi:10.1097/AJP.0000000000000514
27. Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Statistics. 1981;6:107–128. doi:10.3102/10769986006002107
28. Wolfe F, Smythe HA, Yunus MB, et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–172. doi:10.1002/art.1780330203
29. Titze C, Hasenbring MI, Kristensen L, Bendix L, Vaegter HB. Patterns of approach to activity in 851 patients with severe chronic pain: translation and preliminary validation of the 9-item avoidance-endurance fast-screen (AEFS) into Danish. Clin J Pain. 2021;37(3):226–236. doi:10.1097/AJP.0000000000000912
30. Kha TV, Stenager E, Hoang H, et al. Preliminary validity and test-retest reliability of two depression questionnaires compared with a diagnostic interview in 99 patients with chronic pain seeking specialist pain treatment. Scand J Pain. 2020;20(4):717–726. doi:10.1515/sjpain-2020-0042
31. Vaegter HB, Ussing K, Johansen JV, et al. Improvements in clinical pain and experimental pain sensitivity after cognitive functional therapy in patients with severe persistent low back pain. Pain Rep. 2020;5(1):e802. doi:10.1097/PR9.0000000000000802
32. Plesner KB, Vaegter HB. Symptoms of fibromyalgia according to the 2016 revised fibromyalgia criteria in chronic pain patients referred to multidisciplinary pain rehabilitation: influence on clinical and experimental pain sensitivity. J Pain. 2018;19(7):777–786. doi:10.1016/j.jpain.2018.02.009
33. Hansen M, Hyland P, Karstoft KI, et al. Does size really matter? A multisite study assessing the latent structure of the proposed ICD-11 and DSM-5 diagnostic criteria for PTSD. Eur J Psychotraumatol. 2017;8(sup7):1398002. doi:10.1080/20008198.2017.1398002
34. Danish Agency for Digitisation Ministry of Finance. Statistik om digital post; 2021. Available from: https://digst.dk/it-loesninger/digital-post/om-loesningen/tal-og-statistik-om-digital-post/.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.