Back to Journals » International Journal of General Medicine » Volume 13
Small Airways Dysfunction and Bronchial Hyper-Responsiveness in Cough Variant Asthma
Received 10 October 2020
Accepted for publication 18 November 2020
Published 7 December 2020 Volume 2020:13 Pages 1427—1434
DOI https://doi.org/10.2147/IJGM.S286144
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jie Gao,* Hai Gui Wu,* Feng Wu
Department of Pulmonary and Critical Care Medicine, Huizhou the Third People’s Hospital, Guangzhou Medical College, Huizhou 516002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Feng Wu
Huizhou the Third People’s Hospital, 1# Xuebei Ave., Huizhou 516002 Guangdong, People’s Republic of China
Tel +86-13502436132
Fax +86-7522359648
Email [email protected]
Background: Cough variant asthma (CVA) is one kind of atypical asthma. The study was to compare spirometric parameters of small airways and the degree of bronchial hyper-responsiveness (BHR) between CVA and classic asthma (CA), and examine the relationship between BHR and small airways to determine the accuracy of these markers as indicators of CVA.
Methods: A total of 825 asthmatic patients were screened for the study, and 614 were included. All patients performed spirometry and underwent a bronchial challenge with methacholine.
Results: The number of small airways dysfunctions in the CVA group was less than those of the CA group with MMEF% predicted (70% vs 80.91%, P=0.002) and FEF50% predicted (62.71% vs 73.5%, P=0.004). The degree of small airways dysfunction was less in the CVA group compared with the CA group (P< 0.001). Significant positive correlations were observed between the FEV1 level below 20% of the baseline value (PD20) and MMEF% predicted (r=0.282, P< 0.001), FEF50% predicted (r=0.2522, P< 0.001), and FEF75% predicted (r=0.2504, P< 0.001) in patients with CVA. The area under curve (AUC) of MMEF, FEF50, and FEF75 (% predicted) was 0.615, 0.621, and 0.606, respectively. In addition, 0.17 mcg of PD20 was the best diagnostic value for CVA, with an AUC of 0.582 (P=0.001).
Conclusion: Small airway dysfunction is milder in CVA. The value of BHR combined with small airways in CVA prediction, which was significant, but not enough to be clinically useful.
Keywords: small airways, bronchial hyper-responsiveness, cough-variant asthma, classic asthma
Background
Asthma is a heterogeneous disease with chronic airway inflammation of bronchial hyper-responsiveness (BHR) to a variety of stimuli, and variable expiratory airflow limitation that is often reversible either spontaneously or as a result of therapy.1,2 The definition of “classic asthma (CA)” is based on the characteristics and intensity of the respiratory symptoms (wheeze, shortness of breath, tightness, and cough).1
The original definition of cough variant asthma (CVA) was described by Glauser3–5 in 1979. They described patients with asthma with cough as their sole presenting symptom, but whose symptoms improved with bronchodilators alone. The European and American guidelines do not discuss specific diagnostic criteria, but they do highlight the diagnostic value of BHR and recommend that the CVA diagnosis should be determined according to the therapeutic response.1 The 2016 Chinese Cough guidelines take the detailed diagnostic criteria and treatment of CVA, which is the BHR, and successful treatment of bronchodilators and/or inhaled corticosteroids as the basic diagnostic criteria.6
Small airways were defined as the bronchial being less than 2 mm in internal diameter.7 The assessment of small airways dysfunction is a challenging matter, because the distal airway is relatively inaccessible to assess. More specialized tests have been developed, such as spirometry, impulse oscillometry (IOS), breath nitrogen washout (BNW), and body plethysmography, No assessment method is universally and directly representative of small airways function. The value and limitations of each test have been extensively reviewed.8–11 Small airways played a role in the pathobiology of asthma and have a distinct role in specific disease phenotypes, although they are involved in half of all cases of asthma.12–14 The severity of asthma was also associated with inflammatory changes and functional alterations in the small airways.11,15 The role of the small airways in asthma is increasingly recognized as a potential target in optimal control of the disease. Therefore, this study aims to explore the validity of small airways and BHR in the diagnosis of CVA.
Methods
Study Design and Participants
In this retrospective and observational study, we conducted a series of 614 patients with CVA and CA visiting the Third People’s Hospital of Guangzhou Medical College in Huizhou from January 2018 to April 2019.
CA patients were diagnosed according to 1) a clinical record of recurrent wheezing, dyspnea, or/and cough episodes, chest tightness; 2) pulmonary function tests demonstrate variable airflow limitation by means of BHR testing or airway reversibility testing; and 3) a positive to the therapeutic response, which satisfied those of the Chinese national Guidelines on Diagnosis and Management of Asthma (2016).16
CVA patients were described as 1) chronic cough (lasting more than 8 weeks without specific cause) as the only symptom; 2) the cough was usually dry or productive with minimal amounts of clear sputum, and was mainly nocturnal; 3) Normal baseline lung function, some might actually include normal small airway function, and positive test for BHR; 4) No other cause of chronic cough; and 5) a positive to the therapeutic response.6
Inclusion criteria were: age greater than 14 years; no smoking or quitting smoking for more than 1 year; diagnosis of CA and CVA was made according to the guidelines criteria of China;6,16 received initial diagnosis of CA or CVA and were uncontrolled stage; no other apparent causes of cough were present; not used any oral or/and inhaled corticosteroid (ICS) in the previous 4 weeks.
Exclusion criteria were: a history of chronic obstructive pulmonary disease (COPD) or asthma-COPD overlap (ACO), bronchitis, bronchiectasis, lung cancer, cystic fibrosis, or pneumonia; do not continue treatment because of some reasons or diagnosed as other diseases after treatment were excluded.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Huizhou Third People’s Hospital, which absolved the need for written informed consent because of the retrospective study. All personal identification data were anonymized and de-identified before analysis.
Assessments and Spirometry
Pulmonary function tests, induced sputum cell differentials, and CT of the chest were performed on the same day.
Variable airflow limitation was performed by a spirometer (MS-pneumo + APS; JAEGER, Germany). In accordance with recommendations of the Chinese National Guidelines of Pulmonary Function Test (2014): rapid rise in flow/volume curve, duration of expiration more than or equal to six seconds, and visualization of peak expiratory flow (PEF) were required. Repeat at least three times (a reproducible way), the best spirometric values were measured prior to the methacholine challenge. Patient with a percent predicted forced expiratory volume in first second (FEV1%pred) ≥ 60% continued to the BHR test (at baseline). The procedure was terminated when the FEV1 level fell below 20% of the baseline value. The positive response was defined as PD20 ≤2.504 mcg (between NS and 2.504 mcg). The cumulative dose of PD20 was used to assess the degree of BHR.17,18
Small airways dysfunction is the early expression of airway obstruction. FVC, FEV1, and FEV1/FVC are still in normal range, but MMEF, FEF50%, and FEF75% can be significantly decreased. When two of the three indicators were lower than LLN (lower limits of normal), it could be judged as small airway dysfunction.17
Statistical Analysis
All statistical variables were analyzed using SPSS version 22 (IBM Corporation, Armonk, NY, USA). Data were presented as mean±standard deviation (SD), frequencies, and percentages, as appropriate. A t-test for independent samples or a Chi square test was used to observe two groups of CVA and CA patients. The relationship between PD20 and spirometry was detected with the Pearson correlation coefficient. Correlation between tests was assessed by constructing a receiver operating characteristic (ROC) curve. A P-value<0.05 was considered statistically significant.
Results
Characteristics and Pre-Challenge Spirometry of the Patients
A total of 825 asthmatic outpatients attended the diagnosis by pulmonary and critical care medicine physicians and underwent a detailed lung function test. In total, 614 asthmatic patients were eligible and analyzed. Reasons for exclusion were 1) aged <14 years (n=53); 2) combined diagnosis of COPD or ACO (n=8 and n=5, respectively); 3) diagnosis of CA or CVA in the previous (n= 67); and 4) performed by bronchodilator reversibility test (n=78). The strategies of flow-chart are illustrated in Figure 1.
 |
Figure 1 The strategies of flow-chart with asthmatic patients. n: number of subjects. |
There were a total of 825 samples with asthmatic patients to be collected in the retrospective study and 211 were excluded. Demographic parameters of included patients are presented in Table 1. Significant but weaker differences were found in sex ratio between CVA and CA patients (P=0.041).
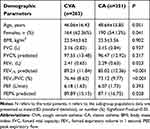 |
Table 1 Baseline Characteristics and Pre-Challenge Spirometry of Patients with CVA and CA (N=614) |
According to the results of spirometry, FEV1% predicted, FEV1/forced expiratory vital capacity (FVC)(FEV1/FVC), and PEF% predicted were higher in CVA compared to CA (P<0.05). We did not find any difference in age, BMI, or history of smoking between the two groups.
Table 2 reflects small airway function. Patients with CVA showed less small airway dysfunction (<65%) than the CA group in MMEF% predicted (70% vs 80.91%, P=0.002) and FEF50% predicted (62.71% vs 73.5%, P=0.004). The degree of small airways dysfunction was significantly milder in the CVA group compared with the CA group (P<0.001).
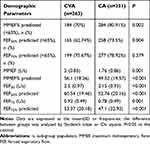 |
Table 2 Baseline Spirometry of Small Airways in Patients with CVA and CA (N=614) |
BHR of the Patients
Responses to bronchial challenge tests with methacholine in CVA and CA patients are shown in Table 3. Significantly higher BHR was found in CA patients compared to in CVA patients (P=0.005). Compared to CVA, CA showed more sensitivity in the degree of FEV1 fall (%) (P=0.002 and P=0.001, respectively).
 |
Table 3 BHR of CVA and CA Patients (N=614) |
Associations between PD20 and FEV1/FVC, Small Airway Function of the Patients
Figure 2 gives an overview of all correlations between PD20 and small airways (MMEF%, FEF50%, FEF75%) with CVA and CA. Significant positive correlations were observed for MMEF% predicted (r=0.282, P<0.0001), FEF50% predicted (r=0.2522, P<0.0001), and FEF75% predicted (r=0.2504, P<0.0001) in patients with CVA (Figure 2A). We also found significant correlations between PD20 and small airways (r=0.2861, r=0.2917, and r=0.2476, respectively) with CA patients (P<0.001) (Figure 2B).
PD20 and proportion parameters in spirometry as predictors to identified CVA from CA were analyzed using the ROC curve (Figure 3). The optimum cut-point for FEV1% predicted was 82.65% with an area under the curve (AUC) of 0.602 (P<0.001), and the AUC of MMEF, FEF50, and FEF75 (% predicted) was 0.615, 0.621, and 0.606, respectively. In addition, 0.17 mcg of PD20 was the best diagnostic value for CVA with an AUC of 0.582 (P=0.001). Data of sensitivity and specificity are shown in Table 4. The AUC of PD20 combined with MMEF (% predicted) was 0.616, and that combined with MEF50 (% predicted) and MEF75 (% predicted) was 0.625 and 0.606, respectively (Figure 4).
 |
Table 4 Results of ROC Analysis of PD20 and Spirometry |
 |
Figure 3 ROC curve analysis of PD20, FEV1, MMEF, FEF50, and FEF75 (%predicted) for CVA diagnosis. |
Discussion
The retrospective study showed that more patients with CVA were associated with differences in levels of sex, FEV1% predicted, FEV1/FVC, compared to patients with CA. Milder BHR and small airway dysfunction are shown in CVA, and the correlation between them were observed weak but significant differences. BHR and small airways were poor to distinguish patients with CVA, respectively. We also evaluated the value of BHR combined with small airways in CVA prediction, which was reflected as significant, but not enough to be clinically useful.
Chronic cough is the sole presenting symptom of CVA. Previous literatures have studied that chronic persistent non-productive cough is more frequent in females, and females are more easily troubled by the symptom.19,20 Females exceed males in number among patients attending specialist respiratory clinics.21 The cough threshold is lower in females than in males, illustrating that the cough sensitivity is heightened in females.22,23 In our study, the difference of gender is weak. The numbers of females was a little frequent in CVA (P=0.041). The mechanism of the gender differences is still unclear, and additional studies are needed to better understand gender differences.
Spirometry is the fundamental diagnostic method and the most widely non-invasive, easy to assess the airflow limitation associated with asthma. Parameters such as FEV1 and PEF are frequently used to evaluate proximal airway obstruction. In the study, we found that patients with CVA were more likely to have a better FEV1 and FEV1% predicted (89.21% vs 85.02, P<0.001). Spirometric values indices are almost independent of patient’s activity if the expiration is forced; they depend only on the properties of the respiratory system because of the airflow limitation phenomenon.24–26 Evaluation of forced spirometry results begins with analysis of whether bronchial airflow capacity quantified by means of the FEV1.
Previously, asthma was understood to be a disease primarily of the central airways. However, surgical lung specimens with living chronic asthma and autopsy specimens with fatal asthma reveal mucus plugging and inflammatory involvement of both the small and large airways.27,28 It is an inflammatory characterized by increased T cells, activated eosinophils, and major basic protein in the small airways, which was similar to the inflammation of the central airways.28 The intensity of the inflammation may be even higher in the small airways compared with central airways.29 These observation confirm that the chronic inflammation of asthma involves the entire lung, from the large proximal to the small distal airways. Assessment of small airways pathology can be subdivided in tests measuring flow, airway resistance, inhomogeneity of ventilation distribution, and hyperinflation or air trapping by pulmonary function machines. Flow measures of small airways commonly used in forced expiratory flow at 50% (FEF50%), at 75% (FEF75%), and at 25–75% (FEF25–75%/MMEF) of FVC. Among these parameters, FEF25–75%is the most commonly adopted, although the literature supporting its reliability is not conclusive.
The pathophysiological features of CVA are similar to those of CA. CVA shows similar levels of eosinophilic airway inflammation and a milder degree of airway remodeling, such as sub-epithelial thickening, goblet cell hyperplasia, and vascular proliferation.30–32 The maximal airway response on the dose–response curve is a clinically relevant component of BHR because it reflects the potential degree of airway obstruction. BHR of CVA is caused by the type of airway inflammation and airway structural changes, which are shown to be similar to CA.31–34 Nearly 30% of CVA patients eventually develop CA, sometimes severe enough to require continuous treatment.5,35 Some researches indicate that increasing BHR has a pathogenetic role in the development of wheezing during the course of CVA on exposure to an allergic or a non-allergic stimulus.36,37 Given these studies, CVA is considered to be the initial stage of asthma.37 Some patients with CVA will evolve into continuous CA. Therefore, the early diagnosis and treatment is recommended to attenuate the inflammation and remodeling. In our study, the spirometric parameters for small airways (FEF50%, FEF75%, and MMEF) with the CVA group were better than those with the CA group. Small airway dysfunction was present in a large proportion of asthma patients at baseline, and the number of small airways dysfunction in the CVA group was less than those of the CA group. Our study has provided a positive relationship between PD20 and small airways in both CVA and CA. The optimum cut-point for MMEF, FEF50, and FEF75 (% predicted) were 47.85%, 47.79%, and 38.2%, and the AUC of them were 0.615, 0.621, and 0.606, respectively. It has been shown that 0.17 mcg of PD20 may help identify CVA with an AUC of 0.582 (P=0.001). The correlation and ROC analyses demonstrated a relatively poor, although significant relationship for PD20 and small airways to predict CVA in statistics. Thus, it is difficult to use BHR and small airways to predict CVA.
Limitation of the Study
Our study reported on a clinical study with 825 patients with asthma to investigate the diagnostic validity of small airways and BHR in cough-variant asthma. A positive relationship showed between PD20 and small airways. However, overall the association appeared weak with low AUCs for the prediction of BHR and small airways to CVA. While these correlations might show statistical significance, none of these appear convincing and potentially clinically relevant judged. Also, the low AUC values are very unreliable and, very likely, not helpful to inform clinical decision-making. Statistically significant does not always imply clinical significance, but the level of association needs to be considered as well.
Another major limitation was the smoking cigarettes factor. In addition to the possibility that this could be a cause of the cough, smoking is associated with small airway dysfunction which (except for non-causally related cough) is usually asymptomatic until values are very obstructed. Further investigation of smoking or no smoking factor in CVA would also benefit from bigger sample sizes.
Conclusions
This study demonstrates that small airway dysfunction and BHR are milder in patients with CVA, and the relationship of them is weak. Small airways and BHR may not be potentially useful as diagnostic measures to CVA patients. Based on these weak correlations and poor prediction values, further investigations would be required.
Abbreviations
BHR, bronchial hyper-responsiveness; GINA, the Global Initiative for Asthma; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; PEF, peak expiratory flow; MMEF, maximum mid expiratory flow; MEF, maximal expiratory flow. CVA, cough-variant asthma; CA, classic asthma.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Huizhou Third People’s Hospital, which absolved the need for written informed consent because of the retrospective study. All personal identification data were anonymized and de-identified before analysis.
Author Contributions
All authors contributed to the data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
These authors contributed equally to this work and should be considered co first authors: Jie Gao and Haigui Wu.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Global Initiative for Asthma. Global strategy for asthma management and prevention. Available from: https://ginasthma.org/pocket-guide-for-asthma-management-and-prevention/.
2. Program NAEaP. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;5:S94–S138.
3. Glauser FL. Variant asthma. Ann Allergy. 1972;30:457–459.
4. McFadden ER
5. Corrao WM, Braman SS, Irwin RS. Chronic cough as the sole presenting manifestation of bronchial asthma. N Engl J Med. 1979;300:633–637. doi:10.1056/NEJM197903223001201
6. Asthma Workgroup of Chinese Society of Respiratory Diseases (CSRD), Chinese Medical Association. The Chinese national guidelines on diagnosis and management of cough (2015). Chin J Tuberc Respir Dis. 2016;39:321–339.
7. Contoli M, Bousquet J, Fabbri LM, et al. The small airways and distal lung compartment in asthma and COPD: a time for reappraisal. Allergy. 2010;65:141–151. doi:10.1111/j.1398-9995.2009.02242.x
8. Usmani OS. Small airways dysfunction in asthma: evaluation and management to improve asthma control. Allergy Asthma Immunol Res. 2014;6:376–388. doi:10.4168/aair.2014.6.5.376
9. van den Berge M, Ten Hacken NH, Cohen J, et al. Small airway disease in asthma and COPD: clinical implications. Chest. 2011;139:412–423. doi:10.1378/chest.10-1210
10. Anderson WJ, Zajda E, Lipworth BJ. Are we overlooking persistent small airways dysfunction in community-managed asthma? Ann Allergy Asthma Immunol. 2012;109:185–189. doi:10.1016/j.anai.20j12.06.022
11. Perez T, Chanez P, Dusser D, et al. Small airway impairment in moderate to severe asthmatics without significant proximal airway obstruction. Respir Med. 2013;107:1667–1674. doi:10.1016/j.rmed.2013.08.009
12. Bonini M, Usmani OS. The role of the small airways in the pathophysiology of asthma and chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2015;9:281–293. doi:10.1177/1753465815588064
13. Farah CS, Keulers LA, Hardaker KM, et al. Association between peripheral airway function and neutrophilic inflammation in asthma. Respirology. 2015;20:975–981. doi:10.1111/resp.12550
14. Contoli M, Santus P, Papi A. Small airway disease in asthma: pathophysiological and diagnostic considerations. Curr Opin Pulm Med. 2015;21:68–73. doi:10.1097/MCP.0000000000000122
15. Alfieri V, Aiello M, Pisi R, et al. Small airway dysfunction is associated to excessive bronchoconstriction in asthmatic patients. Respir Res. 2014;15:86. doi:10.1186/s12931-014-0086-1
16. Asthma Workgroup of Chinese Society of Respiratory Diseases (CSRD), Chinese Medical Association. The Chinese national guidelines on diagnosis and management of asthma (2016). Chin J Tuberc Respir Dis. 2016;39:675–697.
17. Pulmonary Function Workgroup of Chinese Society of RespiratoryDiseases (CSRD), Chinese Medical Association. The Chinese national guidelines of pulmonary function test (2014). Chin J Tuberc Respir Dis. 2014;37:566–571.
18. Miller MR, Hankinson J, Brusasco V, et al.; ATS/ERS Task Force. Standardization of spirometry. Eur Respir J. 26;2005:319–338. doi:10.1183/09031936.05.00034805
19. Fujimura M, Sakamoto S, Matsuda T. Bronchodilator-resistive cough in atopic patients: bronchial reversibility and hyperresponsiveness. Intern Med. 1992;31:447–452. doi:10.2169/internalmedicine.31.447
20. Fujimura M, Kamio Y, Hashimoto T, et al. Cough receptor sensitivity and bronchial responsiveness in patients with only chronic nonproductive cough. In view of effect of bronchodilator therapy. J Asthma. 1994;31:463–472. doi:10.3109/02770909409089488
21. Irwin RS, Boulet LP, Cloutier MM, et al. Managing cough as a defense mechanism and as a symptom: a consensus panel report of the American College of Chest Physicians. Chest. 1998;114:133S–81S. doi:10.1378/chest.114.2_Supplement.133S
22. Fujimura M, Kasahara K, Kamio Y, et al. Female gender as a determinant of cough threshold to inhaled capsaicin. Eur Respir J. 1996;9:1624–1626. doi:10.1183/09031936.96.09081624
23. Kastelik JA, Thompson RH, Aziz I, et al. Sex-related differences in cough reflex sensitivity in patients with chronic cough. Am J Respir Crit Care Med. 2002;166:961–964. doi:10.1164/rccm.2109061
24. Mead J, Turner JM, Macklem PT, et al. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi:10.1152/jappl.1967.22.1.95
25. Pride NB, Permutt S, Riley RL, Bromberger-Barnea B. Determinants of maximal expiratory flow from the lungs. J Appl Physiol. 1967;23:646–662. doi:10.1152/jappl.1967.23.5.646
26. Golczewski T, Darowski M. Virtual respiratory system for education and research: simulation of expiratory flow limitation for spirometry. Int J Artif Organs. 2006;29:961–972. doi:10.1177/039139880602901007
27. Kuyper LM, Pare PD, Hogg JC, et al. Characterization of airway plugging in fatal asthma. Am J Med. 2003;115:6–11. doi:10.1016/S0002-9343(03)00241-9
28. Hamid Q, Song Y, Kotsimbos TC, et al. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100:44–51. doi:10.1016/S0091-6749(97)70193-3
29. Balzar S, Wenzel SE, Chu HW. Transbronchial biopsy as a tool to evaluate small airways in asthma. Eur Respir J. 2002;20:254–259. doi:10.1183/09031936.02.00261102
30. Niimi A, Amitani R, Suzuki K, et al. Eosinophilic inflammation in cough variant asthma. Eur Respir J. 1998;11:1064–1069. doi:10.1183/09031936.98.11051064
31. Niimi A, Matsumoto H, Minakuchi M, et al. Airway remodelling in cough-variant asthma. Lancet. 2000;356:564–565. doi:10.1016/S0140-6736(00)02584-8
32. Niimi A, Torrego A, Nicholson AG, et al. Nature of airway inflammation and remodeling in chronic cough. J Allergy Clin Immunol. 2005;116:565–570. doi:10.1016/j.jaci.2005.07.010
33. Koh YY, Park Y, Kim CK. Maximal airway response in adolescents with long-term asthma remission and persisting airway hypersensitivity: its profile and the effect of inhaled corticosteroids. Chest. 2002;122:1214–1221. doi:10.1378/chest.122.4.1214
34. Irwin RS, Ownbey R, Cagle PT, et al. Interpreting the histopathology of chronic cough: a prospective, controlled, comparative study. Chest. 2006;130:362–370. doi:10.1378/chest.130.2.362
35. Fujimura M, Nishizawa Y, Nishitsuji M, et al. Longitudinal decline in pulmonary function in atopic cough and cough variant asthma. Clin Exp Allergy. 2003;33:588–594. doi:10.1046/j.1365-2222.2003.01658.x
36. Koh YY, Park Y, Kim CK. The importance of maximal airway response to methacholine in the prediction of wheezing development in patients with cough-variant asthma. Allergy. 2002;57:1165–1170. doi:10.1034/j.1398-9995.2002.23602.x
37. Kang H, Koh YY, Yoo Y, et al. Maximalairwayresponsetomethacholineincough-variant asthma: comparison with classic asthma and its relationship to peak expiratory flow variability. Chest. 2005;128:3881–3887. doi:10.1378/chest.128.6.3881
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


