Back to Journals » Nature and Science of Sleep » Volume 6
Sleep–wake disturbances in cancer patients: narrative review of literature focusing on improving quality of life outcomes
Authors Dickerson S, Connors L, Fayad A, Dean G
Received 29 June 2013
Accepted for publication 9 April 2014
Published 12 July 2014 Volume 2014:6 Pages 85—100
DOI https://doi.org/10.2147/NSS.S34846
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Suzanne S Dickerson, Laurie M Connors, Ameera Fayad, Grace E Dean
School of Nursing, State University of New York, University at Buffalo, NY, USA
Purpose: Evidence suggests a high prevalence of sleep–wake disturbances in patients with cancer, occurring at diagnosis, during treatment, and continuing to survivorship. Yet associations between sleep–wake disturbances and the impact on quality of life outcomes is less clear. The purpose of this narrative review of the literature is to evaluate sleep–wake disturbances in patients with cancer, to describe the influence of poor sleep on quality of life as an outcome, and to evaluate the evidence to recommend future interventions.
Framework and methods: This review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) approach. Four databases (CINAHL, MEDLINE, PsycINFO, and Embase) were searched using terms "cancer OR neoplasm", "sleep, sleep disturbance, sleep disorders or insomnia", and "quality of life"; the search included all years, English language, and peer-reviewed articles on research studies. Studies included measurements of sleep and quality of life in cancer patients at a minimum of two time points and demonstrated relationships between sleep and quality of life. Data were collected on date, patient demographics, cancer type and treatment, timeframe, design, measurement, variables, and results.
Results: This narrative review demonstrates that sleep–wake disturbance is a major problem/symptom in patients with cancer. Of the 18 studies included, measurement of sleep-related variables included objective and subjective measures; however, direct measurement of the associations between sleep and quality of life was not common. Cognitive behavioral therapy for insomnia and mind–body interventions demonstrated feasibility when implemented into cancer care settings. In addition, the majority of interventions exhibited moderate effectiveness in improving sleep–wake disturbance and quality of life outcomes.
Conclusion: The studies predominantly reported that poor sleep negatively impacts quality of life. The intervention studies included nonpharmacologic interventions such as cognitive behavioral treatment and mind–body and exercise interventions with moderate-to-high levels of evidence for improvement in sleep measures and quality of life.
Keywords: sleep–wake disturbance, quality of life, cancer, cognitive behavioral therapy, mind-body interventions, insomnia
Introduction
Sleep disturbances are commonly experienced by patients with cancer and are often overlooked due to other diagnostic and treatment-related concerns.1–3 Patients describe problems falling asleep (sleep latency), problems staying asleep (awakenings), having restless sleep (quality of perceived sleep), and/or having trouble staying awake during the day (excessive daytime sleepiness).2 The issues of sleep–wake disturbances are often combined in symptom clusters, especially with cancer-related fatigue4,5 and insomnia,6 which occur pretreatment,3 exacerbate during treatment, and continue through survivorship. During cancer treatment, many patients nap during the day, leading to circadian rhythm problems, which exacerbate sleep problems.2 While there is some awareness of sleep–wake disturbances in the context of symptom clusters5,7–9 during a patient’s cancer trajectory, what is not known is the impact of sleep on the physical, functional, and social aspects of quality of life (QOL).10
Adequate sleep is a biologic requirement for healthy physical, cognitive, and psychological functioning; however, acute and chronic sleep deprivation results in negative health consequences such as compromised immunological, cardiovascular, and neuropsychiatric functioning that are exacerbated by cancer diagnosis and treatments, thus influencing the patients’ QOL.10 QOL is a concept that encompasses how cancer and its treatments affect dimensions of physical (health-related), functional, and mental/psychological/emotional well-being. QOL has been extensively studied in cancer patients, yet sleep disturbance has often not been included as a main study variable.11 Vena et al10 suggest using the two-process model of sleep regulation12 to understand sleep–wake mechanisms in cancer patients, which includes a homeostatic process influenced by previous sleep and wake states and a circadian process that determines the propensity for falling asleep and consolidating sleep. This model can then be used to discuss cancer-related factors that influence a patient’s risk for developing sleep problems, such as: age and sex; cancer and treatment; lifestyle; and psychological well-being. There is a need to understand current knowledge of sleep–wake disturbances and the influence of these factors on QOL, as well as evidence-based interventions to improve sleep–wake disturbance and QOL. Therefore, the purpose of this paper was to systematically conduct a narrative review of the literature on sleep–wake disturbances and QOL in patients with cancer and to synthesize the evidence of longitudinal descriptive and intervention studies to improve sleep and QOL. The review is organized by study design (longitudinal descriptive and intervention), measurement issues, and discussion, and concludes with suggestions for future practice and research.
Methods
Framework and search methods
This narrative review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) approach13 and Berger et al’s methodological review findings.11 Four electronic databases (CINHAL, MEDLINE, PsycINFO, and Embase) were searched using the terms “cancer OR neoplasm”, “sleep, sleep disturbance, sleep disorders or insomnia, and quality of life”; the search included all years, English language, and peer-reviewed articles of original studies. Figure 1 demonstrates the process of search and review. Data collected included: date, patient demographics, cancer type, treatment/timeframe, study design, measurement variables, and results. Inclusion criteria were: 1) peer-reviewed original research or secondary analysis of subjects with a cancer diagnosis (omitting review articles and theses), in which the variables were measured at two or more time points;12 2) QOL and sleep-wake disturbance measures that demonstrated an association between sleep–wake disturbance and QOL (head and neck cancers were omitted due to potential obstructive airway issues); and 3) studies written in the English language, limited to those involving quantitative methods.11
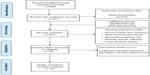  | Figure 1 Flow diagram of the search and review process. |
The review process followed procedures from the PRISMA method modified for narrative analysis. After the search was conducted, manuscripts were appraised by a team of four to reach consensus on applicability and quality. The team included: the first author, an academic nurse educator and sleep researcher; the second author, a nurse educator and advanced practice nurse in oncology; the third author, a PhD student researching sleep in cancer; and the fourth author, a nurse educator and sleep researcher focusing on sleep and fatigue in cancer patients. Any discrepancies were resolved by returning to the original manuscripts and holding discussion to reach consensus. Tables of data were created and validated by consensus. In the synthesis of results and rating of the quality of the interventions, the team assessed the risk of bias, including evaluating each study for recruitment processes, sample size, methods, funding, journal impact, measurement, and study outcomes (effect estimates for intervention studies and confidence intervals for intervention studies when possible). The PRISM checklist was completed to ensure rigor (Table 1).
  | Table 1 PRISMA checklist modified for narrative analysis |
Results
The initial search included 682 manuscripts and a further six were identified from hand searches of reference lists. Eighteen studies met the eligibility criteria, including three longitudinal descriptive and 15 intervention studies, all of which were published in peer-reviewed journals. The reviewed studies included 1,340 cancer patients with a mean age of 55.5 years. The majority were female (88%) with breast cancer (66%) and mixed diagnosis (33%).
Study design: longitudinal descriptive
The three included studies that met the criteria for a descriptive longitudinal design with sleep–wake disturbance and QOL correlations are shown in Table 2. In a recent study by Liu et al,14 QOL and subjective sleep quality were the primary variables in 166 patients undergoing chemotherapy for breast cancer. Liu et al found that poor nocturnal sleep and longer naps were associated with poorer physical QOL, and that the mental component of QOL was associated with the subjective reports of poor sleep but not by objective sleep time. The authors concluded that subjective reports logically concur with mental components and that poor sleep and naptime (longer than 30 minutes) may reflect poor sleep habits that contribute to poor sleep quality.14 This finding suggests that including sleep hygiene information in the interventions to improve sleep in breast cancer patients may ultimately improve QOL. Similarly, Clevenger et al15 studied sleep quality and QOL as primary variables in 173 ovarian cancer patients. Disturbed global sleep was found at all-time points (pre-surgery, at 6 months, and at 1 year) and was associated with depression, pain, sleep medications, and declines in QOL.15 Beck et al16 studied elderly survivors (n=52) with an average age of 72.7 years and reported that fatigue and sleep symptoms occurred together. Fifty-eight percent had poor sleep quality, with significant correlations between sleep quality and physical, mental, and functional QOL.16 These findings demonstrate the importance of developing interventions to improve sleep quality, especially in older adults with a high rate of comorbidities.
These longitudinal studies showed that, when comprehensive sleep measures were implemented in the study design, a clear understanding emerged regarding the influence of poor sleep and sleep behaviors on QOL. Poor sleep quality was moderately associated with poor health-related QOL. Sleep behaviors such as prolonged daytime napping was associated with poor QOL. Such findings suggest the need for interventions that support improvement in sleep and QOL outcomes.
Study design: interventions
Included in this review are 15 studies that tested interventions to evaluate the feasibility and efficacy of improving sleep and QOL (see Table 3). All studies demonstrated an effect of the intervention on sleep and QOL outcomes, although only one study reported an association between sleep and QOL. Interventions included seven cognitive behavioral interventions and eight mind–body interventions (including three meditation, one yoga, one Qigong, one relaxation, one biofeedback, and one exercise).
Cognitive behavioral therapy for insomnia (CBT-I)
Seven interventions in this review utilized CBT-I, which improved sleep efficiency as well as QOL, depression, and fatigue. Of these studies, five were randomized controlled trials (RCTs) (including three pilots) and two were repeated-measures studies. Davidson et al17 tested a group treatment format for 12 patients with insomnia with no control group, utilizing a sleep diary, Sleep Impairment Index, and European Organisation for Research and Treatment of Cancer (EORTC QLQ-C30) to measure sleep disturbance and QOL. There were significant improvements over baseline at weeks 4 and 8 related to sleep efficiency, wake after sleep onset, and total sleep time, with fatigue improving by week 8; however, the QOL role-functioning improvement was not significant. With no comparison group and a small sample size, the study was rated lower in quality of evidence as rated by the review team.
Quesnel et al18 tested an 8-week CBT-I intervention in a small sample (n=10) of breast cancer patients with no control group using Pittsburgh Sleep Quality Index (PSQI) and polysomnography to corroborate with sleep diary data for total wake time and sleep efficiency and found moderate-to-strong effects on global QOL, cognitive QOL, and Insomnia Severity Index. The treatment significantly improved sleep, which was sustained over time (6 months), and reduced depression and fatigue. The small sample size and lack of control influenced the quality; however, high-quality sleep measurement identified moderate-to-strong effect sizes in pre-/posttreatment comparisons.
Savard et al19 tested CBT-I in an RCT of 57 breast cancer patients with insomnia with a waitlist crossover design and reported that the treatment group showed decreased medication use (15.3%) and improved sleep efficiency (by 56%); this group also had decreased anxiety and depression and improved QOL. Measurement included rigorous subjective and objective sleep measures, although there were no reported means and standard deviations by which to calculate effect size. However, results indicated that the CBT-I approach to improving sleep also improved QOL and emotional distress. Savard et al20 more recently tested a 6-week self-help treatment for insomnia (n=11; no control group) using a sleep diary and other measures with moderate-to-large effect sizes pre-/posttreatment on most sleep variables and QOL, suggesting some benefit to using the self-help format; however, the 18% drop out rate is of some concern for applicability.
Ritterband et al21 tested an online CBT-I intervention (n=28) with a waitlist control group and found that the treatment group had reduced insomnia and increased sleep efficiency, although there was no change in QOL; however, the low sample size limited the level of evidence. Dirksen and Epstein22 also tested 10-week CBT-I in an RCT with 72 breast cancer outpatients and found that treatment and control groups improved. The CBT group improved on fatigue, anxiety, depression, and QOL with low-to-moderate effect sizes. The CBT treatment group received a program with stimulus control, sleep restriction, and sleep education that included sleep hygiene, whereas the control groups received only sleep hygiene education. Improvement in both groups may have been a result of the sleep hygiene education. Espie et al23 more rigorously tested CBT-I in an RCT comprising a group of 150 breast and prostate cancer patients and a usual-care control group and found moderate effects in terms of decreased insomnia as well as increased physical and functional QOL. The study utilized both subjective and objective measurements of sleep with actigraphy, sleep diaries, and Epworth Sleepiness Scale.
Overall, the evidence suggests moderate-to-strong effects of CBT-I in reducing insomnia symptoms and improving QOL. Small sample sizes in 42% of the studies and lack of control groups in 42% of the studies limits the strength of the evidence for CBT-I.
Mind–body interventions
Eight studies included in this review involved mind–body interventions. Simeit et al,24 in a study of a German cancer rehabilitation program (n=229), longitudinally tested two groups of cancer patients, implementing sleep management training (cognitive, sleep restriction, and stimulus control) with relaxation training. Patients were identified by the physicians as having sleep problems and self-selected progressive relaxation (n=80) or autogenic training (n=71) with a control of 78 who had no training. There were significant improvements, with moderate-to-strong effects on sleep latency, duration, efficiency, sleep quality, and daytime dysfunction, with improvement in QOL over time. This study showed little difference between relaxation groups; however, improvements reflected the overall value of the rehabilitation program and patient preferences. The sample size was appropriate but lacked randomization.
Mind–body approaches have also been used during cancer treatment. Carlson et al25 tested a mindfulness-based stress reduction intervention in 69 breast and prostate cancer patients. Pre- and posttreatment changes were seen in overall QOL, stress, and sleep quality, but were not correlated with dose (hours of practice). This study also utilized a biologic measure of stress (cortisol), dehydroepiandrosterone sulfate, and melatonin; however, the only change was the change in the levels of dehydroepiandrosterone sulfate throughout the day and QOL improvements associated with decreases in afternoon cortisol levels. This study had no control group for comparison.
Another mind–body approach study was conducted by Dhruva et al26 in a pilot RCT crossover design study (n=16) of pranayama yoga breathing techniques to improve chemotherapy-related symptoms. Increased dose, as measured by hours of practice, was associated with improvements in symptoms (fatigue, sleep disturbance, anxiety, depression, and stress) and mental QOL. The approach was feasible and showed effectiveness, but the small sample size influenced quality.
Chen et al27 conducted an RCT of Qigong, a mind–body practice, in 96 breast cancer patients with an average age of 45 years in China during radiation treatment and found less depressive symptoms over time than the control, less fatigue, and overall improved QOL. No difference in sleep was measured by PSQI and cortisol slopes, yet, unlike in other studies, comorbid insomnia was not an inclusion criterion for the study.
Mind–body techniques have been used in cancer survivors to improve symptoms. Milbury et al28 conducted an RCT of a 6-week program of Tibetan sound meditation (n=47) for cognitive dysfunction and found improved memory, sleep (effect size [ES] 0.23 and at 1 month 0.32), and QOL. Another mind–body approach study by Alvarez et al29 prospectively followed 23 breast cancer patients with cognitive impairment who received electroencephalogram biofeedback to reduce cognitive impairment and were compared to a normative sample. Strong significant improvements on all cognitive measures impacted QOL, fatigue, and sleep scales (sleep quality, daytime dysfunction, global sleep, sleep latency and, sleep disturbance as measured by PSQI). The study by Alvarez et al was the only study in this category to report correlations among the subscales of sleep and impact on QOL; the significant results demonstrated that greater dysfunction in sleep quality correlated with poor performance in QOL.
Nakamura et al30 conducted an RCT (n=57) in cancer survivors with sleep disturbance to test the efficacy of a mind–body bridging and mindfulness meditation intervention compared to sleep hygiene education, and found that both mind–body bridging and mindfulness meditation led to diminished sleep-related symptoms than the sleep hygiene education group, as well as reduced depression and improved mindfulness and well-being.
These nonpharmacologic interventions involved different types of mind–body interventions, but they all involved patient engagement in relaxation or meditative practice, which show some promise for improving multiple symptoms related to QOL.
Exercise interventions have been used to improve sleep and QOL. Wang et al31 tested a 6-week walking program in Taiwan during treatment for breast cancer (n=72) and demonstrated that increased physical activity decreased fatigue and sleep disturbances and improved QOL, although no correlations between sleep and QOL were reported.
Mind–body interventions demonstrated low-to-high effect sizes for sleep and QOL measures. The quality of the study designs was good overall, with 62% having a sample size >50 and 62% utilizing an RCT design.
In the overall summary of the 15 intervention studies that included outcome variables on sleep–wake disturbance and QOL, the issues of sample size (46% had below 50 subjects) and variety in settings and populations, cancer treatments and interventions, sleep and QOL measures, and study designs (40% lacked control group) overall limited the generalizability of the findings. However, effectiveness of the behavioral interventions on sleep and QOL demonstrated the usefulness of these approaches in patients with cancer. While most of these studies did not report correlations between sleep and QOL, they did demonstrate some effectiveness in improving sleep and QOL outcomes.
Measurement of sleep–wake disturbance and QOL
Historically, measurement of sleep–wake disturbance was a single question on a symptom and QOL scales that asked “Do you have trouble sleeping?”; however, as the complexity of sleep disturbance became known,3,12 more comprehensive subjective and objective sleep measures were utilized, as delineated in Table 4. Using both subjective and objective measures is helpful in measuring sleep disturbance, since perceived sleep may not correlate with the actual sleep parameters. Only a few studies25,27 used biologic measures that added insight into the physiological mechanisms, inflammatory responses, and the role of stress influencing sleep. Berger et al3 recommend nine parameters to fully assess sleep: total sleep time; sleep latency; awakenings; wake after sleep onset; napping; sleepiness in daytime; perceived sleep; circadian rhythms; and sleep efficiency.
  | Table 4 Sleep measurement scales used in review sample |
The assessment of QOL measurements as outlined in Table 5 shows that multiple dimensions such as health-related, social, and psychological functions related to well-being were used in the studies. Most of the QOL measures have been validated in the cancer literature and show good reliability and validity. Cancer-specific QOL tools were used in 78% of the studies, including the Functional Assessment of Cancer Therapy (FACT), which was used in 50% of the studies, and FACT gives consideration to treatment and disease-related symptoms.
Discussion
After synthesizing findings from this narrative review, the evidence suggests an interrelationship between increasing sleep–wake disturbances and poorer QOL in cancer patients. Only 2% of the identified literature met the parameters for inclusion, illustrating the dearth of studies that included both sleep–wake disturbances and QOL. While the majority of studies suggested that poor sleep was associated with poorer QOL, only 30% of the reviewed studies reported the actual correlations between sleep and QOL. The majority of the studies involved women with breast cancer, which limits applicability to men and other cancer types. In addition, the studies were heterogeneous, with varied study designs, sample sizes, settings, cancer types and stages, and types of treatment, which limits generalizability.
This review provides a comprehensive assessment of QOL; however, the evaluation of sleep–wake disturbances varied among studies and sometimes included subjective and objective measures as outlined in Tables 4 and 5. In addition, researchers identified multifactorial components influencing sleep–wake disturbances, including anxiety, depression, pain, and fatigue, which have been shown to occur in clusters.6,8,32 Nonetheless, QOL measures offer a way to encompass a more holistic perspective of the outcomes of sleep–wake disturbance that includes social, mental, emotional, and physical measures.
The reviewed interventions demonstrate feasible approaches to improving sleep–wake disturbance in cancer patients that result in improved QOL outcomes, but the evidence is not enough to make a strong recommendation (see Table 6 for a summary of intervention components). In standard CBT-I, five elements are included, ie, cognitive restructuring, stimulus control, sleep restriction, relaxation, and sleep hygiene education. Most CBT-I interventions in the reviewed studies included stimulus control and sleep restriction; however, relaxation was only used in one study. The reviewed studies showed moderate-to-strong effect sizes in reducing insomnia symptoms and improving QOL, although the quality ratings given to the studies ranged from low to high, limiting recommendation. Mind–body interventions included behavioral components such as relaxation/breathing, mindfulness meditation, and movements such as walking and yoga. These studies demonstrate varied effects (from low to high) on improving sleep and QOL, and were given quality ratings ranging from low to high. Similarities between CBT-I and mind–body interventions included behavioral and cognitive processes to reduce stress.
  | Table 6 Intervention components used in studies to improve sleep in cancer patients |
In this review, it was noted that cancer patients’ prolonged daytime napping patterns and shorter nighttime sleep negatively influenced QOL, suggesting congruence with the two-process model, as suggested by Vena et al,11 in evaluating sleep–wake disturbance. The CBT-I component of sleep restriction and sleep hygiene offered some support in reducing napping, supporting the development of sleep pressure, and consolidating sleep at night. The model also infers the importance of circadian rhythms in promoting good sleep. Relatively unstudied is the implication that disrupted circadian rhythms result in decrements in QOL and mortality. It is important to monitor circadian rhythms during treatment, since patients are often encouraged to nap as a result of side effects and increasing fatigue,33 which may ultimately weaken their circadian rhythms and homeostatic sleep drives. This was also evident when patients reported having their nights and days mixed up (Dickerson, unpublished data, 2014). Actigraphy and sleep diaries are effective instruments for measuring this effect. A study tested interventions that strengthen circadian rhythm function are beginning to demonstrate the protective effect of treatments such as bright light therapy during chemotherapy in patients with breast cancer.34 In addition, implementation of future basic sleep hygiene education in patients with cancer regarding the benefits of avoiding excessive daytime napping (>30 minutes) may improve sleep and QOL; however, this requires further validation research in cancer patients.
Implications for the practice setting
From this review, it is evident that sleep disturbance in patients with cancer is problematic and affects their QOL. Cognitive behavioral interventions showed some moderate effectiveness on improving sleep, as did mind–body interventions. Based on this narrative review, the recommendations for practice include:
- Providers routinely including sleep assessments35 as part of the care for patients with cancer, thereby increasing awareness of sleep patterns and potential for disruptions.
- Providers teaching basic sleep hygiene as a standard of care and advising cancer patients (in treatment) to avoid excessive napping (>30 minutes) to avoid circadian rhythm disruption and promote consolidated sleep at night.
- Prescribing CBT-I or mind–body approaches for patients with insomnia.
Since sleep is often not a priority for patients dealing with cancer,2 the onus is on the providers to institute the recommendations into routine clinical practice/care.
Future research
Given the prevalence of sleep–wake disturbance in cancer patients, this review demonstrates the need for research studies to include sleep and QOL instruments that comprehensively assess sleep quality versus the use of a single-item assessment. Use of the subjective and objective measurement tools expands the knowledge of the influence of sleep on QOL. Future research endeavors should also explore the role of circadian rhythms in sleep–wake disturbances, which will facilitate understanding of the effect of sleep disturbance on the multiple components of QOL. Future research should extend the work on the interventions reviewed herein and determine whether these interventions are sustainable and applicable to other cancer diagnoses. Since breast cancer was the main diagnosis in the studies (in eight of the 15 studies), males have been underrepresented. Additionally, intervention studies must be expanded to other cancer diagnostic groups and replicated in larger samples. Overall recommendations are: to establish standard instruments for QOL and sleep that could be used in clinical trials and practice to measure sleep and facilitate meta-studies; to expand the study of sleep and QOL to more populations and ethnic groups; and to further test the interventions in larger trials to improve the level of evidence.
Limitations of this review
In this review, the research team reviewed and cross-validated the results and synthesized the data. However, it is possible that some studies were overlooked by, for example, limiting the search to English-language databases and to studies that had both sleep disturbance and QOL measures. The overall level of evidence was determined to be moderate.
Conclusion
Poor sleep negatively impacts quality of life, as evidenced in the studies included in this review. The intervention studies included nonpharmacologic interventions such as cognitive behavioral treatment and mind–body and exercise interventions, with moderate-to-high levels of evidence for improvement in sleep measures and QOL.
Disclosure
The authors report no conflicts of interest in this work.
References
Moher D, Liberati A, Tetzlaff J, Altman DG; P RISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. | |
Dickerson SS, Sabbah EA, Ziegler P, Chen H, Steinbrenner LM, Dean G. The experience of a diagnosis of advanced lung cancer: sleep is not a priority when living my life. Oncol Nurs Forum. 2012;39:492–499. | |
Berger AM, Parker KP, Young-McCaughan S, et al. Sleep wake disturbances in people with cancer and their caregivers: state of the science. Oncol Nurs Forum. 2005;32:E98–E126. | |
Clark J, Cunningham M, McMillan S, Vena C, Parker K. Sleep-wake disturbances in people with cancer part II: evaluating the evidence for clinical decision making. Oncol Nurs Forum. 2004;31:747–771. | |
Ancoli-Israel S, Moore PJ, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care (Engl). 2001;10:245–255. | |
Dodd MJ, Miaskowski C, Paul SM. Symptom clusters and their effect on the functional status of patients with cancer. Oncol Nurs Forum. 2001;28:465–470. | |
Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Sch Inq Nurs Pract. 2000;14:275–290. | |
Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs. 2010;14:101–110. | |
Dodd MJ, Cho MH, Cooper BA, et al. Identification of latent classes in patients who are receiving biotherapy based on symptom experience and its effect on functional status and quality of life. Oncol Nurs Forum. 2011;38:33–42. | |
Miaskowski C, Cooper B, Paul SM, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13:1172–1187. | |
Vena C, Parker K, Cunningham M, Clark J, McMillan S. Sleep-wake disturbances in people with cancer part I: an overview of sleep, sleep regulation, and effects of disease and treatment. Oncol Nurs Forum. 2004;31:735–746. | |
Berger AM, Sankaranarayanan J, Watanabe-Galloway S. Current methodological approaches to the study of sleep disturbances and quality of life in adults with cancer: a systematic review. Psychooncology. 2007;16:401–420. | |
Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. | |
Liu L, Fiorentino L, Rissling M, et al. Decreased health-related quality of life in women with breast cancer is associated with poor sleep. Behav Sleep Med.2013;11:189–206. | |
Clevenger L, Schrepf A, Degeest K, et al. Sleep disturbance, distress, and quality of life in ovarian cancer patients during the first year after diagnosis. Cancer. 2013;119:3234–3241. | |
Beck SL, Towsley GL, Caserta MS, Lindau K, Dudley WN. Symptom experiences and quality of life of rural and urban older adult cancer survivors. Cancer Nurs. 2009;32:359–369. | |
Davidson JR, Waisberg JL, Brundage MD, MacLean AW. Nonpharmacologic group treatment of insomnia: a preliminary study with cancer survivors. Psychooncology. 2001;10:389–397. | |
Quesnel C, Savard J, Simard S, Ivers H, Morin CM. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J Consult Clin Psychol. 2003;71:189–200. | |
Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23:6083–6096. | |
Savard J, Villa J, Simard S, Ivers H, Morin CM. Feasibility of a self-help treatment for insomnia comorbid with cancer. Psychooncology. 2011;20:1013–1019. | |
Ritterband LM, Bailey ET, Thorndike FP, Lord HR, Farrell-Carnahan L, Baum LD. Initial evaluation of an Internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;21:695–705. | |
Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. J Adv Nurs. 2008;61:664–675. | |
Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26:4651–4658. | |
Simeit R, Deck R, Conta-Marx B. Sleep management training for cancer patients with insomnia. Support Care Cancer. 2004;12:176–183. | |
Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress and levels of cortisol, dehydroepiandrosterone sulfate (DHEAS) and melatonin in breast and prostate cancer outpatients. Psychoneuroendocrinology. 2004;29:448–474. | |
Dhruva A, Miaskowski C, Abrams D, et al. Yoga breathing for cancer chemotherapy-associated symptoms and quality of life: results of a pilot randomized controlled trial. J Altern Complement Med. 2012;18: 473–479. | |
Chen Z, Meng Z, Milbury K, et al. Qigong improves quality of life in women undergoing radiotherapy for breast cancer: results of a randomized controlled trial. Cancer. 2013;119:1690–1698. | |
Milbury K, Chaoul A, Biegler K, et al. Tibetan sound meditation for cognitive dysfunction: results of a randomized controlled pilot trial. Psychooncology. Epub May 9, 2013. | |
Alvarez J, Meyer FL, Granoff DL, Lundy A. The effect of EEG biofeedback on reducing postcancer cognitive impairment. Integr Cancer Ther. 2013;12:475–487. | |
Nakamura Y, Lipschitz DL, Kuhn R, Kinney AY, Donaldson GW. Investigating efficacy of two brief mind-body intervention programs for managing sleep disturbance in cancer survivors: a pilot randomized controlled trial. J Cancer Surviv. 2013;7:165–182. | |
Wang YJ, Boehmke M, Wu YW, Dickerson SS, Fisher N. Effects of a 6-week walking program on Taiwanese women newly diagnosed with early-stage breast cancer. Cancer Nurs. 2011;34:E1–E13. | |
Miaskowski C, Cooper BA, Paul SM, et al. Subgroups of patients with cancer with different symptom experiences and quality-of-life outcomes: a cluster analysis. Oncol Nurs Forum. 2006;33:E79–E89. | |
Fernandes R, Stone P, Andrews P, Morgan R, Sharma S. Comparison between fatigue, sleep disturbance, and circadian rhythm in cancer inpatients and healthy volunteers: evaluation of diagnostic criteria for cancer-related fatigue. J Pain Symptom Manage. 2006;32:245–254. | |
Neikrug AB, Rissling M, Trofimenko V, et al. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behav Sleep Med. 2012;10:202–216. | |
Lee KA, Ward TM. Critical components of a sleep assessment for clinical practice settings. Issues Ment Health Nurs. 2005;26:739–750. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



