Back to Journals » Nature and Science of Sleep » Volume 14
Sleep Quality is an Independent Predictor of Blood Glucose and Gestational Diabetes Mellitus: A Longitudinal Study of 4550 Chinese Women
Authors Chen H , He Y , Zeng X , Chen Q , Zhou N , Yang H , Zhou W , Zhang L , Yang R , Huang Q , Zhang H
Received 14 December 2021
Accepted for publication 24 March 2022
Published 11 April 2022 Volume 2022:14 Pages 609—620
DOI https://doi.org/10.2147/NSS.S353742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ahmed BaHammam
Hongyan Chen,1,* Yang He,2,* Xiaoling Zeng,3,* Qing Chen,3 Niya Zhou,3 Huan Yang,3 Wenzheng Zhou,1 Liwen Zhang,3 Rong Yang,4 Qiao Huang,4 Hua Zhang5
1Quality Management Department, Chongqing Health Center for Women and Children, Chongqing, People’s Republic of China; 2Operating Room, Chongqing Health Center for Women and Children, Chongqing, People’s Republic of China; 3Key Lab of Medical Protection for Electromagnetic Radiation, Ministry of Education of China, Institute of Toxicology, College of Preventive Medicine, Third Military Medical University (Army Medical University), Chongqing, People’s Republic of China; 4Obstetric Outpatient Department, Chongqing Health Center for Women and Children, Chongqing, People’s Republic of China; 5Administration Office, Chongqing Health Center for Women and Children, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hua Zhang, Administration Office, Chongqing Health Center for Women and Children, No. 120, Longshan Road, Yubei District, Chongqing, People’s Republic of China, Email [email protected] Qiao Huang, Obstetric Outpatient Department, Chongqing Health Center for Women and Children, No. 120, Longshan Road, Yubei District, Chongqing, People’s Republic of China, Email [email protected]
Purpose: To investigate whether pregnant women’s subjective sleep quality during the first trimester independently predicted blood glucose and gestational diabetes mellitus (GDM).
Methods: A total of 4550 pregnant women in the first trimester were enrolled in Chongqing Health Center for Women and Children, China, from January to October 2020.The Pittsburgh Sleep Quality Index (PSQI) was used to measure subjective sleep quality. Depression symptoms and anxiety were measured with the Patient Health Questionnaire-9 (PHQ-9) and General Anxiety Disorder-7 (GAD-7). Oral glucose tolerance tests (OGTT) and blood glucose area under the curve (AUC) were used for estimation of blood glucose and diagnosis of GDM during the second trimester. Linear, mixed model, and logistic regression were used to analyze the association between PSQI and blood glucose as well as GDM.
Results: 946/4550 were diagnosed with GDM (20.8%). In the mixed model analysis, the blood glucose level of the highest-scoring group (PSQI score = 18) was 1.94 (95% CI: 0.45∼ 3.43, P = 0.011) mmol/L higher than that of the lowest-scoring group (PSQI score = 0). After adjusting for potential confounders, a one-point PSQI score increase was associated with a 0.014 (95% CI: 0.001∼ 0.027, P = 0.039) mmol/L increase in blood glucose level. Blood glucose AUC was also positively associated with PSQI scores (β = 0.034, 95% CI: 0.003∼ 0.064, P = 0.030). The results for the logistic regression model showed that PSQI was marginal positively correlated with GDM (OR = 1.146, 95% CI: 0.995∼ 1.321, P = 0.059) when age and BMI were not controlled for. When investigating the association between PSQI and the GDM-diagnosed time window, the 1-h diagnosed GDM had a borderline positive correlation with PSQI (OR = 1.182, 95% CI: 0.993∼ 1.405, P = 0.060).
Conclusion: Sleep quality during the first trimester may be a risk factor for elevated blood glucose and GDM later in gestation.
Keywords: gestational diabetes mellitus, PSQI, blood glucose AUC, sleep disturbance
Introduction
Gestational diabetes mellitus (GDM) is a form of glucose intolerance of varying severity that occurs or is first detected during pregnancy. As one of the most common pregnancy complications, GDM can adversely affect maternal and neonatal health.1–3 It can increase the risk for obesity, type 2 diabetes mellitus (T2DM), and cardiovascular disease of mothers, and the risk of preterm birth, intrauterine growth delay, neonatal hypoglycemia, T2DM, obesity, and metabolic syndrome of the offspring.4–6 According to the International Diabetes Federation, the global incidence of GDM is approximately 14%, ranging from 9% in Africa and 12.6% in North America to 21% in Asia in 2017.7 And the incidence of GDM in China has risen sharply due to economic development and improvements in living standards, coupled with changed dietary patterns, physical inactivity, and increasing emphasis on GDM screening.8
Sleep disturbances are associated with a myriad of health problems, including neuromuscular disease, depression, and anxiety.9–12 Both epidemiological studies and animal experiments have confirmed that chronic sleep deprivation is a risk factor for metabolism-related diseases, including impaired glucose regulation and diabetes.13 Studies have shown that poor sleep quality is associated with the development of T2DM.14 And people with self-reported good sleep quality had significantly lower HbA1c levels than those who reported poor sleep quality among patients with type 1 diabetes mellitus (T1DM).15 Compared with the general population, pregnant women are more likely to suffer from sleep disturbances due to endocrine and physical changes during pregnancy.16,17 Recently, there have been several studies concerning whether sleep disturbances during pregnancy lead to hyperglycemia and GDM,18–21 but the results are not consistent. A study with 38 pregnant and 22 non-pregnant women recruited in the United States found that poor sleep quality of pregnant women was positively correlated with higher level of HbA1c, which reflected a higher average blood glucose concentration.22 Another case-control study in the United States (n = 125) found that sleep disturbance was associated with increased risk of GDM (OR = 4.71, 95% CI: 1.05~21.04).20 A prospective study (n = 542) in China also showed that sleep deficiency in early pregnancy was a risk factor for the occurrence of GDM (OR = 7.38, 95% CI: 2.25~24.17).21 However, some studies did not observe an association between sleep quality and GDM risk when monitoring the daily sleep of pregnant women.21,23 Notably, most of these previous studies were cross-sectional or had case-control designs, and thus weak causal inference strength and recall bias were inevitable Previous studies have shown that the prevalence of insulin resistance and diabetes mellitus varies with ethnicity.24 These findings have been reported in both T1DM and T2DM studies.15,25 Given the differences in diabetes susceptibility, therefore, studies of the association between sleep disturbance and blood glucose in other populations are necessary. The present study aims to explore the relationship between first-trimester sleep quality and second-trimester blood glucose and GDM risk based on a longitudinal design with a large sample size of 4550 women in China.
Materials and Methods
Study Population
This longitudinal study was conducted in Chongqing Health Center for Women and Children, China between January 2020 and October 2020. The study participants were recruited from among the women who came to the hospital for their first antenatal care visit. The recruiting criteria were as following: Chinese women aged 18–45 years old; during the first trimester (8–13 weeks of gestation). Those who had any of the following conditions were excluded: diagnosed with T1DM or T2DM or previous GDM during or before the first trimester; had a family history of diabetes; did not complete the oral glucose tolerance test (OGTT) in the health center. A total of 4550 pregnant women were enrolled in the study.
Assessment of Sleep Quality
The Pittsburgh Sleep Quality Index (PSQI) is a self-reported questionnaire evaluating sleep disturbances. The Chinese version of the PSQI has been determined to have good reliability (r = 0.82–0.83). It can be a sensitive, reliable, and valid outcome assessment tool.26 Seven underlying dimensions of sleep were assessed: (i) subjective sleep quality (very good to very bad), (ii) sleep latency (≤ 15 minutes to > 60 minutes), (iii) sleep duration (≥ 7 hours to < 5 hours), (iv) sleep efficiency (≥ 85% to < 65% hours sleep/hours in bed), (v) sleep disturbances (not during the past month to ≥ 3 times per week), (vi) use of sleep medications (none to ≥ 3 times a week), and (vii) daytime dysfunction (not a problem to a very big problem).27 The sum of all dimension scores is the global score of PSQI, which ranges from 0 to 21. A global PSQI score > 5 provided a sensitive and specific measure of poor sleep quality, and higher scores indicate lower sleep quality and more sleep disturbances.28 In the present study, the PSQI global score was divided into four grades: a score of 0–5 indicates very good sleep; 6–10 indicates good sleep; 11–15 indicates fair sleep, and 16–21 indicates very poor sleep.29
Measurement of Blood Glucose and Diagnosis of GDM
The blood glucose of the participants was measured by OGTT test with the glucose oxidase method (Hitachi 7600-110 Automatic biochemical analyzer, Japan) at 24–28 weeks of gestation. After 8–12h fasting, venous blood was collected from the participants between 7:00 and 9:00 AM for the measurement of blood glucose. After the blood collection, 75g glucose was taken orally, and venous blood collection was conducted again 1 hour post and 2 hours post, respectively. The diagnosis of GDM was made based on the criteria of the International Association of Diabetes and Pregnancy Study Group30 when any of the following conditions was met: fasting glucose ≥ 5.1 mmol/L (92 mg/dL), or 1-h post glucose ≥ 10.0 mmol/L (180 mg/dL), or 2-h post glucose ≥ 8.5 mmol/L (153 mg/dL).The blood glucose area under the curve (AUC), an effective indicator to reflect the comprehensive level of fasting, 1-h post and 2-h post blood glucose,31 was also calculated for the participants.
Measurement of Demographics, Gravidity and Other Potential Risk Factors
As a routine clinical process, the following information was recorded in the Maternity Information Management System of the hospital: age, pre-pregnancy body mass index (BMI), gravidity, tobacco smoking, alcohol consumption. Pre-pregnancy BMI was categorized according to the World Health Organization’s cut-off points: low weight (BMI < 18.5 kg/m2) normal weight (BMI < 18.5 kg/m2, BMI ≥ 18.5 and < 25.0 kg/m2), overweight (BMI ≥ 25.0 and < 30.0 kg/m2), and obesity (BMI ≥ 30.0 kg/m2). Additionally, the psychological status of the pregnant women was routinely estimated with the Patient Health Questionnaire-9 (PHQ-9) and General Anxiety Disorder-7 (GAD-7). The PHQ-9 is a nine-item scale designed and validated for the diagnosis of depression and assessment of its severity.32 The GAD-7 is used to screen anxiety status and assess its severity.33
Statistical Analysis
Continuous variables were expressed as mean ± SD (standard deviation), and categorical variables were expressed as frequency (n) and percentage (%). For the continuous variables in this paper, the Shapiro–Wilk test and histograms were used to assess normality. Chi-square tests or t-tests were used for univariate analysis. Mixed model regression was used to analyze the association between PSQI score and OGTT blood glucose concentrations. Multiple linear regression was used to analyze the association between PSQI score and blood glucose AUC. A multivariate logistic regression model was developed to analyze the association between PSQI grade and GDM risk. Moreover, the associations between PSQI grade and GDM risk confirmed only by fasting blood glucose, 1-h post or 2-h post were analyzed, respectively. In the multivariate analyses, the following potential confounders were controlled: age, pre-pregnancy BMI, gravidity, parity, PHQ-9 score, GAD-7 score, tobacco smoking, and alcohol consumption. The confounders were selected according to literature review and expert knowledge.34,35 Missing values accounted for no more than 1.6% in a small number of variables. The missing values were replaced with the median of the related variables. The statistical analyses were performed with SPSS version 25.0 and R version 4.1.1. P-values of < 0.05 (two-tailed) were considered statistically significant.
Results
The demographic characteristics of the participants are shown in Table 1. Of the 4550 participants recruited in the first trimester, 61 (1.3%) women have the lowest score (PSQI score = 0), 2 women have the highest score (PSQI score = 18), 2449 (53.8%) people reported very good sleep quality, 1829 (40.2%) reported good sleep, 260 (5.7%) reported fair sleep and 12 (0.3%) reported poor sleep. In the second trimester, 946 (20.8%) of the participants were diagnosed with GDM. Among the 4550 women, the average fasting blood glucose was 4.46 ± 0.01mmol/L, the average 1-h post glucose was 7.99 ± 0.03mmol/L, and the average 2-h post glucose was 6.88 ± 0.02mmol/L. The maximum blood glucose AUC value was 28.00 mmol/(L·h), the minimum was 7.50 mmol/(L·h), and the average was 13.66 ± 0.04 mmol/(L·h). There were 121 (2.7%) women who had blood glucose concentrations above the standard at three time points, and 568 (12.5%) women had blood glucose concentrations above the standard at two time points, 297 (6.5%) women with fasting glucose ≥ 5.1 mmol/L (92 mg/dL), 597 (13.1%) women with 1-h post glucose ≥ 10.0 mmol/L (180 mg/dL), and 620 (13.6%) women with 2-h post glucose ≥ 8.5mmol/L (153 mg/dL).
 |
Table 1 General Characteristics of Pregnant Women with GDM and Non-GDM |
Compared with the non-GDM group, the GDM group had a higher proportion of women with advanced age (≥ 35 years) (16.4% versus 7.0%, χ2 = 163.16, P < 0.001), with pre-pregnancy BMI ≥ 25 kg/cm2 (15.4% versus 6.8%, χ2 = 95.81, P < 0.001), multipara (34.6% versus 27.9%, χ2 = 18.68, P < 0.001). No significant difference was observed in smoking and drinking consumption history. Frequency and percentage of scores for the PSQI, PHQ-9 and GAD-7 scales are shown in Table 1.
In the mixed model, the three time-specific blood glucose values of OGTT were integrated to analyze the association between blood glucose and time-specific PSQI scores, and the results are shown in Figure 1. The blood glucose concentration of the group with the highest score (PSQI score = 18) was 1.938 (95% CI: 0.447~3.428, P = 0.011) mmol/L higher than that of the group with the lowest (PSQI score = 0). In the univariate model, there was no significant correlation between PSQI and blood glucose concentrations (β = −0.003, 95% CI: −0.014~0.007, P = 0.533). However, after adjusting for age, pre-pregnancy BMI, gravidity, parity, PHQ-9 score, GAD-7 score, tobacco smoking, and alcohol consumption history, a one-point increase in PSQI scores was associated with a 0.014 (95% CI: 0.001~0.027, P = 0.039) mmol/L increase in blood glucose. According to the results for the fixed effects, we found that age, pre-pregnancy BMI and PHQ-9 score had the greatest effect on the results. When we did not adjust for age and pre-pregnancy BMI, two factors that were known to have an effect on the occurrence of GDM, the result was 0.023 (95% CI: 0.009~0.037, P = 0.001). The results of linear regression analysis of PSQI score and blood glucose AUC also showed that there was a significant positive correlation between PSQI score and blood glucose AUC after adjusting potential variables (β = 0.034, 95% CI: 0.003~0.064, P = 0.030) (Table 2). We also investigated how was PSQI associated with fasting, 1-h post and 2-h post blood glucose level. We found that 1-h post blood glucose level and 2-h post blood glucose level were significantly associated with PSQI. But the association between fasting blood glucose and PSQI was not observed. Only marginal positivity was observed in the correlation between fasting blood glucose level and PSQI in the univariate model (Table S1).
 |
Table 2 Multiple Linear Regression Analysis for PSQI and Blood Glucose AUC |
Logistic regression analysis was used to analyze the relationship between the grade of PSQI scores and GDM; the results are shown in the Table 3. When all the variables included in this study were controlled for, there was only a slightly but not significantly increased risk (OR = 1.087, 95% CI: 0.939~1.258, P = 0.265). When age and pre-pregnancy BMI were not controlled for, the association of PSQI grade and GDM became marginally significant (OR = 1.146, 95% CI: 0.995~1.321, P = 0.059). When investigating the association between PSQI grade and the GDM-diagnosed time window, the 1-h diagnosed GDM was found to have a borderline positive correlation with PSQI (OR = 1.182, 95% CI: 0.993~1.405, P = 0.060) when potential confounders were controlled for, while fasting and 2-h diagnosed GDM were not associated with a significant increase in risk (OR = 1.077, 95% CI: 0.845~1.371, P = 0.549 and OR = 1.065, 95% CI: 0.897~1.266, P = 0.472, respectively).
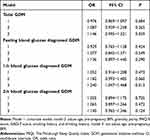 |
Table 3 Multiple Logistic Regression Analysis for PSQI and GDM |
We then analyzed the association between PSQI and blood glucose as well as GDM in subgroups with different ages, obesity levels, pregnancy statuses and different populations. The results are shown in Table 4. The participants were divided into women with age < 35 years (4144/91.1%) versus age ≥ 35 years (406/8.9%) or pre-pregnancy BMI < 25 (4160/91.5%) versus pre-pregnancy BMI ≥ 25 (386/8.5%) or singleton pregnancy (4452/98.8%) versus multiple pregnancies (98/2.2%) or GDM (946/20.8%) versus non-GDM (3604/79.2%). For the younger (< 35 years) and the non-overweight (BMI < 25) women, those with singleton pregnancies, and those that did not have GDM, there were positive correlations with PSQI score and blood glucose concentration (younger: β = 0.013, 95% CI: −0.001~0.027, P = 0.061; singleton pregnancy: β = 0.014, 95% CI: 0.000~0.027, P = 0.045; non-GDM: β = 0.013, 95% CI: 0.002~0.025, P = 0.023); and blood glucose AUC (younger: β = 0.030, 95% CI: −0.001~0.062, P = 0.058; non-overweight: β = 0.028, 95% CI: −0.003~0.060, P = 0.077; singleton pregnancy: β = 0.035, 95% CI: 0.004~0.066, P = 0.025; non-GDM: β = 0.032, 95% CI: 0.008~0.056, P = 0.010); and 1-h blood glucose (younger: β = 0.031, 95% CI: 0.008~0.055, P = 0.009; non-overweight: β = 0.030, 95% CI: 0.006~0.053, P = 0.014; singleton pregnancy: β = 0.035, 95% CI: 0.012~0.059, P = 0.003; non-GDM: β = 0.024, 95% CI: 0.005~0.043, P = 0.016); and 2-h blood glucose (younger: β = 0.019, 95% CI: −0.001~0.039, P = 0.058; non-overweight: β = 0.023, 95% CI: 0.003~0.042, P = 0.027; singleton pregnancy: β = 0.024, 95% CI: 0.004~0.044, P = 0.017; non-GDM: β = 0.015, 95% CI: 0.001~0.030, P = 0.041). But non-significant in fasting blood glucose. The associations between PSQI grade and GDM were not significant in any of the subgroups (younger: OR = 1.070, 95% CI: 0.912~1.254, P = 0.407; elder: OR = 1.162, 95% CI: 0.794~1.700, P = 0.441; non-overweight: OR = 1.047, 95% CI: 0.896~1.224, P = 0.563; overweight: OR = 1.385, 95% CI: 0.899~2.134, P = 0.140; singleton pregnancy: OR = 1.088, 95% CI: 0.938~1.263, P = 0.263; multiple pregnancies: OR = 1.055, 95% CI: 0.382~2.916, P = 0.918). When the association between 1-h post blood glucose diagnosed GDM and PSQI grade in the six sub-groups was investigated, there were significant positive associations for elder (≥ 35 years) women and women with singleton pregnancies (elder: OR = 1.544, 95% CI:1.022~2.335, P = 0.039; singleton pregnancy: OR = 1.205, 95% CI:1.011~1.437, P = 0.037).
 |
Table 4 Sensitivity Analysis: Potential Effect Modification for the Impact of PSQI Score in First Trimester on GDM |
Discussion
Based on a longitudinal design with large sample size in Chinese women, the present study explored the association between sleep quality (by PSQI) in the first trimester and blood glucose concentrations and GDM risk in the second trimester in Chinese women. After adjusting for relevant confounders, PSQI was positively associated with elevated blood glucose and a suggestive increased risk of GDM.
Several studies have investigated the association between sleep quality and GDM, but the findings have not been consistent. Some studies have found no significant association between sleep quality and GDM,36–38 and only two of them used the PSQI scale to evaluate sleep quality.36,37 In a Swiss cross-sectional study, 203 pregnant women were recruited at 24–30 weeks into their pregnancy and, as in our study, classified sleep quality in four categories (very good, fairly good, fairly bad and very bad). However, they did not exclude participants with a family history of diabetes or a previous history of GDM.36 In a study with 209 participants in India, the association between PSQI and GDM was analyzed by using the frequency of PSQI score > 5 among the results of enrollment/first/second/third trimester during pregnancy. They did not examine the relationship between sleep quality in the first trimester alone and the occurrence of GDM in detail.37 There were also several studies that suggested a link between sleep disturbance and GDM.25,39,40 Among these three studies, two used the PSQI for evaluating sleep quality,39,40 and one study adopted self-designed single choice questionnaires.25 All the studies ultimately confirmed GDM by OGTT test with the same diagnostic criteria, but in one of them, participants underwent a round of 50-g glucose challenge test screening first.40 The OGTT test was performed only when the first blood glucose test result was higher than 7.8mmol/L. Only one study investigated the association between sleep quality in the first trimester and GDM diagnosed in the second trimester.39 However, they did not specify the gestational age for the early pregnancy. Two studies excluded women with multiple pregnancies.25,40 In the present study, not only was the relationship between sleep quality and GDM studied, but also the relationship between sleep quality and blood glucose or the blood glucose AUC. Sensitivity analyses were also conducted to explore the influence of other possible risk factors. The association between PSQI and blood glucose level was significantly positive, especially for postprandial blood glucose. However, only a slightly increased but non-significant risk for GDM was observed. The results of this large-scale Chinese population study may greatly help with investigating the association between sleep quality and GDM.
In univariate analysis of PSQI and blood glucose concentration, no significant correlation was observed, but positive results emerged after variable adjustment. We found that age, pre-pregnancy BMI and PHQ-9 score had a significant effect on the results, but the analysis showed that there was no statistically significant correlation between PSQI score and age or pre-pregnancy BMI, so these two factors could not be simply assumed to be confounders. However, PHQ-9 score was found to be strongly correlated with PSQI score and blood glucose concentration, so it was a clear confounder. In the research on PSQI score and GDM, an increased significant association of risk was observed if not age and pre-pregnancy BMI were not adjusted for. So, we speculated that the correlation between GDM and PSQI score was weak, and once age and pre-pregnancy BMI, two variables strongly correlated with GDM, were controlled for, the effect of PSQI score on GDM would no longer appear. The results for time-specific diagnosed GDM might be due to the moderate or the inconsistent effect of PSQI on blood glucose at different time points. We found that PSQI was associated with 1-h diagnosed GDM but not with the GDM diagnosed at the other two time points. The reliability of these results needs to be corroborated in future research.
Pregnant women often have poor sleep quality and short sleep duration due to hormonal changes, physical discomfort, and psychological stress.41,42 Increased muscle tone, increased excitability of nerve cells, dyspnea, increased nocturia frequency, and excessive psychological stress throughout pregnancy all disturb pregnant women’s sleep.43–45 Previous studies have found that sleep disturbances of pregnant women can commence with the onset of pregnancy in the first trimester, and sleep quality gradually decreases during the remainder of the pregnancy.46,47 Mechanism-related animal experiments and limited epidemiological research has observed that poor sleep quality might trigger a series of pathophysiological responses resulting in increased insulin resistance, glucose intolerance, and eventually GDM.25,47–49 Possible pathways included that the sleep disturbance may lead to hyperactivation of the hypothalamic-pituitary-adrenal axis, thereby increasing the production of glucocorticoid cortisol, which was involved in the occurrence and progression of insulin resistance. Sleep disturbance may also cause the level of circulating inflammatory mediators, such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α), to increase, leading to increased inflammatory response,13,21,40 then decreasing insulin sensitivity and downstream insulin signaling.45 Another possible pathway was Inducing oxidative stress which can decrease glucose transporter type 4 (GLUT4) expression by impairing nuclear proteins to the insulin responsive element in the GLUT4 promoter or cause inflammatory response.50
Strengths and Limitations
In this large-scale prospective observational study, probability of selection bias was reduced by including pregnant women all who came to our hospital for regular antenatal examinations and got OGTT results. The longitudinal study design is a robust means of clarifying the temporal relationship between sleep quality and the sequential occurrence of GDM. The present study used the PSQI questionnaire, the reliability and validity of which have been verified in the Chinese population, to evaluate sleep quality rather than a self-made questionnaire or simple questions. The present study also has several major limitations. With the progress of pregnancy, the sleep quality of pregnant women may become poorer, while our study only analyzed sleep quality during the first trimester. Moreover, we did not collect information on weight gain during pregnancy, diet, exercise, napping, and macrosomia history, so the influence of these confounders on sleep quality or GDM may not be controlled. In addition, although PSQI can better reflect the subjective sleep quality of pregnant women, it can be combined with objective measurement methods to obtain more comprehensive results in further studies.
Summary
The present study found that unfavorable PSQI status in the first trimester was associated with increased blood glucose concentrations and blood glucose AUC in the subsequent trimester. The risk of GDM, especially the 1-h diagnosed GDM, was significantly associated with PSQI score. These results suggest that more attention should be paid to the sleep quality of pregnant women in the first trimester. Screening with PSQI and related interventions may help to prevent GDM risk.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author based on reasonable needs. The data are not publicly available due to privacy or ethical restrictions.
Ethics Approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study had been approved by the Ethics Committee of Chongqing Health Center for Women and Children (No.2018-20-2). Informed consent was obtained from all the participants.
Acknowledgments
This study was supported by Chongqing Science and Technology Bureau Project (cstc2018jscx-mszdX0021) and Key Program of National Science Foundation (82130097). We also thank all the pregnant women and staff who participated in the project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–S31.
2. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi:10.1136/bmj.m1361
3. Lowe WL
4. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16(1):7. doi:10.1007/s11892-015-0699-x
5. Silva-Zolezzi I, Samuel TM, Spieldenner J. Maternal nutrition: opportunities in the prevention of gestational diabetes. Nutr Rev. 2017;75(suppl 1):32–50. doi:10.1093/nutrit/nuw033
6. Bianco ME, Josefson JL. Hyperglycemia during pregnancy and long-term offspring outcomes. Curr Diab Rep. 2019;19(12):143. doi:10.1007/s11892-019-1267-6
7. Liu A. Sleep Training. Pediatr Ann. 2020;49(3):e101–e105. doi:10.3928/19382359-20200218-01
8. Juan J, Yang H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health. 2020;17(24):9517. doi:10.3390/ijerph17249517
9. Kloss JD, Perlis ML, Zamzow JA, Culnan EJ, Gracia CR. Sleep, sleep disturbance, and fertility in women. Sleep Med Rev. 2015;22:78–87. doi:10.1016/j.smrv.2014.10.005
10. Tang Y, Zhang J, Dai F, et al. Poor sleep is associated with higher blood pressure and uterine artery pulsatility index in pregnancy: a prospective cohort study. BJOG. 2021;128(7):1192–1199. doi:10.1111/1471-0528.16591
11. Shibata R, Murohara T. [Sleep disorder and lifestyle-related disease]. Nihon Rinsho. 2015;73(6):1046–1048. Japanese.
12. Nicolle MW. Sleep and neuromuscular disease. Semin Neurol. 2009;29(4):429–437. doi:10.1055/s-0029-1237119
13. Myoga M, Tsuji M, Tanaka R, et al. Impact of sleep duration during pregnancy on the risk of gestational diabetes in the Japan environmental and Children’s study (JECS). BMC Pregnancy Childbirth. 2019;19(1):483. doi:10.1186/s12884-019-2632-9
14. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi:10.2337/dc09-1124
15. Reutrakul S, Thakkinstian A, Anothaisintawee T, et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep Med. 2016;23:26–45. doi:10.1016/j.sleep.2016.03.019
16. Wang L, Jin F. Association between maternal sleep duration and quality, and the risk of preterm birth: a systematic review and meta-analysis of observational studies. BMC Pregnancy Childbirth. 2020;20(1):125. doi:10.1186/s12884-020-2814-5
17. Herring SJ, Nelson DB, Pien GW, et al. Objectively measured sleep duration and hyperglycemia in pregnancy. Sleep Med. 2014;15(1):51–55. doi:10.1016/j.sleep.2013.07.018
18. Twedt R, Bradley M, Deiseroth D, Althouse A, Facco F. Sleep duration and blood glucose control in women with gestational diabetes mellitus. Obstet Gynecol. 2015;126(2):326–331. doi:10.1097/AOG.0000000000000959
19. Reutrakul S, Zaidi N, Wroblewski K, et al. Sleep disturbances and their relationship to glucose tolerance in pregnancy. Diabetes Care. 2011;34(11):2454–2457. doi:10.2337/dc11-0780
20. Izci Balserak B, Pien GW, Prasad B, et al. Obstructive sleep apnea is associated with newly diagnosed gestational diabetes mellitus. Ann Am Thorac Soc. 2020;17(6):754–761. doi:10.1513/AnnalsATS.201906-473OC
21. Zhou FM, Yang LQ, Zhao RP, et al. [Effect of sleep in early pregnancy on gestational diabetes: a prospective study]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2016;47(6):964–968. Chinese.
22. Chirwa S, Nwabuisi CR, Ladson GM, et al. Poor sleep quality is associated with higher hemoglobin A1c in pregnant women: a pilot observational study. Int J Environ Res Public Health. 2018;15(10):2287. doi:10.3390/ijerph15102287
23. Facco FL, Grobman WA, Reid KJ, et al. Objectively measured short sleep duration and later sleep midpoint in pregnancy are associated with a higher risk of gestational diabetes. Am J Obstet Gynecol. 2017;217(4):447e441–447 e413. doi:10.1016/j.ajog.2017.05.066
24. Yano Y, Gao Y, Johnson DA, et al. Sleep characteristics and measures of glucose metabolism in blacks: the Jackson heart study. J Am Heart Assoc. 2020;9(9):e013209. doi:10.1161/JAHA.119.013209
25. Zhong C, Chen R, Zhou X, et al. Poor sleep during early pregnancy increases subsequent risk of gestational diabetes mellitus. Sleep Med. 2018;46:20–25. doi:10.1016/j.sleep.2018.02.014
26. Tsai PS, Wang SY, Wang MY, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res. 2005;14(8):1943–1952. doi:10.1007/s11136-005-4346-x
27. Jurado-Fasoli L, Mochon-Benguigui S, Castillo MJ, Amaro-Gahete FJ. Association between sleep quality and time with energy metabolism in sedentary adults. Sci Rep. 2020;10(1):4598. doi:10.1038/s41598-020-61493-2
28. Tomfohr LM, Buliga E, Letourneau NL, Campbell TS, Giesbrecht GF. Trajectories of sleep quality and associations with mood during the perinatal period. Sleep. 2015;38(8):1237–1245. doi:10.5665/sleep.4900
29. Tian R, Bai Y, Guo Y, Ye P, Luo Y. Association between sleep disorders and cognitive impairment in middle age and older adult hemodialysis patients: a cross-sectional study. Front Aging Neurosci. 2021;13:757453. doi:10.3389/fnagi.2021.757453
30. Waters TP, Dyer AR, Scholtens DM, et al. Maternal and neonatal morbidity for women who would be added to the diagnosis of GDM using IADPSG criteria: a secondary analysis of the hyperglycemia and adverse pregnancy outcome study. Diabetes Care. 2016;39(12):2204–2210. doi:10.2337/dc16-1194
31. Khan SH, Manzoor R, Baig AH, Sobia F, Fazal N, Niazi NK. Glucose tolerance versus HbA1c results as depictive of gestational diabetes mellitus. J Coll Physicians Surg Pak. 2019;29(4):333–336. doi:10.29271/jcpsp.2019.04.333
32. Wang W, Bian Q, Zhao Y, et al. Reliability and validity of the Chinese version of the Patient Health Questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. 2014;36(5):539–544. doi:10.1016/j.genhosppsych.2014.05.021
33. Sousa TV, Viveiros V, Chai MV, et al. Reliability and validity of the Portuguese version of the Generalized Anxiety Disorder (GAD-7) scale. Health Qual Life Outcomes. 2015;13(1):50. doi:10.1186/s12955-015-0244-2
34. Zhang C, Rawal S, Chong YS. Risk factors for gestational diabetes: is prevention possible? Diabetologia. 2016;59(7):1385–1390. doi:10.1007/s00125-016-3979-3
35. Lewandowska M. Gestational Diabetes Mellitus (GDM) risk for declared family history of diabetes, in combination with BMI categories. Int J Environ Res Public Health. 2021;18(13):13. doi:10.3390/ijerph18136936
36. Horsch A, Kang JS, Vial Y, et al. Stress exposure and psychological stress responses are related to glucose concentrations during pregnancy. Br J Health Psychol. 2016;21(3):712–729. doi:10.1111/bjhp.12197
37. Sharma SK, Nehra A, Sinha S, et al. Sleep disorders in pregnancy and their association with pregnancy outcomes: a prospective observational study. Sleep Breath. 2016;20(1):87–93. doi:10.1007/s11325-015-1188-9
38. Xu X, Liu Y, Liu D, et al. Prevalence and determinants of gestational diabetes mellitus: a cross-sectional study in China. Int J Environ Res Public Health. 2017;14(12):1532. doi:10.3390/ijerph14121532
39. Wang W, Meng H, Liu Y, et al. Effects of sleep duration and sleep quality in early pregnancy and their interaction on gestational diabetes mellitus. Sleep Breath. 2022;26(1):489–496. doi:10.1007/s11325-021-02391-3
40. Wang H, Leng J, Li W, et al. Sleep duration and quality, and risk of gestational diabetes mellitus in pregnant Chinese women. Diabet Med. 2017;34(1):44–50. doi:10.1111/dme.13155
41. Lu Q, Zhang X, Wang Y, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi:10.1016/j.smrv.2021.101436
42. Rawal S, Hinkle SN, Zhu Y, Albert PS, Zhang C. A longitudinal study of sleep duration in pregnancy and subsequent risk of gestational diabetes: findings from a prospective, multiracial cohort. Am J Obstet Gynecol. 2017;216(4):399e391–399 e398. doi:10.1016/j.ajog.2016.11.1051
43. Soares CN, Murray BJ. Sleep disorders in women: clinical evidence and treatment strategies. Psychiatr Clin North Am. 2006;29(4):1095–1113. doi:10.1016/j.psc.2006.09.002
44. Yang Y, Mao J, Ye Z, et al. Determinants of sleep quality among pregnant women in China: a cross-sectional survey. J Matern Fetal Neonatal Med. 2018;31(22):2980–2985. doi:10.1080/14767058.2017.1359831
45. Conlon RPK, Wang B, Germeroth LJ, Cheng Y, Buysse DJ, Levine MD. Demographic, pregnancy-related, and health-related factors in association with changes in sleep among pregnant women with overweight or obesity. Int J Behav Med. 2021;28(2):200–206. doi:10.1007/s12529-020-09887-4
46. Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95(1):14–18. doi:10.1016/s0029-7844(99)00486-x
47. Izci-Balserak B, Pien GW. The relationship and potential mechanistic pathways between sleep disturbances and maternal hyperglycemia. Curr Diab Rep. 2014;14(2):459. doi:10.1007/s11892-013-0459-8
48. de Fraissinette A, Dezutter-Dambuyant C, Guyotat D, Schmitt D. High level of CD1a putative peripheral blood precursors of epidermal Langerhans cells after bone marrow transplantation. Thymus. 1991;18(2):129–132.
49. Ferrari U, Kunzel H, Trondle K, et al. Poor sleep quality is associated with impaired glucose tolerance in women after gestational diabetes. J Psychiatr Res. 2015;65:166–171. doi:10.1016/j.jpsychires.2015.02.012
50. Zhang H, Wang Q, He S, et al. Ambient air pollution and gestational diabetes mellitus: a review of evidence from biological mechanisms to population epidemiology. Sci Total Environ. 2020;719:137349. doi:10.1016/j.scitotenv.2020.137349
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

