Back to Journals » Journal of Pain Research » Volume 16
Sleep Disturbance in Musculoskeletal Conditions: Impact of a Digital Care Program
Authors Scheer JK, Costa F , Janela D , Molinos M, Areias AC , Moulder RG, Lains J , Bento V, Yanamadala V, Correia FD
Received 22 October 2022
Accepted for publication 16 December 2022
Published 5 January 2023 Volume 2023:16 Pages 33—46
DOI https://doi.org/10.2147/JPR.S394421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jonathan Greenberg
Justin K Scheer,1 Fabíola Costa,2 Dora Janela,2 Maria Molinos,2 Anabela C Areias,2 Robert G Moulder,3 Jorge Lains,4,5 Virgílio Bento,2 Vijay Yanamadala,2,6,7 Fernando Dias Correia2,8
1Department of Neurological Surgery, University of California, San Francisco, CA, USA; 2Sword Health, Inc, Draper, UT, USA; 3Institute for Cognitive Science, University of Colorado Boulder, Boulder, CO, USA; 4Rovisco Pais Medical and Rehabilitation Centre, Tocha, Portugal; 5Faculty of Medicine, Coimbra University, Coimbra, Portugal; 6Department of Surgery, Quinnipiac University Frank H. Netter School of Medicine, Hamden, CT, USA; 7Department of Neurosurgery, Hartford Healthcare Medical Group, Westport, CT, USA; 8Neurology Department, Centro Hospitalar e Universitário do Porto, Porto, Portugal
Correspondence: Fernando Dias Correia, Sword Health Inc, 13937 Sprague Lane Ste 100, Draper, UT, 84020, USA, Tel +1 385-308-8034, Fax +1 801-206-3433, Email [email protected]
Background: Musculoskeletal (MSK) pain is highly prevalent worldwide, resulting in significant disability, and comorbid sleep disturbances. Digital therapy for MSK pain can provide significant improvements in care access, alongside pain and disability reductions. However, studies on the effect of such programs on sleep are lacking.
Purpose: To evaluate the impact on pain-related sleep impairment after a 12-week remote multimodal digital care program (DCP) for MSK conditions.
Patients and Methods: This is an ad-hoc analysis of a decentralized single-arm study into engagement and clinical outcomes after a DCP for MSK rehabilitation. Patients were stratified by baseline sleep disturbance, based on sleep questions in the questionnaires: Oswestry Disability Index, Neck Disability Index, and the Quick Disabilities of the Arm, Shoulder and Hand questionnaire. Additional outcomes were pain, Generalized Anxiety Disorder 7-item scale, Patient Health 9-item questionnaire, Work Productivity, and Activity Impairment, and program engagement.
Results: At baseline, 5749 patients reported sleep disturbance (78.0% of eligible patients). These reported significantly worse clinical outcomes at baseline than patients without sleep disturbance (all p< 0.001). Patients with comorbid sleep disturbance showed improvements in sleep, with a significant proportion reporting full recovery at program completion: 56% of patients with upper limb conditions (including 10% of patients with severe sleep disturbance at baseline), and 24% with spine conditions. These patients also reported significant improvements in all clinical outcomes at program completion. Engagement and satisfaction were high, and also higher than in patients without sleep impairment.
Conclusion: This is the first study of its kind investigating the effect of a completely remote DCP for MSK pain on sleep. Patients reporting comorbid sleep disturbance had significant improvement in sleep, alongside pain, mental health and work productivity at program completion. The results suggest that a DCP for MSK pain can improve sleep disturbances in patients with upper limb and spine conditions.
Keywords: pain, physical therapy, eHealth, telerehabilitation, remote care
Introduction
High quality restful sleep is essential to one’s health and well-being. Sleep deprivation may arise from different etiologies and it is associated with many medical conditions, such as obesity,1,2 cancer,3,4 type 2 diabetes,5 and cardiovascular disease.6,7 Poor sleep and pain interaction share a complex and reciprocal relationship: poor sleep can exacerbate pain, and pain can disrupt restorative nights of sleep.8–10 Moreover, poor sleep is associated with the development of chronic pain, especially musculoskeletal (MSK) conditions.11–13 On the other hand, MSK conditions result in significant disability and suffering, with a very high worldwide prevalence of 1.71 billion.14,15 Sleep disorders are highly comorbid with MSK conditions with a reported prevalence of 72.1% for chronic back pain, 65.4% for rheumatoid arthritis, 70.3% for osteoarthritis, and 95.5% for fibromyalgia.12 Patients with both chronic pain and sleep disturbances have a longer duration of pain and greater pain severity16 and in addition, these patients have more mental health comorbidities, such as pain catastrophizing, anxiety, and depression.16
Currently, exercise-based physical therapy is the mainstay initial treatment for MSK conditions.14,17–19 Not only is it generally effective in addressing MSK pain, it can also improve sleep quality.20–24 A recent meta-analysis by Kelley et al demonstrated that exercise interventions resulted in significant improvements in overall sleep quality, subjective sleep, and sleep latency.22 Moreover, a recent randomized controlled trial by Tseng et al found that moderate-intensity exercise training had a significant beneficial effect on sleep quality and cardio-autonomic function.21
Within physical therapy interventions, there is a recent trend towards telerehabilitation and digital physical therapy.25–27 These programs are effective alternatives to traditional physical therapy28–34 and can increase access to treatment, as well as affordability by reducing travel limitations, time constraints, and geographic restrictions. Digital therapy can also increase patient compliance, empowerment, and self-management.27,35 Overall, telerehabilitation has been well-accepted by patients with MSK conditions, as suggested by the reported high satisfaction rates, similar to those reported after in-person rehabilitation.36,37 To our knowledge, however, no study has been conducted to evaluate the impact of a telerehabilitation program for MSK conditions on pain-related sleep disturbances. In prior studies, we have reported that a multimodal digital care program (DCP) combining exercise-based physical therapy with a psychoeducational component provides a comprehensive approach to pain management with improvements in pain, mental health, and work impairment.31–33,38,39
The purpose of the present study was to evaluate the impact on sleep impairment related to MSK pain after a 12-week remote, multimodal digital care program (DCP) for MSK conditions, with the hypothesis that patients would experience an improvement in sleep disturbance following completion of the program.
Material and Methods
Study Design
The current study is a post-hoc analysis of a decentralized, single-arm investigation to address the impact of a home-based, multimodal DCP on sleep in patients reporting MSK pain in the upper limb or spine. This study is part of a trial that was prospectively approved by the New England Institutional Review Board (number 120190313) and registered on ClinicalTrials.gov (NCT04092946) on September 17th 2019. The study was conducted in accordance with the Declaration of Helsinki. The DCP was administered at the patients’ home and was delivered between June 29th 2020 and November 4th 2021.
Population
The study population included adults (>18 years of age) participating in employer health plans from a total of 50 states and the District of Columbia in the US. Patients that presented MSK pain were invited to apply for the Sword Health’s DCP (Draper, Utah, USA) through a dedicated enrollment website (pre-selecting those with ability to interact with technologies). All participants completed a baseline form providing demographic data and details regarding the clinical condition, alongside specific questions to screen for potential clinical red flags, which was posteriorly assessed by an assigned physical therapist (PT) through an onboarding video call. Any patient that selected any level of MSK-related sleep problem at baseline on the validated metrics were included in the present study. Exclusion criteria included the following: (1) a health condition (eg cardiac, respiratory) or any contra-indication incompatible with performing at least 20 minutes of light to moderate exercise; (2) serious injury or clinical red flags not cleared by a physician; (3) receiving treatment for active cancer; and (4) reporting rapidly progressive loss of strength and/or numbness in the arms/legs or unexplained change in bowel or urinary function in the previous 2 weeks; (5) inability to understand simple and complex motor commands; and (6) not reporting sleep problems. Informed consent was obtained from all patients. Since the recommended validated scales to assess hip or knee conditions by International Consortium for Health Outcomes Measurement (ICHOM) – Hip or Knee and Osteoarthritis Outcome Score – do not account specifically for the sleep domain, we decided to not include patients with lower limb MSK conditions in the present study and include only those with spine or upper limb pain. Any patient that selected any level of MSK-related sleep problem at baseline on the validated metrics were included in the present study. Patients not reporting sleep problems were excluded.
Intervention
The DCP has been previously described in a number of studies.33,39 In brief, the program consists of a 4-, 8-, or 12-week telerehabilitation intervention that includes exercise, education and cognitive behavioral therapy (CBT). This program digitally interfaced between the patient and an assigned physical therapist (PT) who monitors the patients for the study duration. An FDA-listed class II medical device that consists of inertial motion trackers, a dedicated tablet with a mobile app, and a cloud-based portal was made available to all patients. The exercise sessions were designed following current guidelines and were prescribed and tailored by the PT according to the patient’s condition and progression. Exercise consisted of gradual progressive movement exposure, including exercises focused on mobility/stretching, strength, and balance. Progress would include an increase of: (i) number of repetitions and sets, (ii) external load, (iii) range of motion, and (iv) inclusion of multi-articular and multidirectional exercises. Patients performed personalized exercise sessions independently at their convenience through the tablet display, with 3 sessions per week being recommended as a base scenario. The tablet displayed an audio-video explanation prior to each exercise set. During the exercise, an execution interface was shown, with real-time audio-video biofeedback based on the motion trackers data. The devices connected to a cloud-based portal, enabling asynchronous and remote monitoring by the assigned PT, who performed data-driven adjustments to the exercise program as needed. The education and CBT components of the program were developed according to current clinical guidelines and prior research.40–43 Education was also personalized according to each MSK condition addressing the following topics: anatomy, physiology, symptoms, evidence-based treatments, fear-avoidance, and active coping skills, including dealing with feelings of anxiety, depression, and sleep hygiene. The CBT program was based on third generation CBT techniques – mindfulness, acceptance and commitment therapy, and empathy-focused therapy. The education and CBT materials were delivered to patients through written articles, audio content and interactive modules. Bi-directional communication with the assigned PT was ensured through a built-in secure chat within a smartphone application and video calls. Patients who did not engage in any exercise session for 28 consecutive days were considered dropouts.
Demographic Data
Demographic data collected included age, body mass index (BMI), patient gender, and employment status. The gender category included man, woman, and non-binary. The employment status categories were defined as employed (full time or part time) or unemployed.
Clinical Outcomes
Outcomes were collected at baseline, 4, 8 and 12 weeks.
- Primary outcome was sleep metrics from the following validated questionnaires:
- Quick Disabilities of the Arm, Shoulder, and Hand questionnaire (QuickDASH), an 11-item questionnaire with a Likert scale addressing disability and symptom severity. Scores range from 0% to 100% (higher scores indicating poorer functioning).44
- Sleep question: “During the past week, how much difficulty have you had sleeping because of the pain in (your condition)? 1 – No difficulty; 2 – Mild difficulty; 3 – Moderate difficulty; 4 – Severe difficulty; 5 – So much difficulty that I can’t sleep.”
- Oswestry disability index (ODI), a 10-item questionnaire with a Likert scale addressing the impact of back pain on multiple dimensions of the patients’ life. Scores range from 0% to 100% (higher scores indicate more disability).45
- Sleep question: “Sleeping: 1) My Sleep is never disturbed by pain; 2) My sleep is occasionally disturbed by pain; 3) Because of pain I have less than 6 hours sleep; 4) Because of pain I have less than 4 hours sleep; 5) Because of pain I have less than 2 hours sleep; 6) Pain prevents me from sleeping at all.”
- Neck Disability Index (NDI), a 10-item questionnaire with a Likert scale addressing the impact of neck pain on multiple dimensions of the patients’ life. Scores range from 0 to 50 (higher scores indicate more disability).46
- Sleep question: “Sleeping: 1) I have no trouble sleeping; 2) My sleep is slightly disturbed (less than 1 hour sleepless); 3) My sleep is mildly disturbed (1–2 hours sleepless); 4) My sleep is moderately disturbed (2–3 hours sleepless); 5) My sleep is greatly disturbed (3–5 hours sleepless); 6) My sleep is completely disturbed (5–7 hours sleepless).”
- Quick Disabilities of the Arm, Shoulder, and Hand questionnaire (QuickDASH), an 11-item questionnaire with a Likert scale addressing disability and symptom severity. Scores range from 0% to 100% (higher scores indicating poorer functioning).44
- Secondary outcomes:
- Self-reported pain, using the Numerical Pain Rating Scale (NPRS), through the question: “Please rate your average pain over the last 7 days” from 0 (no pain at all) to 10 (worst pain imaginable)”.
- Generalized Anxiety Disorder 7-item scale (GAD-7) (range 0–21)47 to assess anxiety, and Patient Health 9-item questionnaire (PHQ-9) (range 0–27) to assess depression (higher scores indicate worse mental health).48
- Work Productivity and Activity Impairment (WPAI) for general health questionnaire, evaluated employed patients to assess overall work impairment (WPAI overall: total presenteeism and absenteeism from work), presenteeism (WPAI work), absenteeism (WPAI time) and non-work-related activities impairment (WPAI activity). Scores range from 0% to 100% (higher scores indicate declined productivity).49
- Engagement: measured through the following: A) completion of the program (considered as the retention rate); B) total number of completed exercise sessions; C) number of sessions per week, D) total time spent performing exercise sessions; E) total articles read, F) total messages exchanged between the physical therapist and the patient, and G) overall satisfaction through the question: “On a scale from 0 to 10, how likely is it that you would recommend this intervention to a friend or neighbor?”.
Statistical Analysis
Descriptive statistics were applied to characterize the study population and included continuous variables being described with the mean and standard deviation. Categories were described with frequencies (percentage). Comparison of baseline means between the groups initially included an analysis of variance (ANOVA). Frequency analyses for categorical variables were conducted via Pearson’s χ2 analysis. Patients that completed the program were defined as “completers” and those who did not were defined as “non-completers”. Comparisons were made between completers and non-completers.
For the longitudinal data, latent growth curve analysis (LGCA) was used to estimate clinical outcome trajectories across the digital program based on the individual patient trajectories and considering time as a continuous variable.50 This methodology is in the same family of linear mixed-effects modeling however, it is estimated as a structural equation model.51 This type of model considers repeated measures on the same individual to be correlated. LGCA includes measures of model fit and full information maximum likelihood (FIML) to deal with any missing data.52 FIML considers all available data at each time point from all patients. Prior studies have demonstrated the superiority of FIML compared to other imputation methods.52 For the sleep disturbance analysis, ordinal regression was conducted with the R package “MASS”, taking into account the different item-ranged questions. Ordinal regression is a method for modeling dependent variables that are ordinal in nature by assuming an underlying latent continuous distribution behind the observed ordinal data. This latent distribution is assumed to be Gaussian and has a mean of 0 and a standard deviation of 1. Ordinal regression models determine threshold values on this latent distribution that best estimate the observed proportions of each ordinal category. Independent variables were tested for statistical significance by allowing them to influence the location of the mean of this distribution, thus changing the observed proportions of each ordinal category based upon the estimated thresholds. Significant independent variables indicate a reliable shift in the underlying latent distribution greater than expected by chance. In the case of the current data, we evaluated if the latent distribution of sleep quality ordinal categories changed significantly from pre to post.
To facilitate interpretation of the results, 3 subgroups were created post-analysis. For QuickDASH: No disturbance = 1; Mild/Moderate disturbance = 2 and 3, and Severe disturbance = 4 and 5. For ODI and NDI: No disturbance =1; Mild/Moderate disturbance = 2 and 3, and Severe disturbance = 4, 5 and 6.
All statistical analyses were conducted using commercially available software (SPSS v22, IBM, Armonk, NY) or coded using R (version 1.4.1717, R Foundation for Statistical Computing). The level of significance was set at p < 0.05 for all tests.
Results
A total of 5749 patients reported some level of sleep disturbance at baseline (78.0%) and were therefore selected for the study. From these, 1250 (21.7%) were dropouts and 448 (7.8%) were excluded, with 4051 (70.5%) of patients completing the program. The study flow diagram is presented in Figure 1. No serious adverse events53,54 were reported during the study.
 |
Figure 1 Flowchart of the study. |
Baseline Characteristics
Baseline demographics of both the entire cohort and stratified by upper limb or spine pain are presented in Table 1. Overall, patients were middle aged (50.8±12.7 years), overweight, with mean BMI 29.1±6.5, majorly composed of women (56.7%) and employed individuals (81.9%, Table 1). In addition, a majority of patients reported chronic MSK pain (79.1%).
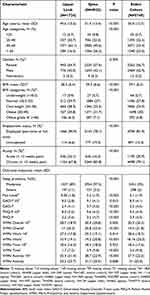 |
Table 1 Baseline Characteristics of Study Patients |
Patients with upper limb conditions (wrist/hand, elbow and shoulder), were significantly younger (49.6±10.6 vs 51.4±13.4 years, p<0.001), had a lower mean BMI (28.5±6.4 vs 29.3±6.6, p<0.001), and a higher proportion of acute conditions than patients with spine pain (32.1% vs 16.2%, p<0.001). By contrast, there was a significantly larger proportion of unemployed individuals within those with spine (neck and back) conditions (19.3% vs 6.6%, respectively, p<0.001). This group also reported worse mean mental health scores at baseline, particularly when filtering for scores ≥5 (GAD 9.1±4.1 vs 8.5±3.8 and PHQ 9.6±4.5 vs 8.5±3.5, p=0.01 and p<0.001, respectively) and work impairment (WPAI overall>0 30.9±20.8 vs 28.7±18.9, p=0.005 Table 1).
Comparing completers with non-completers, the latter were significantly younger (46.2±11.0 vs 51.8±12.5 years, p<0.001) and reported higher levels of BMI (30.1±7.2 vs 28.6±6.2, p<0.001, Supplementary Table S1), with no additional demographic differences between groups (p>0.05 for all).
When comparing patients with and without sleep disturbance at baseline, the former were significantly older, had higher mean BMI, and presented higher proportions of women, unemployed patients, chronic pain sufferers and conditions affecting the spine (p<0.001 for all, Supplementary Table S2). They also reported worse baseline mean scores for pain, anxiety, depression, and WPAI (p<0.001 for all, Supplementary Table S2).
Improvement in Sleep Disturbance
As depicted in Figure 2 and Table 2, we observed an improvement in patients reporting baseline sleep disturbance, with a shift in distribution onto lower sleep impairment categories. The highest improvements were observed in those with upper limb conditions, with 56% of patients reporting total recovery on sleep impairment, with 46% corresponding to patients with moderate disturbance at baseline and 10% to patients reporting severe disturbance at baseline. Among patients with spine conditions, 24% reported complete recovery, all from the group reporting moderate baseline disturbance.
 |
Table 2 Ordinal Regression Analysis of the Pre-Post Proportions of the Sleep Categories Distribution |
Clinical Outcomes
Overall, patients reported improvements in all clinical outcomes at program end. Pain decreased on average 2.42 points (95% CI2.33; 2.50), while anxiety and depression (among those with scores ≥5) decreased by 4.15 (95% CI3.83; 4.49) and 4.42 (95% CI3.98; 4.86) points, respectively. Regarding work productivity, among those with WPAI scores>0 at baseline, we observed an improvement of 15.32 (95% CI 14.03; 16.61) points, as well as an improvement of 19.48 (95% CI18.47; 20.49) points in non-work-related activity impairment (Supplementary Table S3).
Importantly, greater mean changes were observed for pain and impairment in both work- and non-work-related activities in patients with sleep disturbance at baseline, in comparison with those without sleep impairment (Supplementary Table S4).
Impact of Covariates on the Sleep Categories Distribution
The impact of baseline characteristics on changes in sleep disturbance category are presented in Table 3. Patients with higher baseline pain scores presented lower chances of improvement on sleep disturbance (Table 3). In addition, within patients with spine conditions, those with chronic pain, higher depression (PHQ-9) scores at baseline and women were less likely to improve (Table 3). None of the other demographic characteristics had a significant impact on sleep disturbance changes.
 |
Table 3 Impact of Covariates (Demographic and Clinical) on Sleep Disturbance Changes |
Engagement Outcomes
The engagement outcomes for the entire cohort and stratified by upper limb and spine conditions are listed in Table 4. Similar engagement was observed across the different body areas, except for the communication with the PT, where those with spine conditions presented a higher frequency of contacts. Similar satisfaction values were observed between therapy areas (p=0.126).
 |
Table 4 System Usability-Related Outcomes |
Notably, compared to those with no comorbid sleep disturbance, patients with baseline sleep impairment had a significantly higher mean number of sessions per week, total number of sessions, number of messages with the PT and total articles read (p<0.05 for all, Supplementary Table S5). They also had a significantly higher mean satisfaction score than those without baseline sleep disturbance (8.6±1.7 vs 8.5±1.8, respectively, p<0.001).
Discussion
Main Findings
This is the first study of its kind investigating the effect of a completely remote digital care plan for MSK pain on sleep improvement. There was a prevalence of baseline sleep disturbance of 78%. Overall, patients with comorbid sleep disturbance showed a recovery pattern, with a significant proportion of these patients reporting complete sleep impairment recovery at program completion. Patients with upper limb conditions had the highest improvements in sleep, with 56% of patients reporting total recovery of sleep impairment (including 10% of patients with severe sleep disturbance at baseline), while 24% of patients with spine conditions recovered completely from their baseline sleep disturbance, despite the higher prevalence of poor prognostic factors in this cohort (female gender, having chronic pain and higher depression scores at baseline). Chances of improvement in sleep disturbance were negatively impacted by baseline pain severity.
Overall, patients with comorbid sleep disturbance at baseline reported significant improvement in mean pain, anxiety, depression and work productivity scores at program completion. Engagement and satisfaction were high in these patients (higher than observed among patients without sleep impairment at baseline).
Comparison with Prior Work
Sleep disturbances are highly prevalent and comorbid with a number of different medical conditions.1,5,11,12
Despite more wide investigation on the association between sleep and MSK pain in chronic widespread pain and chronic low back pain, evidence indicates that this association also exists for a broader range of conditions,55–57 including those affecting the upper limb.55,58,59 In fact, sleep disturbance is one of the main complaints among patients with shoulder disorders.60,61 Within MSK pain sufferers, sleep disorders have a reported prevalence between 65.4% and 95.5%.12 The present study reported an overall prevalence of 78%, which is in line with the current literature.
MSK conditions with related comorbid sleep disturbance have been associated with poor clinical outcomes including longer pain duration and greater pain severity, as well as higher disability.16 This was also found in the present study, as patients with comorbid sleep disturbance had significantly worse mean pain, anxiety/depression, and work impairment scores, as well as a higher proportion of chronic pain sufferers, than those without sleep disturbance at baseline.
It has been well established that traditional exercise programs significantly improve MSK pain and sleep.20,22 A meta-analysis with a total of 950 patients found a statistically significant improvement in several sleep outcomes with exercise.22 Another meta-analysis conducted by Kovacevic et al gathering 13 different studies found significant improvements in subjective sleep duration and sleep quality.20 In the present study, patients reported improved outcomes in pain, with a mean reduction greater than the 2-points recommended by the IMMPACT guidelines as the minimal clinically important difference.62 Additionally, a high proportion of patients reported improvements in pain-related sleep disturbances, in line with the current literature on exercise and sleep improvement. A greater recovery of sleep impairment was observed in patients with upper limb conditions. Patients with spine conditions presented several poor prognostic factors, such as chronic conditions,16 higher baseline pain severity,63 poorer mental state64 and a larger proportion of women.65 Women have been reported to have higher prevalence of MSK conditions,66 associated with higher pain, mental distress and sleep disorders.67–69 These results emphasize the opportunity of taking advantage of the adaptability of digital multimodal programs, to better address the specific needs of high-risk patients.
Patients also reported improvement in both anxiety and depression, which are domains highly comorbid with both chronic MSK pain and sleep disorders,11,12,70,71 and whose association tends to result in lower improvement and worse outcomes.11,72 This is in agreement with the reported in the present study, as patients with spine pain and more severe mental health issues were more likely to end up in a more severe sleep category at the program completion. It is important to note that all of these comorbidities are likely interrelated, with varying synergistic effects on one another. This highlights the importance of adopting a biopsychosocial view in the management of MSK conditions, considering other aspects involved which influence health and recovery besides pain. Improving both pain and mental status on patients, through a DCP including not only exercise, but also education and CBT, might have contributed for synergistic improvements in all clinical outcomes measured.
Overall engagement in the program was high, with a high satisfaction rate similarly reported by patients with spine or upper limb pain. Patients with comorbid sleep disturbances had a significantly higher engagement in the program than those without baseline sleep disturbances at baseline, specifically with regard to more sessions, exercising time, interactions with the PT, and articles read. We speculate that higher baseline pain levels, together with existing sleep disturbances may have positively influenced the motivation of these patients to engage in the program.
Strengths and Limitations
There are many strengths to this study, one of which is the large sample size from a real-world context. Patients were enrolled through their employers’ health plans from 50 states and the District of Columbia in the U.S. allowing for a diverse population and better generalizability. Another strength is the DCP itself, which is a multimodal approach that includes exercise, utilizing real-time biofeedback, regular communication with the same PT, as well as education and CBT, in a digital format. All these factors favor accessibility, engagement and can therefore maximize outcomes, as well as address both physical and mental health aspects jointly. An additional strength is the use of self-reported sleep outcomes, which are integrated in widely used and validated outcome scales, allowing for translational application and generalizability to other studies. Lastly, the novel idea of investigating sleep disturbance within a digital therapy program allows for a foundation for future work in this field, and helps identify areas of improvement for future telerehabilitation programs.
The major limitation of the study is the lack of a control group, which does not allow for any causal effect to be determined on sleep improvement. However, given the real-world context in which this was performed, the most obvious comparator would be a “waiting list” control, which was not ethical considering the high accessibility this technology affords. In addition, because the program includes patients that are beneficiary to their employers’ health benefits, the current population may not be an exact representation of the general population in the U.S.. Thus any applicability of the results to clinical settings in which there are more uninsured, elderly, or work-disabled patients may be limited. In addition, patients with lower limb MSK conditions were excluded since ICHOM recommended validated metrics do not account specifically for the sleep domain. It is therefore unknown if these results apply to that population. However, given the improvement in pain and sleep in the other conditions, it is likely they will benefit too. Further studies on this population is warranted to confirm the present findings in that specific population. The sleep metrics used for analysis were from self-reported validated surveys completed by the patient and thus are subjective in nature by definition. However, no specific and dedicated scale was used that focused on sleep disturbance alone. Therefore, more research is needed to better characterize the types of sleep disturbance and improvements in patients with MSK, particularly under a telerehabilitation context. Finally, long-term follow-up was also not available to ascertain the benefits of the program at later time points and to see if any of the sleep improvements remained. Further prospective controlled studies are warranted to better characterize the effect of digital therapy on sleep disturbance outcomes.
Conclusion
This is the first study of its kind investigating the effect of a completely remote digital care plan for MSK pain on sleep. Patients reporting comorbid sleep disturbance had significant improvement in MSK pain-related sleep disturbance, as well as pain, mental and work productivity. The results suggest that a digital care program for MSK pain can improve sleep disturbances in patients with upper limb and spine conditions.
Abbreviations
MSK, Musculoskeletal; DCP, Digital care program; US, United States; ICHOM, International Consortium for Health Outcomes Measurement; CBT, Cognitive behavioral therapy; PT, Physical Therapist; FDA, United States Food and Drug Administration; BMI, Body Mass Index; QuickDASH, Quick Disabilities of the Arm, Shoulder and Hand questionnaire; NPRS, Numerical Pain Rating Scale; GAD-7, Generalized Anxiety Disorder 7-item scale; PHQ-9, Patient Health 9-item questionnaire; WPAI, Work Productivity and Activity Impairment; ANOVA, Analysis of variance; LGCA, Latent growth curve analysis; FIML, Full information maximum likelihood; NDI, Neck Disability Index; ODI, Oswestry Disability Index; IMMPACT, Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.
Data Sharing Statement
All data relevant to the study are included in the article or are available as Digital Content at Supplementary Materials. Only de-identified individual participant data is provided. Further information, including the study protocol, can be found at ClinicalTrials.gov (NCT04092946).
Ethics Approval and Informed Consent
The study was approved by the New England IRB (protocol number 120190313) and prospectively registered in ClinicalTrials.gov, NCT04092946, 17/09/2019. This study was conducted in accordance with the approved guidelines. All patients were informed about the purpose and procedures of the study and provided informed consent.
Acknowledgments
The authors acknowledge the team of physical therapists responsible for the management of participants. The authors also acknowledge the contributions of João Tiago Silva and Guilherme Freches in data validation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Disclosure
Fabíola Costa, Dora Janela, Maria Molinos, Anabela C. Areias, Virgílio Bento, Vijay Yanamadala and Fernando Dias Correia are employees at Sword Health, the study sponsor. Robert G. Moulder is an independent scientific consultant responsible for statistical analysis, while Jorge Lains and Justin K. Scheer are independent scientific/clinical consultants who were funded by Sword Health in connection with the development and execution of this article. Fernando Dias Correia, Vijay Yanamadala and Virgílio Bento also hold equity from Sword Health. The authors report no other conflicts of interest in this work.
References
1. Fatima Y, Doi SA, Mamun AA. Sleep quality and obesity in young subjects: a meta-analysis. Obes Rev. 2016;17(11):1154–1166. doi:10.1111/obr.12444
2. St-Onge MP. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev. 2017;18(Suppl 1):34–39. doi:10.1111/obr.12499
3. Wang P, Ren FM, Lin Y, et al. Night-shift work, sleep duration, daytime napping, and breast cancer risk. Sleep Med. 2015;16(4):462–468. doi:10.1016/j.sleep.2014.11.017
4. Zhao H, Yin JY, Yang WS, et al. Sleep duration and cancer risk: a systematic review and meta-analysis of prospective studies. Asian Pac J Cancer Prev. 2013;14(12):7509–7515. doi:10.7314/apjcp.2013.14.12.7509
5. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. doi:10.2337/dc09-1124
6. Bertisch SM, Pollock BD, Mittleman MA, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: sleep Heart Health Study. Sleep. 2018;41(6):zsy047. doi:10.1093/sleep/zsy047
7. Wang D, Li W, Cui X, et al. Sleep duration and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. 2016;219:231–239. doi:10.1016/j.ijcard.2016.06.027
8. Skarpsno ES, Mork PJ, Nilsen TIL, Nordstoga AL. Influence of sleep problems and co-occurring musculoskeletal pain on long-term prognosis of chronic low back pain: the HUNT Study. J Epidemiol Community Health. 2020;74(3):283–289. doi:10.1136/jech-2019-212734
9. Nitter AK, Pripp AH, Forseth K. Are sleep problems and non-specific health complaints risk factors for chronic pain? A prospective population-based study with 17 year follow-up. Scand J Pain. 2012;3(4):210–217. doi:10.1016/j.sjpain.2012.04.001
10. Gerhart JI, Burns JW, Post KM, et al. Relationships between sleep quality and pain-related factors for people with chronic low back pain: tests of reciprocal and time of day effects. Ann Behav Med. 2017;51(3):365–375. doi:10.1007/s12160-016-9860-2
11. Whale K, Gooberman-Hill R. The importance of sleep for people with chronic pain: current insights and evidence. JBMR Plus. 2022;6(7):e10658. doi:10.1002/jbm4.10658
12. Sun Y, Laksono I, Selvanathan J, et al. Prevalence of sleep disturbances in patients with chronic non-cancer pain: a systematic review and meta-analysis. Sleep Med Rev. 2021;57:101467. doi:10.1016/j.smrv.2021.101467
13. Herrero Babiloni A, De Koninck BP, Beetz G, De beaumont L, Martel MO, Lavigne GJ. Sleep and pain: recent insights, mechanisms, and future directions in the investigation of this relationship. J Neural Transm. 2020;127(4):647–660. doi:10.1007/s00702-019-02067-z
14. El-Tallawy SN, Nalamasu R, Salem GI, Jak L, Pergolizzi JV, Christo PJ. Management of musculoskeletal pain: an update with emphasis on chronic musculoskeletal pain. Pain Ther. 2021;10(1):181–209. doi:10.1007/s40122-021-00235-2
15. Cieza A, Causey K, Kamenov K, Hanson SW, Chatterji S, Vos T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2021;396(10267):2006–2017. doi:10.1016/S0140-6736(20)32340-0
16. Husak AJ, Bair MJ. Chronic pain and sleep disturbances: a pragmatic review of their relationships, comorbidities, and treatments. Pain Med. 2020;21(6):1142–1152. doi:10.1093/pm/pnz343
17. George SZ, Fritz JM, Silfies SP, et al. Interventions for the management of acute and chronic low back pain: revision 2021. J Orthop Sports Phys Ther. 2021;51(11):CPG1–CPG60. doi:10.2519/jospt.2021.0304
18. Gianola S, Bargeri S, Del Castillo G, et al. Effectiveness of treatments for acute and subacute mechanical non-specific low back pain: a systematic review with network meta-analysis. Br J Sports Med. 2022;56(1):41–50. doi:10.1136/bjsports-2020-103596
19. Stochkendahl MJ, Kjaer P, Hartvigsen J, et al. National Clinical Guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J. 2018;27(1):60–75. doi:10.1007/s00586-017-5099-2
20. Kovacevic A, Mavros Y, Heisz JJ, Fiatarone Singh MA. The effect of resistance exercise on sleep: a systematic review of randomized controlled trials. Sleep Med Rev. 2018;39:52–68. doi:10.1016/j.smrv.2017.07.002
21. Tseng TH, Chen HC, Wang LY, Chien MY. Effects of exercise training on sleep quality and heart rate variability in middle-aged and older adults with poor sleep quality: a randomized controlled trial. J Clin Sleep Med. 2020;16(9):1483–1492. doi:10.5664/jcsm.8560
22. Kelley GA, Kelley KS. Exercise and sleep: a systematic review of previous meta-analyses. J Evid Based Med. 2017;10(1):26–36. doi:10.1111/jebm.12236
23. Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother. 2012;58(3):157–163. doi:10.1016/S1836-9553(12)70106-6
24. Hartescu I, Morgan K, Stevinson CD. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: a randomized controlled trial. J Sleep Res. 2015;24(5):526–534. doi:10.1111/jsr.12297
25. Grona SL, Bath B, Busch A, Rotter T, Trask C, Harrison E. Use of videoconferencing for physical therapy in people with musculoskeletal conditions: a systematic review. J Telemed Telecare. 2018;24(5):341–355. doi:10.1177/1357633X17700781
26. Levy CE, Silverman E, Jia H, Geiss M, Omura D. Effects of physical therapy delivery via home video telerehabilitation on functional and health-related quality of life outcomes. J Rehabil Res Dev. 2015;52(3):361–370. doi:10.1682/jrrd.2014.10.0239
27. Seron P, Oliveros MJ, Gutierrez-Arias R, et al. Effectiveness of telerehabilitation in physical therapy: a rapid overview. Phys Ther. 2021;101(6). doi:10.1093/ptj/pzab053
28. Horsley S, Schock G, Grona SL, et al. Use of real-time videoconferencing to deliver physical therapy services: a scoping review of published and emerging evidence. J Telemed Telecare. 2020;26(10):581–589. doi:10.1177/1357633X19854647
29. Mecklenburg G, Smittenaar P, Erhart-Hledik JC, Perez DA, Hunter S. Effects of a 12-week digital care program for chronic knee pain on pain, mobility, and surgery risk: randomized controlled trial. J Med Internet Res. 2018;20(4):e156. doi:10.2196/jmir.9667
30. Nelson M, Bourke M, Crossley K, Russell T. Telerehabilitation is non-inferior to usual care following total Hip replacement - A randomized controlled non-inferiority trial. Physiotherapy. 2020;107:19–27. doi:10.1016/j.physio.2019.06.006
31. Correia FD, Nogueira A, Magalhaes I, et al. Home-based rehabilitation with a novel digital biofeedback system versus conventional in-person rehabilitation after total knee replacement: a feasibility study. Sci Rep. 2018;8(1):11299. doi:10.1038/s41598-018-29668-0
32. Correia FD, Nogueira A, Magalhaes I, et al. Medium-term outcomes of digital versus conventional home-based rehabilitation after total knee arthroplasty: prospective, parallel-group feasibility study. JMIR Rehabil Assist Technol. 2019;6(1):e13111. doi:10.2196/13111
33. Correia FD, Molinos M, Luis S, et al. Digitally assisted versus conventional home-based rehabilitation after arthroscopic rotator cuff repair: a randomized controlled trial. Am J Phys Med Rehabil. 2022;101(3):237–249. doi:10.1097/PHM.0000000000001780
34. Russell TG, Buttrum P, Wootton R, Jull GA. Internet-based outpatient telerehabilitation for patients following total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2011;93(2):113–120. doi:10.2106/jbjs.I.01375
35. Cottrell MA, Russell TG. Telehealth for musculoskeletal physiotherapy. Musculoskelet Sci Pract. 2020;48:102193. doi:10.1016/j.msksp.2020.102193
36. Bennell KL, Marshall CJ, Dobson F, Kasza J, Lonsdale C, Hinman RS. Does a web-based exercise programming system improve home exercise adherence for people with musculoskeletal conditions?: a randomized controlled trial. Am J Phys Med Rehabil. 2019;98(10):850–858. doi:10.1097/phm.0000000000001204
37. Adamse C, Dekker-Van Weering MG, van Etten-Jamaludin FS, Stuiver MM. The effectiveness of exercise-based telemedicine on pain, physical activity and quality of life in the treatment of chronic pain: a systematic review. J Telemed Telecare. 2018;24(8):511–526. doi:10.1177/1357633x17716576
38. Correia FD, Molinos M, Neves C, et al. Digital rehabilitation for acute ankle sprains: prospective longitudinal cohort study. JMIR Rehabil Assist Technol. 2021;8(3):e31247. doi:10.2196/31247
39. Costa F, Janela D, Molinos M, et al. Telerehabilitation of acute musculoskeletal multi-disorders: prospective, single-arm, interventional study. BMC Musculoskelet Disord. 2022;23(1):29. doi:10.1186/s12891-021-04891-5
40. Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–530. doi:10.7326/m16-2367
41. Joypaul S, Kelly F, McMillan SS, King MA. Multi-disciplinary interventions for chronic pain involving education: a systematic review. PLoS One. 2019;14(10):e0223306. doi:10.1371/journal.pone.0223306
42. NICE. Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain. Chronic pain (primary and secondary) in over 16s: assessment of all chronic pain and management of chronic primary pain. London: National Institute for Health and Care Excellence (NICE); 2021. PMID: 33939353.
43. Wong JJ, Côté P, Sutton DA, et al. Clinical practice guidelines for the noninvasive management of low back pain: a systematic review by the Ontario Protocol for Traffic Injury Management (OPTIMa) Collaboration. Eur J Pain. 2017;21(2):201–216. doi:10.1002/ejp.931
44. Mintken PE, Glynn P, Cleland JA. Psychometric properties of the shortened disabilities of the arm, shoulder, and hand questionnaire (QuickDASH) and numeric pain rating scale in patients with shoulder pain. J Shoulder Elbow Surg. 2009;18(6):920–926. doi:10.1016/j.jse.2008.12.015
45. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25(22):2940–52;discussion 2952. doi:10.1097/00007632-200011150-00017
46. Vernon H, Mior S. The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14(7):409–415.
47. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi:10.1001/archinte.166.10.1092
48. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
49. Ospina MB, Dennett L, Waye A, Jacobs P, Thompson AH. A systematic review of measurement properties of instruments assessing presenteeism. Am J Manag Care. 2015;21(2):e171–e185.
50. Duncan TE, Duncan SC. The ABC’s of LGM: an introductory guide to latent variable growth curve modeling. Soc Personal Psychol Compass. 2009;3(6):979–991. doi:10.1111/j.1751-9004.2009.00224.x
51. McNeish D, Matta T. Differentiating between mixed-effects and latent-curve approaches to growth modeling. Behav Res Methods. 2018;50(4):1398–1414. doi:10.3758/s13428-017-0976-5
52. Pfaffel A, Kollmayer M, Schober B, Spiel C, Missing Data A. Approach to correct for direct and indirect range restrictions with a dichotomous criterion: a simulation study. PLoS One. 2016;11(3):e0152330. doi:10.1371/journal.pone.0152330
53. Niemeijer A, Lund H, Stafne SN, et al. Adverse events of exercise therapy in randomised controlled trials: a systematic review and meta-analysis. Br J Sports Med. 2020;54(18):1073–1080. doi:10.1136/bjsports-2018-100461
54. What is a Serious Adverse Event?. U.S. Food and Drug Administration. Published 2022. Accessed 5 December, 2022. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event
55. Maestroni L, Marelli M, Gritti M, Civera F, Rabey M. Is rotator cuff related shoulder pain a multidimensional disorder? An exploratory study. Scand J Pain. 2020;20(2):297–305. doi:10.1515/sjpain-2019-0108
56. Harrison L, Wilson S, Munafò MR. Exploring the associations between sleep problems and chronic musculoskeletal pain in adolescents: a prospective cohort study. Pain Res Manag. 2014;19(5):e139–e145. doi:10.1155/2014/615203
57. Chun MY, Cho BJ, Yoo SH, Oh B, Kang JS, Yeon C. Association between sleep duration and musculoskeletal pain: the Korea National Health and Nutrition Examination Survey 2010–2015. Medicine. 2018;97(50):e13656. doi:10.1097/md.0000000000013656
58. Longo UG, Facchinetti G, Marchetti A, et al. Sleep disturbance and rotator cuff tears: a systematic review. Medicina. 2019;55(8):453. doi:10.3390/medicina55080453
59. Cho CH, Jung SW, Park JY, Song KS, Yu KI. Is shoulder pain for three months or longer correlated with depression, anxiety, and sleep disturbance? J Shoulder Elbow Surg. 2013;22(2):222–228. doi:10.1016/j.jse.2012.04.001
60. Khazzam MS, Mulligan EP, Brunette-Christiansen M, Shirley Z. Sleep quality in patients with rotator cuff disease. J Am Acad Orthop Surg. 2018;26(6):215–222. doi:10.5435/jaaos-d-16-00547
61. Mulligan EP, Brunette M, Shirley Z, Khazzam M. Sleep quality and nocturnal pain in patients with shoulder disorders. J Shoulder Elbow Surg. 2015;24(9):1452–1457. doi:10.1016/j.jse.2015.02.013
62. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
63. Nieminen LK, Pyysalo LM, Kankaanpää MJ. Prognostic factors for pain chronicity in low back pain: a systematic review. Pain Rep. 2021;6(1):e919. doi:10.1097/pr9.0000000000000919
64. Hayden JA, Dunn KM, van der Windt DA, Shaw WS. What is the prognosis of back pain? Best Pract Res Clin Rheumatol. 2010;24(2):167–179. doi:10.1016/j.berh.2009.12.005
65. Lavigne GJ, Okura K, Abe S, et al. Gender specificity of the slow wave sleep lost in chronic widespread musculoskeletal pain. Sleep Med. 2011;12(2):179–185. doi:10.1016/j.sleep.2010.07.015
66. Wijnhoven HA, de Vet HC, Picavet HS. Prevalence of musculoskeletal disorders is systematically higher in women than in men. Clin J Pain. 2006;22(8):717–724. doi:10.1097/01.ajp.0000210912.95664.53
67. Stubbs D, Krebs E, Bair M, et al. Sex differences in pain and pain-related disability among primary care patients with chronic musculoskeletal pain. Pain Med. 2010;11(2):232–239. doi:10.1111/j.1526-4637.2009.00760.x
68. Zeng LN, Zong QQ, Yang Y, et al. Gender difference in the prevalence of insomnia: a meta-analysis of observational studies. Front Psychiatry. 2020;11:577429. doi:10.3389/fpsyt.2020.577429
69. Louie GH, Tektonidou MG, Caban-Martinez AJ, Ward MM. Sleep disturbances in adults with arthritis: prevalence, mediators, and subgroups at greatest risk. Data from the 2007 National Health Interview Survey. Arthritis Care Res. 2011;63(2):247–260. doi:10.1002/acr.20362
70. Bijker L, Sleijser-Koehorst MLS, Coppieters MW, Cuijpers P, Scholten-Peeters GGM. Preferred self-administered questionnaires to assess depression, anxiety and somatization in people with musculoskeletal pain - a modified delphi study. J Pain. 2020;21(3–4):409–417. doi:10.1016/j.jpain.2019.08.006
71. Freeman D, Sheaves B, Goodwin GM, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry. 2017;4(10):749–758. doi:10.1016/S2215-0366(17)30328-0
72. Morin CM, LeBlanc M, Daley M, Gregoire JP, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–130. doi:10.1016/j.sleep.2005.08.008
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

