Back to Journals » Nature and Science of Sleep » Volume 14
Sleep Apnea and Risk of Influenza-Associated Severe Acute Respiratory Infection: Real-World Evidence
Authors Tsai MS , Chen HC, Li HY , Tsai YT, Yang YH , Liu CY, Lee YC, Hsu CM , Lee LA
Received 30 October 2021
Accepted for publication 29 March 2022
Published 10 May 2022 Volume 2022:14 Pages 901—909
DOI https://doi.org/10.2147/NSS.S346984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sarah L Appleton
Ming-Shao Tsai,1– 4,* Hung-Chin Chen,1,* Hsueh-Yu Li,2,5 Yao-Te Tsai,1,2 Yao-Hsu Yang,4,6,7 Chia-Yen Liu,4 Yi‑Chan Lee,2,8 Cheng-Ming Hsu,1,2 Li-Ang Lee2,5,9
1Department of Otolaryngology – Head and Neck Surgery, Chiayi Chang Gung Memorial Hospital, Chiayi, 613, Taiwan; 2Faculty of Medicine, College of Medicine, Chang Gung University, Taoyuan, 333, Taiwan; 3Graduate Institute of Clinical Medical Sciences, College of Medicine, Chang Gung University, Taoyuan, 333, Taiwan; 4Health Information and Epidemiology Laboratory of Chang Gung Memorial Hospital, Chiayi, 613, Taiwan; 5Department of Otolaryngology – Head and Neck Surgery, Linkou Chang Gung Memorial Hospital, Taoyuan, 333, Taiwan; 6Department of Traditional Chinese Medicine, Chiayi Chang Gung Memorial Hospital, Chiayi, 613, Taiwan; 7School of Traditional Chinese Medicine, College of Medicine, Chang Gung University, Taoyuan, 333, Taiwan; 8Department of Otolaryngology – Head and Neck Surgery, Keelung Chang Gung Memorial Hospital, Keelung, 204, Taiwan; 9School of Medicine, National Tsing Hua University, Hsinchu, 300, Taiwan
*These authors contributed equally to this work
Correspondence: Li-Ang Lee, Department of Otolaryngology – Head and Neck Surgery, Linkou Chang Gung Memorial Hospital, No. 5, Fu-Hsing Street, Gueishan District, Taoyuan City, 33305, Taiwan, Tel +886 3 3281200 ext. 3968, Fax +886 3 3979361, Email [email protected]
Purpose: We executed the presented retrospective cohort study with the purpose of probing the risk of severe acute respiratory infection (SARI) following influenza in patients with sleep apnea.
Materials and Methods: We executed this real-world study by gathering Taiwan National Health Insurance Research Database (NHIRD) data. From a database containing 1 million individuals sampled at random from the NHIRD, we identified all patients aged 20 years or older with a sleep apnea diagnosis between 1997 and 2013 as the study group. We established a comparison cohort of individuals without sleep apnea by randomly matching patients with respect to monthly income, gender, urbanization level, and age at a 1:4 ratio. Follow-up was performed until death or the end of 2015 for both groups. We determined the study outcome to be the occurrence of influenza-associated SARI.
Results: We enrolled 6508 and 26,032 patients into the study and comparison groups, respectively. A significantly higher cumulative incidence of influenza-associated SARI was discovered in the study group (p < 0.001). In our multivariate analysis, sleep apnea, chronic obstructive pulmonary disease, and coronary artery disease were independent risk factors for influenza-associated SARI. The hazard ratio of sleep apnea for influenza-associated SARI was 1.98 (95% CI: 1.26– 3.10) after adjustment for all comorbidities, gender, age, monthly income, and urbanization level.
Conclusion: Sleep apnea increased the risk of influenza-associated SARI. We suggest that physicians be cautious about the development of severe influenza illness in patients with sleep apnea. Vaccination and early oseltamivir administration should be actively considered in this group of patients.
Keywords: sleep apnea, sleep disturbance, influenza, severe acute respiratory infection
Introduction
Influenza viruses result in the contagious respiratory disease named influenza.1 Its prevalence fluctuates, but influenza has been estimated to affect 5% to 10% of the population of the world annually and to lead to 250,000 to 650,000 deaths globally.2–4 Three to five million patients with influenza infection, especially elderly individuals, progress to severe acute respiratory infection (SARI) or die of the infection annually.5–7 SARI is defined as acute respiratory illness (onset within the preceding 10 days) characterized by fever of 38°C or higher, cough, and need for hospitalization.8 Data compiled by Taiwan’s Centers for Disease Control revealed that hospitalization was required in approximately 0.5% of outpatient cases of influenza, and 7% of patients who received inpatient treatment developed severe complications or needed intensive care.9 Free antiviral drugs are given, under the sponsorship of Taiwan’s National Health Insurance (NHI), to those who are detected to be at high risk of influenza complications, including individuals aged younger than 5 years or aged 65 years or older, patients with chronic medical conditions, individuals with morbid obesity (body mass index [BMI] ≥ 30 kg/m2), pregnant women, and patients involved in institutional outbreaks.10
Sleep apnea, reflected by an apnea–hypopnea index (AHI) value of at least 5, was reported to affect approximately 17% (range, 4–50%) and 22% (range, 9–37%) of women and men, respectively.11 Sleep apnea is implicated in damage to multiple organ systems and associated with several major complications that include diabetes mellitus (DM), metabolic syndrome, dementia, coronary artery disease, stroke, and chronic obstructive pulmonary disease (COPD).12–19 Previous literature has mentioned that patients with sleep apnea are more prone to influenza.20 Scholars have also discovered people with sleep apnea to be at a relatively high risk of community-acquired pneumonia.21,22 Patients with severe sleep apnea and obesity also tend to develop chronic hypercapnia, which may lead to deterioration of lung function following respiratory tract infection, and are likely to require hospitalization and ventilator treatment.23,24 According to our review of the literature, how sleep apnea is associated with influenza-associated SARI remains unclear. Accordingly, we executed our present study to probe the SARI risk following influenza in patients with sleep apnea.
Materials and Methods
Data Source and Study Design
This retrospective cohort study was executed by employing data gathered from Taiwan’s National Health Insurance Research Database (NHIRD). In operation since March 1, 1995, the Taiwan NHI program is a compulsory and single-payer health care scheme providing comprehensive coverage to virtually all of Taiwan’s population (99.7%) and medical institutions at all levels of the country.25,26 Consequently, the NHIRD is a favorable resource for nationwide population-based studies. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and Procedure Coding System (ICD-9-PCS) diagnostic and procedure codes are available in the NHIRD.27–29 Further information such as clinic visits, prescription drugs, hospitalizations, radiologic examinations, and types of surgical intervention can also be obtained from the database.30,31 The comprehensiveness of the NHIRD enables scholars to obtain population-based evidence to help support decisions. To ensure personal privacy, names of health care providers, medical institutions, and patients are encrypted with unique and unnamed identifiers.32,33
The data employed in this study were from the Longitudinal Health Insurance Database 2000, a database subset comprising data on 1 million individuals who were picked from among the NHIRD’s Registry for Beneficiaries at random in the year 2000. The random selection was executed through a systematic sampling approach. The database comprises the entirety of claims data for the period 1997 to 2013.32,34,35 National Health Research Institutes reports revealed the NHIRD sample and the LHID2000 sample groups to not differ significantly with respect to monthly income, gender, urbanization level, or age.35 The Chang Gung Medical Foundation Institutional Review Board ratified the current study (Approval No. 201801126B1).
Study Population and Comparison Group
Regarding the patient enrollment process, as displayed by the flowchart in Figure 1, the personal information of enrollees was extracted from the NHIRD. Patients who were 20 years or older and receiving a new sleep apnea diagnosis (ICD-9-CM codes 780.51, 780.53, 780.57) during the period from January 1, 1997, to December 31, 2013, were included;16,36 we excluded 18 patients who developed pneumonia following influenza before being diagnosed as having sleep apnea. We finally enrolled 6508 patients with sleep apnea. To ensure accurate diagnoses, we enrolled only patients for whom the sleep apnea ICD-9-CM codes were recorded in either the inpatient setting or at least three claims for outpatient care.36 This method has been validated in a study using the original medical records of 3766 patients; of these, 3124 (83%) patients had PSG reports, of which 87% were confirmed to have sleep apnea.22
We also randomly selected a comparison cohort of people without sleep apnea throughout the study period from the same database. This cohort was matched with the study group with respect to monthly income, gender, urbanization level, and age in a 1:4 ratio. The enrollment date for the study group was stipulated as that on which a sleep apnea diagnostic code was first recorded. This date also marked the beginning of follow-up for the corresponding controls in the comparison group.
Outcome and Covariate Measurements
For each patient with sleep apnea, the index date was the date sleep apnea was first diagnosed, and the same index date was used for their matched non-sleep apnea control. We executed patients’ follow-up until death or December 31, 2015, the end of the study period. Insurees drop out of the NHI program when they die.37 The primary outcome in our research was influenza-associated SARI, defined as hospital admission with a diagnosis of influenza (ICD-9-CM codes 487.0, 487.1, 487.8, 488.1, 488.8).
The NHIRD constituted the source of sociodemographic data on the patients—monthly income, gender, urbanization level, and age. The levels of urbanization of the enrollees’ residence were graded on a scale of 1 to 4 (grades 1 to 4), and grades were calculated using a range of factors, including the population pyramid, expenditure, industrial structure, mean annual per capita income, population density, manufacturing density, and economic activity index.38
The following comorbidities were recorded using ambulatory or inpatient claims data: DM (ICD-9-CM code 250), obesity (ICD-9-CM code 278), stroke (ICD-9-CM codes 430–438), hypertension (ICD-9-CM codes 401–405), COPD (ICD-9-CM codes 491, 492, 496), coronary artery disease (CAD; ICD-9-CM codes 410–414), and chronic kidney disease (CKD; ICD-9-CM codes 582, 583, 585, 586, 588).16,29,36,39 A comorbid condition was included if the corresponding diagnostic code appeared in either the inpatient setting or at least three claims for ambulatory care.36
Statistical Analyses
The independent Student t test was executed for continuous variables; in addition, the Pearson chi-square test was executed for categorical data. These tests were executed for the purpose of comparing the study and comparison groups with respect to comorbid conditions and sociodemographic characteristics. Adjusted hazard ratios (HRs) for sleep apnea and other potential confounders—age, gender, and comorbidities—for influenza-associated SARI were evaluated using Cox proportional hazards regression. To derive the cumulative incidence of influenza-associated SARI in our two groups, we applied the Kaplan–Meier method; furthermore, we compared curves by employing the Log rank test. We employed SAS version 9.4 (SAS Inc., Cary, NC, USA) for all statistical analyses, with statistical significance being indicated by two-sided p < 0.05.
Results
This study recruited 6508 patients with sleep apnea as the study group and 26,032 individuals without sleep apnea as the comparison group. The study group was determined to have a higher proportion of male patients. Nearly half of the patients in the study group had hypertension and a quarter had DM (Table 1). The study group was also noted to have a higher incidence of the DM, hypertension, obesity, stroke, COPD, CAD, and CKD (Table 1). During the follow-up period, influenza was diagnosed in 10.4% of the study cohort and 7.2% of the comparison cohort, respectively (p < 0.001). The incidence of influenza-associated SARI in the study group and comparison group was 0.5% and 0.2%, respectively (p < 0.001).
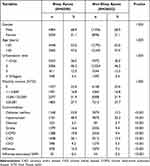 |
Table 1 Sociodemographic Characteristics and Comorbid Conditions of the Study Patients |
Table 2 presents the potential risk factors for influenza-associated SARI. In our executed univariate analysis, patients with sleep apnea, DM, hypertension, stroke, COPD, CAD, CKD and those aged 50 years or older had a higher risk of SARI following influenza. The crude HR for each specified factor in the unadjusted analysis is presented in Table 2. As revealed by our executed multivariate analysis, sleep apnea, COPD, and CAD were independent risk factors for influenza-associated SARI (p < 0.001, 0.021, and 0.014, respectively), with the corresponding HRs (as well as 95% CIs) being 1.98 (1.26–3.10), 1.80 (1.09–2.96), and 1.92 (1.14–3.21). Adjustments were made in the model for monthly income, gender, urbanization level, age, and all comorbidities.
 |
Table 2 Hazard Ratios for Sleep Apnea and Other Potential Confounders for Influenza-Associated SARI |
In our study, we determined the mean (standard deviation) observation duration to be 5.38 (3.81) and 5.45 (3.81) years for the study and comparison groups, respectively. When not considering competing risk events in the Kaplan–Meier approach, we discovered the study group to exhibit a significantly higher cumulative incidence of influenza-associated SARI than did the comparison group (Log rank test: p < 0.001; Figure 2). The 1-, 5-, and 10-year cumulative incidence rates of influenza-associated SARI were 0.08%, 0.46%, and 1.00%, respectively, in the sleep apnea group and 0.03%, 0.20%, and 0.38%, respectively, in the comparison group.
Discussion
Our present research is a nationwide-based study investigating the influenza-associated SARI–sleep apnea relationship. Patients in the study group were discovered to be more likely to develop SARI and require inpatient care following influenza infection than did those in the comparison group. A similar conclusion was reported in a recently executed study, which revealed a higher risk of hospitalization following influenza infection in patients with sleep apnea who did not adhere to continuous positive airway pressure (CPAP) than in those who received CPAP treatment.40 According to these findings, we suggest that patients with sleep apnea, especially those who remain untreated, have a higher probability of severe illness when infected with influenza. In one study involving the risk assessment of severe influenza illness, age higher than 65 years, chronic renal disease, cardiovascular disease, neuromuscular disease, and immunosuppression elevated the influenza mortality risk.41 Moreover, patients with COPD required ventilator support more often.41 Asthma was noted to be associated with a relatively high likelihood of developing pneumonia.41 In our large-scale cohort study, we also discovered COPD and CAD to be associated with a relatively high risk of influenza-associated SARI. In addition, this study also found that the incidence of influenza in sleep apnea patients was higher than that in non-sleep apnea patients, which is also consistent with past literature.20
Patients with sleep apnea experience partial or complete cessation of respiratory airflow during sleep, which is usually accompanied by intermittent nocturnal hypoxemia.42,43 Apnea is then terminated by a forceful inspiratory gasp and brief arousal, which could increase the risk of aspiration.44 The coexistence of sleep apnea and COPD, which was named “overlap syndrome,” was observed in a substantial number of patients14 and found to result in more profound nocturnal oxygen desaturation and a relatively high likelihood of pulmonary hypertension, thereby increasing substantially the likelihood of morbidity and mortality.14 Sleep apnea is well known to be a common comorbidity in patients with obesity. One study reported an association between daytime hypercapnia and relatively high BMI levels in patients with sleep apnea.24 Coexisting obesity reduces lung compliance and capacity, creates intrinsic positive end-expiratory pressure, and increases the work of breathing, which was linked to a relatively high risk of respiratory failure following pulmonary infection.45 However, the results of this study showed that obesity is not an independent risk factor for SARI, which may be due to the fact that the database used in this study does not contain the height and weight of patients. Thus, the patients with obesity could only be identified by ICD-9 codes rather than BMI, which may lead to an underestimation of the number of obesity patients. Further research is needed to clarify the exact association between obesity and SARI.
From the epidemiological viewpoint, a relatively high risk of pneumonia has been discovered in patients with sleep apnea.21,22 In addition, the sleep apnea severity, established according to the AHI, was reported to be positively correlated with pneumonia severity.21 Morimoto et al found that mixed sleep apnea contributed to an elevated risk of death from pneumonia.46 After executing a cohort study of 250,907 patients with pneumonia, Lindenauer et al concluded that sleep apnea was linked to a higher rate of both initial ventilator use and clinical deterioration.47 One study reported that severe obstructive sleep apnea syndrome (OSAS) was an independent predictor of cardiovascular mortality risk.48 Another study revealed OSAS to be associated with a relatively high risk of acute kidney injury in critically ill patients.49 These findings imply that sleep apnea has the potential to induce severe complications involving several vital organs, and this probably explains the elevated risk of severe influenza in our sleep apnea group. Moreover, sepsis is regarded as severe systemic inflammation in response to infections such as influenza. One study discovered that patients with sepsis and sleep apnea had poorer prognosis than did those with sepsis but without sleep apnea.50
For a single medical institute, obtaining an appropriately large sample and ensuring sufficient follow-up duration to accurately evaluate the incidence of influenza-associated SARI after sleep apnea would be difficult. The NHIRD constituted an excellent resource with which we could explore and trace numerous cases of sleep apnea and severe influenza because of the database’s wide coverage of nearly the entire population of Taiwan. Our study demonstrated the study group to exhibit a higher prevalence of influenza-associated SARI compared with the comparison group after a 5.4-year mean follow-up period. To minimize possible bias, we took potential confounding factors—which included obesity, gender, comorbidities, and age—into account. The final result confirmed sleep apnea being an independent risk factor for influenza-associated SARI.
A few possible limitations of our executed study warrant mentioning. First, administrative claims data may constitute a less accurate source of sleep apnea and severe influenza diagnoses than would diagnoses made in a prospective, clinical setting. Second, we were unable to identify a patient’s sleep apnea type (central, obstructive, or mixed) from the ICD-9-CM codes. The respective effects of these types on influenza-associated SARI thus remain unclear. Third, obesity diagnosis was indicated by the ICD-9-CM code (278) instead of actual BMI, which could have led to misclassification bias. Fourth, recorded data from polysomnography could not be obtained, making it difficult to explore how the severity of sleep apnea and occurrence of severe influenza were associated. Fifth, the high prevalence of undiagnosed OSA in the study population would lead to a higher prevalence of SARI in the control group as well. Therefore, this effect may lead to an underestimation of the relationship between OSA and influenza-related SARI. Finally, the number of samples with Influenza-associated SARI was small, and studies with larger sample size and longer follow-up periods are necessary in the future.
The findings from this study may be useful in re-examining the prevention and treatment strategies for influenza infection in patients with sleep apnea. Vaccination and early oseltamivir administration should be actively considered in this group of patients. Additional prospective studies investigating the possible mechanism through which severe influenza is promoted in patients with sleep apnea are warranted.
Conclusion
Our executed research is a population-based cohort study exploring the sleep apnea–influenza-associated SARI relationship. We obtained strong evidence for sleep apnea as an independent risk factor for influenza-associated SARI. In addition, patients with COPD or CAD are also at higher risk of developing influenza-associated SARI. We suggest that physicians be cautious about the development of severe influenza in patients with sleep apnea. Vaccination and early oseltamivir administration should be actively considered in this group of patients.
Acknowledgments
The study was financially supported by grants from the Ministry of Science and Technology, Taiwan (MOST 109-2314-B-182A-054-) and Chang Gung Memorial Hospital, Taoyuan, Taiwan (CMRPG6J0331). We extend our gratitude to the Health Information and Epidemiology Laboratory of Chiayi Chang Gung Memorial Hospital (CLRPG6G0041) for their comments and assistance with data analysis. This manuscript was edited by Wallace Academic Editing.
Disclosure
The authors have no conflicts of interest for this work and no other funding or financial relationships to disclose.
References
1. Thompson WW, Comanor L, Shay DK. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis. 2006;194(Suppl 2):S82–91. doi:10.1086/507558
2. Thompson WW, Weintraub E, Dhankhar P, et al. Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respir Viruses. 2009;3:37–49. doi:10.1111/j.1750-2659.2009.00073.x
3. Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378(9807):1917–1930. doi:10.1016/S0140-6736(11)61051-9
4. Clayville LR. Influenza update: a review of currently available vaccines. Pharm Ther. 2011;36(10):659.
5. Troeger CE, Blacker BF, Khalil IA, et al. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the global burden of disease study 2017. Lancet Respir Med. 2019;7(1):69–89. doi:10.1016/S2213-2600(18)30496-X
6. Loubet P, Samih-Lenzi N, Galtier F, et al. Factors associated with poor outcomes among adults hospitalized for influenza in France: a three-year prospective multicenter study. J Clin Virol. 2016;79:68–73. doi:10.1016/j.jcv.2016.04.005
7. Paget J, Spreeuwenberg P, Charu V, et al. Global mortality associated with seasonal influenza epidemics: new burden estimates and predictors from the GLaMOR Project. J Glob Health. 2019;9(2):12. doi:10.7189/jogh.09.020421
8. Fitzner J, Qasmieh S, Mounts AW, et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ. 2018;96(2):122–128. doi:10.2471/BLT.17.194514
9. Taiwan Centers for Disease Control. Influenza. Available from: https://www.cdc.gov.tw/En/Category/ListContent/bg0g_VU_Ysrgkes_KRUDgQ?uaid=Zvnt3Ff941PorUmUD0-leA.
10. Su CP, Tsou TP, Chen CH, Lin TY, Chang SC. Seasonal influenza prevention and control in Taiwan-Strategies revisited. J Formos Med Assoc. 2019;118(3):657–663. doi:10.1016/j.jfma.2018.12.022
11. Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311–1322. doi:10.3978/j.issn.2072-1439.2015.06.11
12. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi:10.1016/S0140-6736(08)61622-0
13. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi:10.1016/S0140-6736(05)71141-7
14. McNicholas WT. COPD-OSA overlap syndrome: evolving evidence regarding epidemiology, clinical consequences, and management. Chest. 2017;152(6):1318–1326. doi:10.1016/j.chest.2017.04.160
15. Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. doi:10.1378/chest.09-0360
16. Tsai MS, Li HY, Huang CG, et al. Risk of Alzheimer’s disease in obstructive sleep apnea patients with or without treatment: real-world evidence. Laryngoscope. 2020;130(9):2292–2298. doi:10.1002/lary.28558
17. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi:10.1056/NEJMoa043104
18. Li HY, Lee LA, Tsai MS, et al. How to manage continuous positive airway pressure (CPAP) failure -hybrid surgery and integrated treatment. Auris Nasus Larynx. 2020;47(3):335–342. doi:10.1016/j.anl.2020.03.007
19. Li HY, Lee LA, Hsin LJ, et al. Intrapharyngeal surgery with integrated treatment for obstructive sleep apnea. Biomed J. 2019;42(2):84–92. doi:10.1016/j.bj.2019.02.002
20. Chen -TY-T, Chang R, Chiu L-T, Hung Y-M, Wei J-C-C. Obstructive sleep apnea and influenza infection: a nationwide population-based cohort study. Sleep Med. 2021;81:202–209. doi:10.1016/j.sleep.2021.02.034
21. Chiner E, Llombart M, Valls J, et al. Association between obstructive sleep apnea and community-acquired pneumonia. PLoS One. 2016;11(4):e0152749. doi:10.1371/journal.pone.0152749
22. Su VY, Liu CJ, Wang HK, et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. Cmaj. 2014;186(6):415–421. doi:10.1503/cmaj.131547
23. Sampol G, Rodés G, Ríos J, Romero O, Lloberes P, Morell F. [Acute hypercapnic respiratory failure in patients with sleep apneas]. Arch Bronconeumol. 2010;46(9):466–472. Spanish. doi:10.1016/j.arbres.2010.05.009
24. Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest. 2009;136(3):787–796. doi:10.1378/chest.09-0615
25. Cheng TM. Reflections on the 20th anniversary of Taiwan’s single-payer national health insurance system. Health Aff. 2015;34(3):502–510. doi:10.1377/hlthaff.2014.1332
26. Chang GH, Su YC, Lin KM, et al. Deep neck infection in systemic lupus erythematosus patients: real-world evidence. Sci Rep. 2020;10(1):4133. doi:10.1038/s41598-020-61049-4
27. Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308(18):1906–1914. doi:10.1001/2012.jama.11975
28. Tsai MS, Chang GH, Chen WM, et al. The association between decompensated liver cirrhosis and deep neck infection: real-world evidence. Int J Environ Res Public Health. 2019;16:3863.
29. Tsai MS, Lin MH, Lee CP, et al. Chang Gung research database: a multi-institutional database consisting of original medical records. Biomed J. 2017;40(5):263–269. doi:10.1016/j.bj.2017.08.002
30. Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in Taiwan. J Epidemiol. 2014;24(6):500–507. doi:10.2188/jea.JE20140076
31. Chang GH, Ding MC, Chen YC, et al. Real-world evidence for increased deep neck infection risk in patients with rheumatoid arthritis. Laryngoscope. 2020;130(6):1402–1407. doi:10.1002/lary.28272
32. Hsieh CY, Su CC, Shao SC, et al. Taiwan’s national health insurance research database: past and future. Clin Epidemiol. 2019;11:349–358. doi:10.2147/CLEP.S196293
33. Chang GH, Chen YC, Lin KM, et al. Real-World database examining the association between Sjogren's syndrome and chronic rhinosinusitis. J Clin Med. 2019;8(2):155. doi:10.3390/jcm8020155
34. GH Chang, Ding MC, YH Yhang, et al. High risk of deep neck infection in patients with type 1 diabetes mellitus: a nationwide population-based cohort study. J Clin Med. 2018;7(11). doi:10.3390/jcm7110385.
35. Tsai MS, Yang YH, Liu CY, et al. Unilateral vocal fold paralysis and risk of pneumonia: a nationwide population-based cohort study. Otolaryngol Head Neck Surg. 2018;158(5):896–903. doi:10.1177/0194599818756285
36. Tsai MS, Lee LA, Tsai YT, et al. Sleep apnea and risk of vertigo: a nationwide population-based cohort study. Laryngoscope. 2018;128(3):763–768. doi:10.1002/lary.26789
37. Tsai ML, Mao CT, Chen DY, Hsieh IC, Wen MS, Chen TH. Short- and long-term major cardiovascular adverse events in carotid artery interventions: a nationwide population-based cohort study in Taiwan. PLoS One. 2015;10(3):e0121016. doi:10.1371/journal.pone.0121016
38. Chen BK, Yang CY. Differences in age-standardized mortality rates for avoidable deaths based on urbanization levels in Taiwan, 1971–2008. Int J Environ Res Public Health. 2014;11(2):1776–1793. doi:10.3390/ijerph110201776
39. Ho T-W, Ruan S-Y, Huang C-T, Tsai Y-J, Lai F, Yu C-J. Validity of ICD9-CM codes to diagnose chronic obstructive pulmonary disease from national health insurance claim data in Taiwan. Int J Chron Obstruct Pulmon Dis. 2018;13:3055. doi:10.2147/COPD.S174265
40. Mok EM, Greenough G, Pollack CC. Untreated obstructive sleep apnea is associated with increased hospitalization from influenza infection. J Clin Sleep Med. 2020;16(12):2003–2007. doi:10.5664/jcsm.8744
41. Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi:10.1136/bmj.f5061
42. Chen HC, Lee LA, Hsin LJ, et al. Transverse retropalatal collapsibility is associated with obstructive sleep apnea severity and outcome of relocation pharyngoplasty. Otolaryngol Head Neck Surg. 2015;153(6):1056–1063. doi:10.1177/0194599815599527
43. Chen HC, Wang CJ, Lo YL, et al. Parapharyngeal fat pad area at the subglosso-supraglottic level is associated with corresponding lateral wall collapse and apnea-hypopnea index in patients with obstructive sleep apnea: a pilot study. Sci Rep. 2019;9(1):17722. doi:10.1038/s41598-019-53515-5
44. Beal M, Chesson A, Garcia T, Caldito G, Stucker F, Nathan CO. A pilot study of quantitative aspiration in patients with symptoms of obstructive sleep apnea: comparison to a historic control group. Laryngoscope. 2004;114(6):965–968. doi:10.1097/00005537-200406000-00002
45. Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. 1985;2010(108):212–218.
46. Morimoto S, Takahashi T, Okaishi K, et al. Sleep apnoea syndrome as a risk for mortality in elderly inpatients. J Int Med Res. 2012;40(2):601–611. doi:10.1177/147323001204000222
47. Lindenauer PK, Stefan MS, Johnson KG, Priya A, Pekow PS, Rothberg MB. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest. 2014;145(5):1032–1038. doi:10.1378/chest.13-1544
48. Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath. 2017;21(1):181–189. doi:10.1007/s11325-016-1393-1
49. Dou L, Lan H, Reynolds DJ, et al. Association between obstructive sleep apnea and acute kidney injury in critically ill patients: a propensity-matched study. Nephron. 2017;135(2):137–146. doi:10.1159/000453367
50. Huang CY, Chen YT, Wu LA, et al. Sleep apnoea patients have higher mortality when confronting sepsis. Eur J Clin Invest. 2014;44(1):38–45. doi:10.1111/eci.12187
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


