Back to Journals » Nature and Science of Sleep » Volume 12
Sleep, a Governor of Morbidity in PTSD: A Systematic Review of Biological Markers in PTSD-Related Sleep Disturbances
Authors Maguire DG , Ruddock MW , Milanak ME, Moore T , Cobice D , Armour C
Received 4 May 2020
Accepted for publication 6 July 2020
Published 31 July 2020 Volume 2020:12 Pages 545—562
DOI https://doi.org/10.2147/NSS.S260734
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sutapa Mukherjee
Daniel G Maguire,1 Mark W Ruddock,2 Melissa E Milanak,3 Tara Moore,1 Diego Cobice,1 Cherie Armour4
1Biomedical Sciences Research Institute, Ulster University, Coleraine BT52 1SA, Northern Ireland; 2Randox Laboratories Ltd, Clinical Studies, Crumlin, County Antrim BT29 4QY, Northern Ireland; 3Department of Psychiatry & Behavioral Sciences, Medical University of South Carolina, Charleston, SC 29425, USA; 4School of Psychology, David Keir Building, Queen’s University Belfast, Belfast BT9 5BN, Northern Ireland
Correspondence: Mark W Ruddock Tel +44 02894422413
Email [email protected]
Background: Sleep disturbances (SD) are the most impactful and commonly reported symptoms in post-traumatic stress disorder (PTSD). Yet, they are often resistant to primary PTSD therapies. Research has identified two distinct SDs highly prevalent in PTSD; insomnia and nightmares. Those who report SDs prior to a traumatic event are at greater risk for developing PTSD; highlighting that sleep potentially plays a role in PTSD’s pathology. To further understand the pathobiological mechanisms that lead to the development of PTSD, it is first imperative to understand the interplay which exists between sleep and PTSD on a biological level. The aim of this systematic review is to determine if biological or physiological markers are related to SD in PTSD.
Methods: A systematic literature search was conducted on the electronic databases; Medline, Embase, AMED and PsycINFO, using Medical Subject Headings and associated keywords.
Results: Sixteen studies were included in the final analyses. Physiological makers of autonomic function, and biochemical markers of HPA-axis activity; inflammatory processes; and trophic factor regulation were related to the severity of SDs in PTSD.
Conclusion: These findings add to the growing literature base supporting a central focus on sleep in research aiming to define the pathophysiological processes which result in PTSD, as well as emphasising the importance of specifically targeting sleep as part of a successful PTSD intervention strategy. Resolving SDs will not only reduce PTSD symptom severity and improve quality of life but will also reduce all-cause mortality, hospital admissions and lifetime healthcare costs for those with PTSD. Limitations of the current literature are discussed, and key recommendations future research must adhere to are made within.
Keywords: post-traumatic stress disorder, sleep disturbances, insomnia, nightmares, biomarkers
Introduction
Post-traumatic stress disorder (PTSD) is a debilitating condition which may develop following exposure to, witnessing, or learning about, a traumatic event such as; actual or threatened death, actual or threatened serious injury, or actual or threatened sexual violence.1 These events are referred to as criterion A events, as per the diagnostic and statistical manual of mental disorders, fifth edition (DSM-5).1
A diagnosis of PTSD is made based on the presentation of symptoms, which are arranged into four symptom clusters; alterations in arousal and reactivity, intrusions, avoidance and negative alterations in cognitions and mood.1 Lifetime prevalence of PTSD is approximately 2–8%, but this differs by geographical location and the level of trauma exposure experienced in a particular population.2–5 As an example, military and emergency service personnel are exposed to more trauma than the general population, which is reflected in their respective PTSD prevalence rates of 13.4% and 22%.3,4,6-9
Sleep Disturbances as a Core Pathological Factor in PTSD
Difficulty sleeping is considered one of the most commonly experienced symptoms of PTSD,5,10,11 Two distinct sleep disruptions exist, insomnia (persistent difficulty falling asleep or staying asleep) and nightmares. These fall under the DSM−5 PTSD symptom clusters of “alterations in arousal and reactivity” and ‘intrusions’, respectively.1 A recent survey of 2,647 US adults exposed to potentially traumatic events by Milanak et al (2019) found that 92% of those who met the criteria for PTSD experienced at least one symptom of disturbed sleep.10 Although experiencing insomnia and nightmares together accounted for the majority of SDs in PTSD, Milanak et al (2019) noted that where only a single sleep disturbance was endorsed, insomnia was more prevalent than nightmares (56.7% vs 24.9% vs 11.3%).10
A variation in the prevalence of SDs in PTSD is common in the literature.12–14 Some of this variability may be explained by differences in population demographics such as sex and level and type of trauma exposure. However, the effect these, and other population factors have on the endorsement of SDs in PTSD, requires further research. Methodological differences in measuring sleep is a further source of variability. Modern sleep research has capitalized on advancements in wearable technology to provide objective measures of sleep in the free-living environment.15–18 Actigraphy is the most common method, and involves participants wearing a personal accelerometer device, normally on the wrist, to record movement during sleep.19–21 This movement data is then translated to provide information about a participants’ sleep.22–24 Incorporation of objective methods, such as actigraphy, improves upon a sole reliance on subjectively reported sleep.17
Sleep is a central treatment and research objective in PTSD, and may represent a modality through which PTSD treatment uptake can be improved, due to a lack of the stigmatization associated with other PTSD symptomology.11 However, the paradigm of causality between PTSD and disrupted sleep (both nightmares and insomnia) remains ambiguous. Originally considered merely as symptoms of PTSD, recent studies have established a theory of sleep disturbances preceding traumatic exposure as a risk factor for PTSD development.25,26 Longitudinal examination of a Dutch military cohort by Van Liempt et al (2013) revealed that experiencing nightmares before combat deployment, was somewhat predictive of the development of PTSD post-deployment.27 Furthermore, experiencing insomnia immediately following trauma exposure has been found to be predictive of PTSD development a year later, suggesting that a potentially stunted ability of sleep to consolidate memory could contribute to the development of PTSD.28 Moreover, primary therapies specifically targeting PTSD, such as cognitive behavioural therapy (CBT) for PTSD, are often insufficient to completely improve SDs despite a reduction in other PTSD attributable symptoms.29 This would appear to suggest that SDs, both insomnia and nightmares, are a component of the pathology of PTSD, rather than solely a symptom; a hypothesis also postulated for depression and anxiety disorders.30–32
Improving sleep quality has been associated with a clinically relevant reduction in the severity and impact of PTSD symptomology, thus highlighting the importance of specifically targeting sleep as part of a multi-faceted approach for PTSD treatment.33–35 Despite these findings, and high prevalence, a specific mechanistic role of sleep in PTSD, as well as the reciprocal nature of the relationship between SDs and PTSD, remains to be established.25 Furthermore, subjective and objective sleep measures have yielded conflicting reports on the number of awakenings and sleep duration in PTSD, depending on the comparative population used.12,14,36,37 Therefore, to fully establish the directionality of the relationship between PTSD and disordered sleep, and the individual contributions of insomnia and nightmares, there is a need to examine if an interplay exists between these factors on a biological level.
HPA-Axis Regulation and Inflammation in PTSD
To determine any biological interconnection between sleep and PTSD, it is important to first consider each separately. The pathobiology of PTSD remains an active area of research, which to date has focused mainly on two functional systems; 1) the hypothalamic-pituitary-adrenal (HPA) axis and 2) systemic inflammation.
PTSD and the Hypothalamic-Pituitary-Adrenal Axis (HPA-Axis)
Exposure to a threat, which may be either physical or psychological, triggers a physiological response. Termed the “stress response”, this results in the activation of biological mechanisms which allow a reaction to the threat. Central regulation of this process is facilitated by the HPA-axis, functional alterations to which have been linked to psychiatric conditions such as depression, anxiety and PTSD.38,39
Following exposure to a stressor, neurons of the paraventricular nucleus (PVN) in the hypothalamus respond by releasing corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP) into hypophysial portal blood, causing the pituitary gland to release adrenocorticotrophic hormone (ACTH) into the general circulation.40 ATCH acts on the adrenal glands to stimulate the synthesis and release of glucocorticoids, of which cortisol is the primary in humans.40 Regulation of HPA-axis activity is facilitated by cortisol-mediated negative feedback on the anterior pituitary, PVN and hippocampus, resulting in an inhibition of CRH, AVP and ACTH release.40
Two cortisol receptors exist in humans: mineralocorticoid receptors, which function as high-affinity cortisol stores, and functionally active glucocorticoid receptors (GRs).40 FK506 binding protein (FKBP5) has recently been characterised as a functional inhibitor of GRs, preventing their activation by lowering the GRs affinity for cortisol binding.41 Longitudinal examination of FKPB5 mRNA expression in a military cohort, revealed low pre-deployment expression was predictive of PTSD occurrence post deployment, a finding replicated in further studies.41–45 This would appear to suggest that a stunted inhibition of the HPA-axis stress response could contribute to the pathobiology of PTSD. However, further research is required to explore this hypothesis as contradictory findings, such as elevated and attenuated cortisol levels, remain. Albeit, these may be somewhat explained by differences in comparative control populations, gender differences, types of traumatic events experienced and sampling time and conditions.46 Despite controversy over the exact functional response of the HPA-axis in PTSD, inhibitors of FKPB51, the functional protein produced in response to FKBP5 expression, are being developed with the aim to use as novel therapies in the treatment of psychiatric conditions such as PTSD and depression.39 Further understanding of the HPA-axis, GRs and associated molecular partners, in psychiatric disorders, is required before any therapeutic potential of such FKBP51 antagonists can be discussed. However, by inhibiting the activity of FBKP51 without activating GRs themselves, such antagonists may prove useful experimental tools to aid in this endeavour.39
PTSD and Systemic Inflammation
PTSD has been hypothesised to be accompanied by a systemic rise in pro-inflammatory activity.47 Activation of cytotoxic T lymphocytes in response to stress (physical or psychological), results in an inflammatory response dominated by Th2 type cells, producing an unrestrained production of pro-inflammatory cytokines.48,49 It is therefore unsurprising that in a recent meta-analysis of inflammatory markers in PTSD, increased pro-inflammatory interleukin−6 (IL−6), IL−1β, TNF-α and interferon gamma (INF-γ) were observed.50 This is supported by increased inflammation present in the peripheral circulation, adrenal glands and neurological tissue of predator exposure mouse models of PTSD.51
Further fitting this inflammatory hypothesis is the established increased incidence of cardiovascular disease, type 2 diabetes, chronic fatigue syndrome and other metabolic syndromes in PTSD populations, conditions which are progressed by dysregulated inflammatory processes.52–59 Pathological inflammation is also demonstrated to result in neuronal loss, which is interesting considering the loss of hippocampal volume noted in both humans and mouse models of PTSD.60–62
PTSD and Trophic Factors
Trophic factors, such as Brain-Derived Neurotrophic Factor (BDNF), Nerve Growth Factor (NGF) and Vascular Endothelial Growth Factor (VEGF), are central regulators of the CNS, essential for maintaining synaptic plasticity, learning and memory.63–65 In the context of psychiatric disorders, trophic factors have demonstrated altered expression levels in both PTSD and depression, although their exact functional role remains ill-defined.66–68 The literature surrounding trophic factors and PTSD remains particularly unclear, with findings often opposing one and other.69–71 The intertwined relationship between trophic factors and sleep is particularly interesting in this case. BDNF and its molecular coplayers may have a central role in sleep homeostasis, particularly slow-wave sleep.72 Trophic factors may therefore serve as a linchpin in the relationship between PTSD and its related SDs.
Interconnections Between Sleep and PTSD Pathophysiology
The physiological role of sleep remains the focus of much research but elucidated roles include cellular and tissue repair, cognitive and memory processing, maintenance of synaptic plasticity, through a process known as “pruning”, and free radical detoxification.73,74 As such, disruption of sleep through either sudden awakening from nightmares or through reduction in consolidated, sustained sleep from insomnia, may contribute to a host of pathophysiological processes.
Pressure to fall asleep, facilitated by the circadian drive, is enacted through melatonin secretion. Melatonin, under the control of the suprachiasmatic nuclei (SCN), is secreted from the pineal gland through metabolism of serotonin, upon darkness onset.75 Light sensing is facilitated by direct communication of photoreceptive retinal ganglion cells with the SCN via the retinohypothalamic tract. Secretion increases at twilight, reaches a peak during the middle of the night and then begins to decrease to basal daytime levels.76 Metabolization of melatonin primarily occurs in the hepatocytes of the liver where it undergoes the process of hydroxylation, producing 6-hydroxymelatonin which is then sulphated and excreted in urine.77 However, the processes of demethylation, deacetylation and oxidation also occur, albeit to a lesser extent.78 Hypothesised to be resultant from a circadian phase shift, alterations in melatonin production have been noted in a range of neurological and neuropsychological conditions however, this has not yet been noted in PTSD.75,79
Therefore, it would be of interest to investigate the rate and pathway of melatonin metabolization in PTSD, considering the neuroprotective-free radical scavenging properties of melatonin oxidation products and the hypothesised increased oxidative stress in PTSD.78 Moreover, the role of melatonin in blood pressure regulation is interesting considering the persistently reported presence of hypertension diagnoses in PTSD populations.80–83
Occurrence of metabolic and cardiovascular diseases which result in increased mortality, similar to those observed in PTSD populations, has been associated with persistent insomnia in longitudinal analysis.84 Akin to that previously mentioned in PTSD, elevation of inflammatory status has been noted in populations with persistent insomnia, in the form of increased pro-inflammatory C-reactive protein (CRP) and IL-6.85 It would therefore be of interest to examine SDs of PTSD in relation to biological markers, differentiating and examining the individual roles played by insomnia and nightmares. SDs represent targetable symptoms of PTSD that with successful treatment not only have the potential to reduce PTSD symptomology and improve quality of life but may also reduce lifetime all-cause mortality among PTSD populations.
This review aims to systematically examine the literature to determine; 1) are biochemical or physiological markers related to SDs in PTSD, 2) are biochemical or physiological markers related to the degree of SD severity in PTSD and, 3) does improvement in SDs result in a change of biochemical or physiological markers in PTSD populations?
Methodology
Following a broad review of the literature, a search strategy was devised to identify research articles able to address the question, “Are biological and physiological markers related to sleep in PTSD?”. Set out in Table 1, this strategy was used to search the electronic databases; EMBASE, Medline, AMED and PsycINFO using the OVID online platform. Searches were carried out periodically between March 2018 and March 2020.
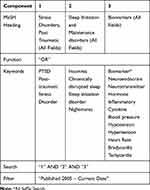 |
Table 1 Literature Search Strategy, Consisting of Medical Subject Headings (MeSH) and Keywords. OVID Online Platform Was to Search EMBASE, Medline, AMED, PsychINFO Databases |
Inclusion Criteria
Studies were eligible for inclusion if they contained (1) a human adult population, (2) a measurement of PTSD (either valid self-assessment methods such as the PTSD checklist (PCL), or clinical diagnosis), (3) a subjective or objective measurement of sleep and (4) either a non-invasive physiological measurement (independent of sleep quantification method) or any biochemical assessment carried out on a biological sample (blood/tissue). Randomized control trials, cohort, case-controlled, and cross-sectional studies were eligible for inclusion and were not limited based on population gender, race, age or trauma exposure. Included studies were limited to peer-reviewed articles published in English between September 2008 and March 2020.
Exclusion Criteria
Articles were excluded if they did not contain: (1) a PTSD subpopulation or PTSD assessment, (2) a subjective or objective measurement of sleep, and (3) a physiological or biochemical measurement, independent of the sleep quantification method. For example, for eligibility assessment electroencephalogram (EEG), recorded as part of polysomnography (PSG), was considered a component of sleep measurement rather than a physiological measurement. However, heart rate (HR) and peripheral oxygen saturation were deemed appropriate as a physiological measurement, if analysed independently of sleep. Pharmacological treatment trials, either for sleep or PTSD symptoms, were excluded. PTSD populations with concurrent traumatic brain injury (TBI) were excluded to minimise confounding of results. Case studies and conference abstracts were also excluded.
Screening and Data Extraction
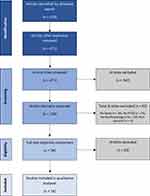
The process of identifying, screening and assessing articles for eligibility criteria is summarized in Figure 1. Using the previously stated search strategy, 513 research entries were identified, and their references imported to a web-based reference management programme (RefWorks), for the purpose of duplicate identification and removal. Removal of duplicates resulted in 471 entries for title and abstract screening. Article titles were screened for relevance to PTSD and sleep, resulting in 129 being retained for abstract review. Abstracts were examined to confirm a PTSD population, a measure of sleep, a biological or physiological measure and that the article was original research. Where any details could not be established, articles were retained for full-text assessment. Following abstract assessment, 38 articles were retained and subject to full-text eligibility assessment. Sixteen articles were deemed to meet the eligibility criteria and were included in the final analyses.
Data extracted from the included articles (n=16) encompassed; the authors, date of publication, study inclusion and exclusion criteria, excluded participants, population size, age, sex, study design, objective sleep measurement, subjective sleep measurement, sleep intervention, PTSD symptom assessment, physiological measurement(s), biochemical measurement(s), gene expression analysis, any additional measures, key findings and key limitations. For each study, the results of this data extraction are set out in Table 2. Full references for each study are available in the bibliography.
 |
Table 2 Summary of Data Extraction from Included Studies |
Quality Assessment
Criteria adapted from the Critical Appraisal Skills Programme (CASP) checklist was used as a quality assessment for each included article.86 Key findings of this review were then subject to a quality of findings assessment, using the Grading of recommendations, assessment, development and evaluations (GRADE) criteria for systematic reviews.87 Results of GRADE analysis are shown in Table 3, produced using GRADEpro Guideline Development Tool [Software], McMaster University, 2015 (developed by Evidence Prime, Inc.), Available from gradepro.org. Summary grades available are as follows; High (Confidence in the study findings is high); Moderate (Confident that the effect observed is close to the real effect but may also be substantially different); Low (The true effect may differ significantly from that observed); and Very Low (The effect is likely to be very different from that observed).
 |
Table 3 Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) Assessment of Evidence Quality. Final GRADE Quality Assessment Possibilities; High, Moderate, Low, Very Low |
Results
A total of 2,838 participants were included across the 16 studies, of which 68% were male and 30% female. No information on sex was available for 2% of participants. Most study populations were “male and female” (10/16), three were “female only” and two “male only”. Sex was not reported in the remaining study. The overall mean age was 37.93 ± 7.35 years, with study mean ages ranging from 21.7 to 51.4 years.
Eight/16 studies consisted exclusive of military populations (active service and veterans), expanding to 9/16 when a further study which incorporated emergency service personnel were included. Of the remaining studies, two utilised an urban population, one a kidney transplant population, and four were population non-specific.
Relationship analysis between biological measures and sleep was routinely performed however, tri-directional analysis with PTSD, or PTSD and biological-specific analyses, was often unexplored or unreported (Tables S1 and S2). Key outcome measures included subjective and objective sleep, PTSD symptoms, depression, HR and variability, blood pressure, inflammatory and anti-inflammatory status, HPA-axis function and mRNA gene expression. Sleep assessment methods varied greatly, with 10 different tools employed across the 16 included studies, as visualised in Figure 2. Although when objective methods were employed, heterogeneity was greatly reduced with only polysomnography or actigraphy utilised.
Most studies were designed primarily to examine the role and relationship of sleep either to psychological symptomology or biological markers. Study designs varied but generally can be divided into the following three categories: epidemiological (Studies 11,13), intervention (Studies 1,3,4,6,7,8,9,10,15) or night-time specific (Studies 2,5,12,14). A further study carried out a baseline group comparison of PTSD vs non-PTSD controls (Study 16).
Physiological Measures – Assessing Autonomic Function
Physiological measurements of the cardiovascular or pulmonary systems were recorded in 11/16 studies (Studies 2,4,5,6,7,9,11,12,13,14,15). Of these, quantification of blood pressure (BP), or BP regulation was the dominant measure (6/11). Baroreceptor sensitivity (BRS), a measure of the ability to regulate BP, was found significantly lower in a PTSD sub-population (Study 7). This negative relationship was found to be exacerbated with declines, and mitigated with gains, in sleep quality, a relationship upheld with direct examination of blood pressure (Studies 2, 9.11 and 13). However, PTSD status alone was found not to predict a “non-dipping” designation of nocturnal blood pressure, an important indicator linking BP and cardiovascular disease (Study 2).88 Although “non-dipping” was related to poorer sleep quality and was associated with a more severe PTSD phenotype, with a greater experience of traumatic events and hyperarousal symptoms.
Used as an estimate of sympathetic and parasympathetic activity, HR and/or heart rate variability (HRV) measures were examined in 6/11 studies (Studies 4,5,6,9,12,14). Standard deviation of the normal heartbeat to beat interval (SDNN), a measure indicative of vagal autonomic influence, decreased following interventions which concurrently increased sleep quality and decreased PTSD symptoms (Studies 4,9). However, a direct relationship with insomnia severity index score was not supported (Study 4). Further measures of HRV included normalised high frequency (nHF) and low frequency to high-frequency ratio (LF/HF) of respiratory sinus arrhythmia (RSA), which correlated with total sleep time in trauma controls but not PTSD (Study 5).89 RSA, indicative of parasympathetic tone, was investigated in study 15 in relation to nightmare or distressing dream occurrence in PTSD.90 Lower sleep period RSA was found predictive of an increased likelihood of endorsing a nightmare or distressing dream. Study 15 also noted nightmares or distressing dreams were associated with an increase in the number of sleep respiratory events (hypopneas or apneas).
Study 6 examined skin conductance level (SCL) reactivity, a measure indicative of sympathetic activity. Decreases in SCL reactivity related to decreased nightmare severity, improvements in self-assessed sleep quality and a reduction in CAPS assessed PTSD symptom severity, following exposure, relaxation and re-scripting therapy (ERRT) for nightmares.
Baseline and night-time HR was evaluated in studies 14 and 12, respectively. An increase in HR was observed immediately preceding or following nightmare occurrence (Study 12), albeit baseline comparison between PTSD and health controls yielded no significance (Study 14). Interestingly, baseline HR was significantly lower in trauma controls compared to PTSD (Study 14). HR was also recorded in study 15 however findings were not reported.
Biological Measures – Gene Expression and Biochemical Quantification
Three main biological processes, or systems, were identified as the focus of the included studies; (1) inflammation, (2) HPA-axis activity and (3) trophic factor regulation. With the aim to elucidate the effect sleep has on these systems in PTSD populations, biochemical and gene expression analyses were the main techniques employed and were performed in 7/16 studies.
Inflammation
Biomarkers pertaining to inflammatory status were investigated in 4/7 studies, in the form of peripheral blood: CRP (Studies 8,9,16), IL-6 (Studies 8, 16), IL-10 (Study 9), TNF-α (Study 16), soluble IL-6 receptor (Study 16), IL-1β (Study 16) and global mRNA gene expression analysis (Study 3). Improvements in baseline sleep resulted in differential mRNA expression in 217 coding genes of which, pro-inflammatory IL-1β, IL-6, IL-8, IL-13, CCL3, CCL4 and CCL5 genes experienced a significant fold decrease. A tandem increase in the expression of the inflammatory regulatory genes TLR1, TLR4, TLR7 and TLR8, was also observed. Taken together this suggests that improving sleep is associated with an increase in the regulation of inflammatory processes, which results in a global decrease of inflammatory status, a hypothesis reflected with decreases in plasma IL-6 and CRP mediated by improved sleep (Studies 8 and 9). However, no relationship between Athens insomnia scale assessed sleep and IL-6, sIL-6R, CRP or TNF-α was noted in Study 16. In relation to PTSD status and inflammation, reductions to PTSD symptom severity as assessed by PCL-M followed improved sleep and were accompanied by a non-significant decrease in the expression of the inflammatory regulatory IL-10 (Study 9). However, no relationship between PTSD symptom severity and the proinflammatory, CRP, IL-6, soluble IL-6 receptor, IL-1β and TNF-α cytokines was substantiated by correlation analysis in Study 16.
HPA-Axis
HPA axis function, considered as a physiological response to physical and/or psychological stress, formed the basis of 2/7 studies, which measured cortisol, 11-deoxycortisol and adrenocorticotrophic hormone (ACTH) (Studies 10, 14). A comparative group analysis of nocturnal or baseline cortisol and ACTH resulted in no significant difference between PTSD, trauma-exposed (TCs) or health controls (HCs) (Studies 10.14). Examination of cortisol: ACTH ratio revealed a significant ratio reduction in PTSD vs TCs, and borderline significance when compared to HCs, a finding accentuated between 07:00 and 08:00 am, i.e. the cortisol awakening response and natural circadian peak. This appears to suggest decreased responsiveness of the adrenal cortex to ACTH (Study 14). A finding further supported by increased 11-deoxycortisol at baseline, with similar ACTH, observed in PTSD when compared with controls (Study 10).
Total nocturnal ACTH was positively related to the number of awakenings, and was an independent, inverse predictor of slow-wave sleep (Study 14). Congruently, the relationship between ACTH and delta power sleep was negative in PTSD, but not controls (Study 10). Suggesting increased ACTH in PTSD negatively affects sleep, a relationship not apparent in control populations.
Administration of a metyrapone challenge to remove negative feedback to the hypothalamus, by inhibiting cortisol synthesis, resulted in a greater reduction in delta power sleep, a greater decrease in cortisol and increase of ACTH levels in PTSD compared to controls (Study 10). This would suggest an amplified response to the negative feedback of cortisol at the level of the hypothalamus in PTSD. Interestingly, improvements to sleep resulted in a fold increase of glucocorticoid inhibitory FKB506 binding protein and FK506 binding protein 15 mRNA expression, potentially relating sleep as an additional element regulating HPA-axis function (Study 3).
Trophic Factors
Trophic factors, insulin-like growth factor-1 (IGF-1) and BDNF, implicated in the maintenance of synaptic plasticity and learning and memory consolidation, were examined following a CBT-I intervention to improve sleep (Study 1). Sleep improvements resulted in a significant increase in IGF-1 but no significant changes in BDNF were observed. At baseline, PTSD subgroup analysis revealed no difference in the concentration of either trophic factor, and CBT-I treatment reductions in PTSD symptoms were unrelated to IGF-1 or BDNF changes.
Depression
A wide range of self and clinician-administered psychological assessments were reported on, of which depression was the most common (11/16). Intervention studies which examined sleep as a function over time reported improvements in sleep were significantly associated with reductions in depression symptomology (Studies 1,3,8). Congruently, declines in sleep over time were associated with an increase in depression ratings. This finding is further supported with a noted relationship between sleep quality and depression in kidney transplant recipients (Study 13). Sleep-related improvements in depression were examined independently to PTSD status and symptoms.
In summary, five main outcomes were identified; (1) SDs regulate BP in PTSD, (2) sympathetic dominance in PTSD is mediated by sleep, (3) sleep regulates global inflammatory status, (4) protection from glucocorticoid overexposure in PTSD may be at the expense of sleep and (5) trophic factor regulation is affected by changes to sleep in PTSD.
Discussion
To understand the biological interplay between PTSD and associated SDs, this review evaluated studies which examined biochemical and/or physiological markers in PTSD populations, with a specific reference to sleep. Inclusion of intervention, night-time specific, epidemiological and baseline studies provided an all-encompassing overview. Differential sleep period assessments, such as single point quantifications and time course comparisons, while not directly comparable, together yield valuable mechanistic insights into altered biological processes potentially associated with sleep in PTSD.
The most commonly reported measure across all studies was BP or associated BRS. Baroreceptor activation responds to increased BP by increasing parasympathetic, and decreasing sympathetic stimulation of the heart and blood vessels.91,92 Findings of reduced BRS in PTSD populations were noted to be influenced by sleep quality. Poorer sleep resulted in further reductions to BRS, which increased with improvements in sleep, suggesting sympathetic dominance in PTSD can be somewhat reduced by alterations to sleep, a finding mirrored by HRV and skin conductance measures. Furthermore, the note that attenuation of sleep RSA was predictive of nightmare/distressing dream occurrence demonstrates this phenomenon is also related to nightmares in PTSD. This is notable considering that hypertension and cardiovascular disease (CVD) rates are higher in PTSD populations, suggesting in this population both sleep quality and nightmares represent targetable and modifiable CVD drivers which with improvements, could reduce CVD development in this vulnerable population.83
Nocturnal BP regulation is emerging as a significant predictor of cardiovascular events in hypertension patients, with non-dipping (defined as less than 10% decrease in nocturnal BP vs daytime BP) associated with increased mortality.93 Non-dipping nocturnal BP was associated with poorer quality sleep (Study 2). However, PTSD was not found to be a predictor of dipping status, opposing the previous observation of reduced BP regulatory ability and sympathetic dominance in PTSD. Nonetheless, non-dipping was associated with a more severe PTSD phenotype which fits with the role of cortisol in BP dipping. Reduced variation in diurnal cortisol is associated with non-dipping designation.94 Thus, a more severe PTSD phenotype which results in a protective inhibition of glucocorticoid response may represent a mechanism capable of accounting for this observation, as discussed further below.93
Findings from this review indicate that a reduced responsiveness of the adrenal cortex to ACTH stimulation may exist as a functionally protective mechanism in PTSD. This results in a reduced cortisol:ACTH ratio, particularly evident during the cortisol awakening response, and a greater responsiveness of the hypothalamus to cortisol negative feedback removal, as demonstrated via a cortisol inhibiting metyrapone challenge. The need to develop such a protective mechanism is unsurprising considering alterations to circadian oscillations of glucocorticoids (cortisol) and chronic exposure have a detrimental effect on synaptic plasticity, learning and memory.95 However, this may be at the expense of sleep quality in PTSD, as ACTH was positively related to the number of night-time awakenings, was an inverse predictor of restorative slow-wave sleep, and had a negative relationship with delta power sleep in PTSD. Improvements to sleep resulted in alterations to glucocorticoid regulatory mRNA expression, further intertwining sleep and HPA-axis regulation in PTSD.
Specific investigations of this mechanism are warranted to establish directionality. Are detrimental effects to sleep resultant from a mechanism aiming to prevent pathologic exposure to glucocorticoids in PTSD? Or, does poor sleep quality result in an inappropriate activation of this mechanism, pre-disposing an individual to PTSD development due to a blunted glucocorticoid responsiveness to stress? Most likely, we hypothesise a combination of the two is likely to be true, representing alternative activation arms of a common mechanism. If validated, this represents a promising research avenue for the design and development of new, specific pharmaceuticals for PTSD treatment protocols. Moreover, reported poor sleep quality could be used to identify PTSD “at risk” individuals within “at risk” populations (such as military and first responders), and therapies shown to improve sleep quality, such as CBT-I for insomnia or nightmare re-scripting therapy, could be used as prophylactic treatments potentially capable of reducing PTSD susceptibility.96
Of further intrigue is the work in mouse models of PTSD, whereby enforced sleep deprivation following traumatic exposure has been found to reduce stress behaviours associated with recall exposure.97 An effect that was dependant on HPA-axis activity.97 Although such mouse models of PTSD are translationally limited, this serves as an additional point reinforcing the importance of the HPA-axis and sleep in PTSD susceptibility, progression and treatment.
An increased inflammatory status, associated with increased mortality risk, has been independently associated with SDs, PTSD and other psychiatric conditions such as depression.49,56,84,85,98,99 Results from this review indicate improvements to sleep result in both a decrease in pro-inflammatory cytokines and mRNA expression, and an increase in inflammatory regulatory mRNA. This suggests disturbed sleep as a driver of systemic inflammation in PTSD populations, capable of contributing to comorbid conditions which are progressed by dysregulated inflammatory processes, such as type 2 diabetes and CVD, to which this population are vulnerable.51–58 However, more research is required to fully establish the role of inflammation and provide a more complete picture of total inflammatory status in PTSD, and the role played by sleep. This review has highlighted that conflictions remain in the literature between studies examining mRNA expression and those which examine functional proteins. Perhaps future studies should aim to examine the role of post-translational modifications. Our hypothesised protective inhibition of the HPA-axis, linked to sleep, is particularly interesting when considered with inflammatory findings. HPA-axis dysfunction has been linked to dysregulated and inappropriate inflammatory responses, suggesting these processes are highly interrelated in PTSD, potentially causative of one another and possibly linked through sleep.100
To confirm any such relationship between sleep, inflammation and PTSD, further research should consider the following limitations of studies included in this review. Foremost, inflammatory expression changes were not directly linked to PTSD symptom assessments, therefore they cannot be assumed to directly relate to PTSD symptom changes. Secondly, it may be necessary to perform tri-directional analyses which consider depression symptom changes. Of the included studies which incorporated a measure of depression, improvements to sleep resulted in reductions to depression symptoms. As depression is an inflammatory disease, it is unclear if inflammation changes are resultant from sleep changes, reductions in depression, or reductions in PTSD symptom severity in this complex population.101–103
Examination of the trophic factors IGF-1 and BDNF noted increases in their expression following improvements to sleep quality, using standard of care sleep therapies. Shown to be decreased in depression, and chronic stress, IGF-1 and BDNF are key regulators which modulate synaptic plasticity, learning, memory and contextual fear response.104,105 This finding adds further weight to the modifiable, central and pathological role of sleep disturbances in PTSD.
Perhaps a surprising observation is the lack of investigation of melatonin and its associated metabolites, considering its central role in sleep promotion.106 Only a single study incorporated a melatonin measurement, noting no significant differences in overall melatonin secretion or time of melatonin secretion onset, between PTSD and comparative populations. However, differences in urine sulphated-melatonin have been observed in PTSD populations when compared to normal controls.107 In future studies, it would be of interest to examine melatonin metabolites in PTSD populations following changes to sleep, considering their antioxidant properties and the advantages this may present in PTSD pathobiology.50,75,108
Significant methodological limitations identified as part of this review are the range of sleep and PTSD assessment methods currently employed in the field, their reliability and most importantly their cross comparability. When comparing biomarkers, having a clear definition of the test condition is key, particularly if studies are to be cross-compared to derive any pathobiological mechanisms. Some of this disparity in PTSD assessment was due to the introduction of DSM-5 in 2013.109 However, a wide range of assessment methods remain in use, which serve to limit the comparability and generalizability of biological results across studies. Therefore, this review recommends that a universal PTSD symptom assessment method should be adopted for biological investigations.
This problem of method heterogeneity extends to sleep. Although actigraphy provides a useful objective measurement of sleep in the free-living environment, a key limitation is the use of different devices in different studies, and unclear reporting of sleep parameter calculation protocols.15,21,23 Actigraphy devices record movements which are translated into sleep parameters using scoring algorithms. To improve the comparability and reproducibility between studies, it would be beneficial for future research to report accelerometer specifications, sleep parameter calculation algorithms and raw movement data.19 It was surprising that none of the studies which employed actigraphy supported this with a valid subjective sleep assessment method such as the ISI. Incorporation of such a measurement would allow for greater comparability with previous studies, and may prove more robust than objective actigraphy alone.17,18,110 Therefore, this review recommends future research should report raw accelerometer movement data, in actigraphy-based studies, where possible. This should also be accompanied by a validated self-report sleep measure.
As noted previously, recent literature has identified differences between the rates of insomnia endorsement and nightmare occurrence in PTSD.10 Considering this, it is important to note a large proportion of the included studies were not able to separate the two, referring instead to a combined “sleep quality” measure. Therefore, in the case of PTSD, and perhaps other mental disorders, employing a combination of sleep quantification methods, so that insomnia and nightmares can be clearly differentiated, may allow any differential effects they have on biological processes to be identified. Further limitations include the lack of tri-directional analysis between PTSD symptom measures, sleep and biological markers and non-universal previous treatment continuation protocols used by intervention studies.
Less than a third of participants included in this review were female, despite a two-fold greater susceptibility of females to the development of PTSD.111 This is most likely attributable to the dominance of military populations within the reviewed studies. Despite this disparity in sex, only five studies consisted of a single-sex population, with the remaining studies incorporating a very small number of female participants. This is not preferable when examining biochemical or physiological markers, which have normal variations due to sex, and should be addressed in future research.112–115
Limitations
Findings of this review are limited by the small number of studies which met all inclusion criteria and an assumed comparability between the different PTSD, subjective sleep, and objective sleep assessment methods used across studies.
Conclusions and Future Directions
The aim of this review was to establish if biochemical and/or physiological markers are related to SDs, severity of the disturbances and their resolution in PTSD. In this regard, SDs were found to directly affect physiological markers relating to autonomic function, and biochemical markers relating to HPA-axis activity, systemic inflammatory processes and trophic factor regulation thus, not only do SDs reduce quality of life, but they also contribute to overall morbidity and mortality associated with PTSD.
Findings pertaining to changes in sleep over time established sleep as a central driver playing a role in, and potentially capable of modifying, many pathological pathways. Improving sleep was shown to decrease sympathetic dominance, increase trophic factor concentrations and reduce pro-inflammatory processes. Improving sleep quality, therefore, has the potential to reduce all-cause lifetime mortality associated with CVD, diabetes and other metabolic conditions in PTSD, and as such should be of central focus when developing treatment regimes. Resolving SDs will not only reduce the overall severity of PTSD symptoms but also has the potential to reduce hospital admission time, total lifetime healthcare costs and improve the overall quality of life for those with PTSD.
Looking into the future, mechanistic insights of this review highlight the importance of carrying out further research into the ability of prophylactic sleep therapies, such as CBT-I, to reduce PTSD risk in populations of high trauma exposure, such as the military and first responders. Such research should be conducted, and findings used in conjunction with those of this review, to inform future policy and occupational health practices in these populations. If this is carried out and appropriate practices implemented, not only could it reduce the risk of developing PTSD, but it may also bring additional economic benefits to employers through reduced sickness-related time off work, and compensation payments associated with occupational-related PTSD.116 However, to allow cross-comparability and expand the generalizability of findings, future research must address and adhere to the methodological recommendations laid out in this review.
Abbreviations
PTSD, post-traumatic stress disorder; SD, sleep disturbances; DSM-5, Diagnostic and statistical manual of mental disorders 5th edition; CBT, cognitive behavioural therapy; CBT-I, cognitive behavioural therapy for insomnia; PCL, PTSD checklist; PVN, paraventricular nucleus; CRH, corticotrophin-releasing hormone; AVP arginine vasopressin; ACTH, adrenocorticotrophic hormone; FKBP5, FK506 binding protein; IL-6, interleukin-6, IL-1β; TNF-α, tumour necrosis factor-α; INF-γ, interferon gamma; SCN, suprachiasmatic nuclei; CRP, C-reactive protein; MeSH, Medical Subject Headings; EEG, electroencephalogram; PSG, polysomnography; HR, heart rate; HRV, heart rate variability; nHF, normalised high frequency; LF, low frequency; BRS, baroreceptor sensitivity; BP, blood pressure; SCL, skin conductance level; ERRT, exposure, relaxation and rescripting therapy; TLR, toll-like receptor; CVD, cardiovascular disease.
Disclosure
MWR is a paid employee of Randox Laboratories but holds no shares in the company. No other authors have any conflicts of interests to declare.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5).
2. Ferry F, Bunting B, Murphy S, O’Neill S, Stein D, Koenen K. Traumatic events and their relative PTSD burden in Northern Ireland: a consideration of the impact of the “Troubles”. Soc Psychiatry Psychiatr Epidemiol. 2014;49(3):435–446. doi:10.1007/s00127-013-0757-0
3. Bunting BP, Ferry FR, Murphy SD, O’Neill SM, Bolton D. Trauma associated with civil conflict and posttraumatic stress disorder: evidence from the Northern Ireland study of health and stress. J Trauma Stress. 2013;26(1):134–141. doi:10.1002/jts.21766
4. Burri A, Maercker A. Differences in prevalence rates of PTSD in various European countries explained by war exposure, other trauma and cultural value orientation. BMC Res Notes. 2014;7(1):1–11. doi:10.1186/1756-0500-7-407
5. Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress. 2013;26(5):537–547. doi:10.1002/jts.21848
6. Dorrington S, Zavos H, Ball H, et al. Trauma, post-traumatic stress disorder and psychiatric disorders in a middle-income setting: prevalence and comorbidity. Br J Psychiatry. 2014;205(5):383–389. doi:10.1192/bjp.bp.113.141796
7. Frissa S, Hatch SL, Gazard B, Fear NT, Hotopf M. Trauma and current symptoms of PTSD in a South East London community. Soc Psychiatry Psychiatr Epidemiol. 2013;48(8):1199–1209. doi:10.1007/s00127-013-0689-8
8. Tanielian T, Jaycox LH, Adamson DM, et al. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Vol. 60. 2008. doi:10.1176/appi.ps.60.2.273
9. Bennett P, Williams Y, Page N, Hood K, Woollard M. Levels of mental health problems among UK emergency ambulance workers. Emerg Med J. 2004;21(2):235–236. doi:10.1136/emj.2003.005645
10. Milanak ME, Zuromski KL, Cero I, Wilkerson AK, Resnick HS, Kilpatrick DG. Traumatic event exposure, posttraumatic stress disorder, and sleep disturbances in a national sample of U.S. adults. J Trauma Stress. 2019;32(1):14–22. doi:10.1002/jts.22360
11. McLay RN, Klam WP, Volkert SL. Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Mil Med. 2010;175(10):759–762. doi:10.7205/MILMED-D-10-00193
12. El-Solh AA, Riaz U, Roberts J. Sleep disorders in patients with posttraumatic stress disorder. Chest. 2018;154:427–439. doi:10.1016/j.chest.2018.04.007
13. Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam Veterans. Am J Psychiatry. 1998;155(7):929–933. doi:10.1176/ajp.155.7.929
14. Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry. 2000;41(6):469–478. doi:10.1053/comp.2000.16568
15. Quante M, Kaplan ER, Cailler M, et al. Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nat Sci Sleep. 2018;10:13–20. doi:10.2147/NSS.S151085
16. Tahmasian M, Khazaie H, Golshani S, Avis KT. Clinical application of actigraphy in psychotic disorders: a systematic review. Curr Psychiatry Rep. 2013;15(6):359. doi:10.1007/s11920-013-0359-2
17. Natale V, Léger D, Martoni M, Bayon V, Erbacci A. The role of actigraphy in the assessment of primary insomnia: a retrospective study. Sleep Med. 2014;15(1):111–115. doi:10.1016/j.sleep.2013.08.792
18. Palesh O, Haitz K, Levi F, et al. Relationship between subjective and actigraphy-measured sleep in 237 patients with metastatic colorectal cancer. Qual Life Res. 2017;26(10):2783–2791. doi:10.1007/s11136-017-1617-2
19. Van Hees VT, Sabia S, Anderson KN, et al. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS One. 2015;10(11):1–13. doi:10.1371/journal.pone.0142533
20. Rosenberger ME, Buman MP, Haskell WL, et al. 24 hours of sleep, sedentary behavior, and physical activity with nine wearable devices. Med Sci Sport Exerc. 2016;48(3):457–465. doi:10.1249/MSS.0000000000000778.24
21. Cellini N, Buman MP, Mcdevitt EA, Ricker AA, Mednick SC. Direct comparison of two actigraphy devices with polysomnographically recorded naps in healthy young adults. Chronobiol Int. 2013;30(5):691–698. doi:10.3109/07420528.2013.782312
22. van Hees VT, Sabia S, Jones SE, et al. Estimating sleep parameters using an accelerometer without sleep diary. bioRxiv. 2018:257972. doi:10.1101/257972
23. Rowlands AV, Yates T, Davies M, Khunti K, Edwardson CL. Raw accelerometer data analysis with GGIR R-package: does accelerometer brand matter? Med Sci Sports Exerc. 2016;48(10):1935–1941. doi:10.1249/MSS.0000000000000978
24. van Hees V, Sabia S, Jones S, et al. Assessment of sleep parameters from raw accelerometry data. Sleep Med. 2017;40:e333. doi:10.1016/j.sleep.2017.11.980
25. Cox RC, Tuck BM, Olatunji BO. Sleep disturbance in post traumatic stress disorder: epiphenomenon or causal factor? Curr Psychiatry Rep. 2017;19(4). doi:10.1007/s11920-017-0773-y
26. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013;36(7):1009–1018. doi:10.5665/sleep.2798
27. Van Liempt S, Van Zuiden M, Westenberg H, Super A, Vermetten E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: a prospective longitudinal cohort study. Depress Anxiety. 2013;30(5):469–474. doi:10.1002/da.22054
28. Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-Year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159(5):855–857. doi:10.1176/appi.ajp.159.5.855
29. Belleville G, Guay S, Marchand A. Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. J Psychosom Res. 2011;70(4):318–327. doi:10.1016/j.jpsychores.2010.09.022
30. Bao YP, Han Y, Ma J, et al. Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: meta-analysis and systematic review. Neurosci Biobehav Rev. 2017;75:257–273. doi:10.1016/j.neubiorev.2017.01.032
31. Cox RC, Olatunji BO. A systematic review of sleep disturbance in anxiety and related disorders. J Anxiety Disord. 2016;37:104–129. doi:10.1016/j.janxdis.2015.12.001
32. Bernert RA, Kim JS, Iwata NG, Perlis ML. Sleep disturbances as an evidence-based suicide risk factor. Curr Psychiatry Rep. 2015;17(3):1–9. doi:10.1007/s11920-015-0554-4
33. Taylor DJ, Peterson AL, Pruiksma KE, et al. Impact of cognitive behavioral therapy for insomnia disorder on sleep and comorbid symptoms in military personnel: a randomized clinical trial. Sleep. 2018:1–11. doi:10.1093/sleep/zsy069
34. Talbot LS, Maguen S, Metzler TJ, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. 2014;37(2):327–341. doi:10.5665/sleep.3408
35. Taylor DJ, Pruiksma KE. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int Rev Psychiatry. 2014;26(2):205–213. doi:10.3109/09540261.2014.902808
36. Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170(4):372–382. doi:10.1176/appi.ajp.2012.12040432
37. Straus LD, Drummond SPA, Nappi CM, Jenkins MM, Norman SB. Sleep variability in military-related PTSD: a comparison to primary insomnia and healthy controls. J Trauma Stress. 2015;28(1):8–16. doi:10.1002/jts.21982
38. Rozpedek W. Neurobiology of posttraumatic stress disorder. Neuropsychiatr I Neuropsychol. 2015;10(1):27–39.
39. Sabbagh JJ, Cordova RA, Zheng D, et al. Targeting the FKBP51/GR/Hsp90 complex to identify functionally relevant treatments for depression and PTSD. ACS Chem Biol. 2018;13(8):2288–2299. doi:10.1021/acschembio.8b00454
40. Stephens MAC, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34(4):468–483. doi:10.1002/brb3.990
41. Touma C, Gassen NC, Herrmann L, et al. FK506 binding protein 5 shapes stress responsiveness: modulation of neuroendocrine reactivity and coping behavior. Biol Psychiatry. 2011;70(10):928–936. doi:10.1016/j.biopsych.2011.07.023
42. Van Zuiden M, Geuze E, Willemen HLDM, et al. Pre-existing high glucocorticoid receptor number predicting development of posttraumatic stress symptoms after military deployment. Am J Psychiatry. 2011;168(1):89–96. doi:10.1176/appi.ajp.2010.10050706
43. Van Zuiden M, Geuze E, Willemen HLDM, et al. Glucocorticoid receptor pathway components predict posttraumatic stress disorder symptom development: a prospective study. Biol Psychiatry. 2012;71(4):309–316. doi:10.1016/j.biopsych.2011.10.026
44. Yehuda R, Cai G, Golier JA, et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the world trade center attacks. Biol Psychiatry. 2009;66(7):708–711. doi:10.1016/j.biopsych.2009.02.034
45. Sarapas C, Cai G, Bierer LM, et al. Genetic markers for PTSD risk and resilience among survivors of the world trade center attacks. Dis Markers. 2011;30(2–3):101–110. doi:10.3233/DMA-2011-0764
46. Daskalakis NP, Cohen H, Nievergelt CM, et al. New translational perspectives for blood-based biomarkers of PTSD: from glucocorticoid to immune mediators of stress susceptibility. Exp Neurol. 2016;284(Pt B):133–140. doi:10.1016/j.expneurol.2016.07.024
47. Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26(5):447–455. doi:10.1002/da.20564
48. Hou N, Zhang X, Zhao L, et al. A novel chronic stress-induced shift in the Th1 to Th2 response promotes colon cancer growth. Biochem Biophys Res Commun. 2013;439(4):471–476. doi:10.1016/j.bbrc.2013.08.101
49. Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8(3):223–246.
50. Passos IC, Vasconcelos-Moreno MP, Costa LG, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2(11):1002–1012. doi:10.1016/S2215-0366(15)00309-0
51. Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS One. 2013;8(10):1–10. doi:10.1371/journal.pone.0076146
52. Maslov B, Marcinko D, Milicevic R, Babic D, Dordevic V, Jakovljevic M. Metabolic syndrome, anxiety, depression and suicidal tendencies in post-traumatic stress disorder and schizophrenic patients. Coll Antropol. 2009;33(Suppl 2):7–10.
53. David D, Woodward C, Esquenazi J, Mellman TA. Comparison of comorbid physical illnesses among veterans with PTSD and veterans with alcohol dependence. Psychiatr Serv. 2004;55(1):82–85. doi:10.1176/appi.ps.55.1.82
54. McFarlane AC. The long-term costs of traumatic stress: intertwined physical and psychological consequences. World Psychiatry. 2010;9(1):3–10. doi:10.1002/j.2051-5545.2010.tb00254.x
55. Beristianos MH, Yaffe K, Cohen B, Byers AL. PTSD and risk of incident cardiovascular disease in aging Veterans. Am J Geriatr Psychiatry. 2016;24(3):192–200. doi:10.1016/j.jagp.2014.12.003
56. Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, Marvar PJ. Autonomic and inflammatory consequences of posttraumatic stress disorder and the link to cardiovascular disease. Am J Physiol Regul Integr Comp Physiol. 2015;309(4):R315–21. doi:10.1152/ajpregu.00343.2014
57. Rohleder N, Karl A. Role of endocrine and inflammatory alterations in comorbid somatic diseases of post-traumatic stress disorder. Minerva Endocrinol. 2006;31(4):273–288.
58. Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141–153. doi:10.1196/annals.1314.011
59. Levine AB, Levine LM, Levine TB. Posttraumatic stress disorder and cardiometabolic disease. Cardiology. 2014;127(1):1–19. doi:10.1159/000354910
60. De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262–2272. doi:10.2337/db13-1954
61. Golub Y, Kaltwasser SF, Mauch CP, et al. Reduced hippocampus volume in the mouse model of posttraumatic stress disorder. J Psychiatr Res. 2011;45(5):650–659. doi:10.1016/j.jpsychires.2010.10.014
62. Bremner JDD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res. 2008;167(07):171–186. doi:10.1016/S0079-6123(07)67012-5.Structural
63. Massicotte C, Scherer SS. Neuropathies—translating causes into treatments. In: Waxman SBT-FNTN, editor. From Neuroscience to Neurology. Burlington: Elsevier; 2005:405–xvii. doi:10.1016/B978-012738903-5/50025-4
64. Andero R, Choi DC, Ressler KJ. Chapter six - BDNF–TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. In: Khan ZU, Muly ECBT-P editors, Molecular Basis of Memory. Vol. 122. Academic Press; 2014:169–192. doi:10.1016/B978-0-12-420170-5.00006-4
65. Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38(3):579–593. doi:10.1007/s10571-017-0510-4
66. Mondal AC, Fatima M. Direct and indirect evidences of BDNF and NGF as key modulators in depression: role of antidepressants treatment. Int J Neurosci. 2019;129(3):283–296. doi:10.1080/00207454.2018.1527328
67. Aksu S, Unlu G, Kardesler AC, Cakaloz B, Aybek H. Altered levels of brain-derived neurotrophic factor, proBDNF and tissue plasminogen activator in children with posttraumatic stress disorder. Psychiatry Res. 2018;268:478–483. doi:10.1016/j.psychres.2018.07.013
68. Arumugam V, John VS, Augustine N, et al. The impact of antidepressant treatment on brain-derived neurotrophic factor level: an evidence-based approach through systematic review and meta-analysis. Indian J Pharmacol. 2017;49(3):236–242. doi:10.4103/ijp.IJP_700_16
69. Aspesi D, Pinna G. Could a blood test for PTSD and depression be on the horizon? Expert Rev Proteomics. 2018;15(12):983–1006. doi:10.1080/14789450.2018.1544894
70. Schmidt U, Kaltwasser SF, Wotjak CT. Biomarkers in posttraumatic stress disorder: overview and implications for future research. Dis Markers. 2013;35(1):43–54. doi:10.1155/2013/835876
71. Rusch HL, Guardado P, Baxter T, Mysliwiec V, Gill JM. Improved sleep quality is associated with reductions in depression and PTSD arousal symptoms and increases in IGF-1 concentrations. J Clin Sleep Med. 2015;11(6):615–623. doi:10.5664/jcsm.4770
72. Bachmann V, Klein C, Bodenmann S, et al. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep. 2012. doi:10.5665/sleep.1690
73. Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol. 2013;23(17):R774–R788. doi:10.1016/j.cub.2013.07.025
74. Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. doi:10.1152/physrev.00032.2012
75. Mahmood D, Muhammad BY, Alghani M, Anwar J, el-Lebban N, Haider M. Advancing role of melatonin in the treatment of neuropsychiatric disorders. Egypt J Basic Appl Sci. 2016;3(3):203–218. doi:10.1016/j.ejbas.2016.07.001
76. Joshi N, Biswas J, Nath C, Singh S. Promising role of melatonin as neuroprotectant in neurodegenerative pathology. Mol Neurobiol. 2015;52(1):330–340. doi:10.1007/s12035-014-8865-8
77. Boks MP, Rutten BPF, Geuze E, et al. SKA2 methylation is involved in cortisol stress reactivity and predicts the development of post-traumatic stress disorder (PTSD) after military deployment. Neuropsychopharmacology. 2016;41(5):1350–1356. doi:10.1038/npp.2015.286
78. Ma X, Idle JR, Krausz KW, Gonzalez FJ. Metabolism of melatonin by human cytochromes P450. Drug Metab Dispos. 2005;33(4):489–494. doi:10.1124/dmd.104.002410.not
79. Benarroch EE, Suprachiasmatic nucleus and melatonin. Neurology. 2008;71(8):594LP- 598. doi:10.1212/01.wnl.0000324283.57261.37
80. Abouzeid M, Kelsall HL, Forbes AB, Sim MR, Creamer MC. Posttraumatic stress disorder and hypertension in Australian veterans of the 1991 Gulf War. J Psychosom Res. 2012;72(1):33–38. doi:10.1016/j.jpsychores.2011.08.002
81. Sumner JA, Kubzansky LD, Roberts AL, et al. Post-traumatic stress disorder symptoms and risk of hypertension over 22 years in a large cohort of younger and middle-aged women. Psychol Med. 2016;46(15):3105–3116. doi:10.1017/S0033291716001914
82. Balint EM, Boseva P, Schury K, Guendel H, Rottbauer W, Waller C. High prevalence of posttraumatic stress in patients with primary hypertension. Gen Hosp Psychiatry. 2016;38:53–58. doi:10.1016/j.genhosppsych.2015.10.002
83. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94. doi:10.1007/s11886-016-0770-5
84. Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268–75.e2. doi:10.1016/j.amjmed.2014.10.015
85. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi:10.1016/j.biopsych.2015.05.014
86. Critical Appraisal Skills Programme. CASP case control study checklist. CASP. 2018;2018:1–6. https://casp-uk.net/wp-content/uploads/2018/01/CASP-Case-Control-Study-Checklist-2018.pdf.
87. Goldet G, Howick J. Understanding GRADE: an introduction. J Evid Based Med. 2013;6(1):50–54. doi:10.1111/jebm.12018
88. de la Sierra A, Gorostidi M, Banegas JR, Segura J, de la Cruz JJ, Ruilope LM. Nocturnal hypertension or nondipping: which is better associated with the cardiovascular risk profile? Am J Hypertens. 2014;27(5):680–687. doi:10.1093/ajh/hpt175
89. Michels N, Sioen I, Clays E, et al. Children’s heart rate variability as stress indicator: association with reported stress and cortisol. Biol Psychol. 2013;94(2):433–440. doi:10.1016/j.biopsycho.2013.08.005
90. Winter M, Tyree K. Polyvagal theory. Encycl Evol Psychol Sci. 2016;74(2):1–5. doi:10.1007/978-3-319-16999-6_2311-1
91. Rowaiye OO, Jankowska EA, Ponikowska B. Baroreceptor sensitivity and diabetes mellitus. Cardiol J. 2013;20(5):453–463. doi:10.5603/CJ.2013.0130
92. La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13(2):191–207. doi:10.1111/j.1542-474X.2008.00219.x
93. Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertens. 2011;57(1):3–10. doi:10.1161/HYPERTENSIONAHA.109.133900
94. Holt-Lunstad J, Steffen PR. Diurnal cortisol variation is associated with nocturnal blood pressure dipping. Psychosom Med. 2007;69(4):339–343. doi:10.1097/PSY.0b013e318050d6cc
95. Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan W-B. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16(6):698–705. doi:10.1038/nn.3387
96. Davidson JR, Dawson S, Krsmanovic A. Effectiveness of group cognitive behavioral therapy for insomnia (CBT-I) in a primary care setting. Behav Sleep Med. 2017;1–13. doi:10.1080/15402002.2017.1318753
97. Cohen S, Kozlovsky N, Matar MA, Kaplan Z, Zohar J, Cohen H. Post-exposure sleep deprivation facilitates correctly timed interactions between glucocorticoid and adrenergic systems, which attenuate traumatic stress responses. Neuropsychopharmacology. 2012;37(11):2388–2404. doi:10.1038/npp.2012.94
98. Prather AA, Vogelzangs N, Penninx BWJH. Sleep duration, insomnia, and markers of systemic inflammation: results from the Netherlands study of depression and anxiety (NESDA). J Psychiatr Res. 2015;60:95–102. doi:10.1016/j.jpsychires.2014.09.018
99. Van Zuiden M, Heijnen CJ, van de Schoot R, et al. Cytokine production by leukocytes of military personnel with depressive symptoms after deployment to a combat-zone: a prospective, longitudinal study. PLoS One. 2011;6(12):e29142. doi:10.1371/journal.pone.0029142
100. Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its behavioural and metabolic correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi:10.1111/j.1749-6632.2012.06633.x.Glucocorticoid
101. Han QQ, Yu J. Inflammation: a mechanism of depression? Neurosci Bull. 2014;30(3):515–523. doi:10.1007/s12264-013-1439-3
102. Slavich GM, Irwin MR. Social signal transduction theory of depression. Psychiatr Bull. 2014;140(3):774–815. doi:10.1037/a0035302.From
103. Allison DJ, Ditor DS. The common inflammatory etiology of depression and cognitive impairment: a therapeutic target. J Neuroinflammation. 2014;11(1):1–12. doi:10.1186/s12974-014-0151-1
104. Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169–192. doi:10.1016/B978-0-12-420170-5.00006-4
105. Johnson-Farley NN, Travkina T, Cowen DS. Cumulative activation of akt and consequent inhibition of glycogen synthase kinase-3 by brain-derived neurotrophic factor and insulin-like growth factor-1 in cultured hippocampal neurons. J Pharmacol Exp Ther. 2006;316(3):1062–1069. doi:10.1124/jpet.105.094433
106. Zhao D, Yu Y, Shen Y, et al. Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol. 2019;10:1–16. doi:10.3389/fendo.2019.00249
107. McFarlane AC, Barton CA, Briggs N, Kennaway DJ. The relationship between urinary melatonin metabolite excretion and posttraumatic symptoms following traumatic injury. J Affect Disord. 2010;127(1–3):365–369. doi:10.1016/j.jad.2010.05.002
108. Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57(2):131–146. doi:10.1111/jpi.12162
109. Hoge CW, Riviere LA, Wilk JE, Herrell RK, Weathers FW. The prevalence of post-traumatic stress disorder (PTSD) in US combat soldiers: a head-to-head comparison of DSM-5 versus DSM-IV-TR symptom criteria with the PTSD checklist. Lancet Psychiatry. 2014;1(4):269–277. doi:10.1016/S2215-0366(14)70235-4
110. McCall C, McCall WV. Objective vs. subjective measurements of sleep in depressed insomniacs: first night effect or reverse first night effect? J Clin Sleep Med JCSM off Publ Am Acad Sleep Med. 2012;8(1):59–65. doi:10.5664/jcsm.1664
111. Lehavot K, Katon JG, Chen JA, Fortney JC, Simpson TL. Post-traumatic stress disorder by gender and Veteran status. Am J Prev Med. 2018;54(1):e1–e9. doi:10.1016/j.amepre.2017.09.008
112. Briant LJB, Charkoudian N, Hart EC. Sympathetic regulation of blood pressure in normotension and hypertension: when sex matters. Exp Physiol. 2016;101(2):219–229. doi:10.1113/EP085368
113. Agushi E, Xhyheri B, Bugiardini R. Sex differences in biomarkers for predicting cardiovascular and coronary events. Curr Vasc Pharmacol. 2013;11(5):785–794. doi:10.2174/1570161111311050017
114. Tothova L, Ostatnikova D, Sebekova K, Celec P, Hodosy J. Sex differences of oxidative stress markers in young healthy subjects are marker-specific in plasma but not in saliva. Ann Hum Biol. 2013;40(2):175–180. doi:10.3109/03014460.2012.754495
115. Newbern D, Gumus Balikcioglu P, Balikcioglu M, et al. Sex differences in biomarkers associated with insulin resistance in obese adolescents: metabolomic profiling and principal components analysis. J Clin Endocrinol Metab. 2014;99(12):4730–4739. doi:10.1210/jc.2014-2080
116. Wise EA, Beck JG. Work-related trauma, PTSD, and workers compensation legislation: implications for practice and policy. Psychol Trauma. 2015;7(5):500–506. doi:10.1037/tra0000039
117. Ulmer CS, Calhoun PS, Bosworth HB, Dennis MF, Beckham JC, Nocturnal blood pressure non-dipping, posttraumatic stress disorder, and sleep quality in women. Behav Med. 2013;39(4):111–121. doi:10.1080/08964289.2013.813434
118. Livingston WS, Rusch HL, Nersesian PV, Baxter T, Mysliwiec V, Gill JM. Improved sleep in military personnel is associated with changes in the expression of inflammatory genes and improvement in depression symptoms. Front Psychiatry. 2015;6. doi:10.3389/fpsyt.2015.00059
119. Zucker TL, Samuelson KW, Muench F, Greenberg MA, Gevirtz RN. The effects of respiratory sinus arrhythmia biofeedback on heart rate variability and posttraumatic stress disorder symptoms: a pilot study. Appl Psychophysiol Biofeedback. 2009;34(2):135–143. doi:10.1007/s10484-009-9085-2
120. Kobayashi I, Lavela J, Mellman TA. Nocturnal autonomic balance and sleep in PTSD and resilience. J Trauma Stress. 2014;27(6):712–716. doi:10.1002/jts.21973
121. Davis JL, Rhudy JL, Pruiksma KE, et al. Physiological predictors of response to exposure, relaxation, and rescripting therapy for chronic nightmares in a randomized clinical trial. J Clin Sleep Med. 2011;7(6):622–631. doi:10.5664/jcsm.1466
122. Ulmer CS, Calhoun PS, Edinger JD, Wagner HR, Beckham JC. Sleep disturbance and baroreceptor sensitivity in women with post-traumatic stress disorder. J Trauma Stress. 2009;22(6):643–647. doi:10.1002/jts.20464
123. Heinzelmann M, Lee H, Rak H, et al. Sleep restoration is associated with reduced plasma C-reactive protein and depression symptoms in military personnel with sleep disturbance after deployment. Sleep Med. 2014;15(12):1565–1570. doi:10.1016/j.sleep.2014.08.004
124. Tegeler CL, Gerdes L, Shaltout HA, et al. Successful use of closed-loop allostatic neurotechnology for post-traumatic stress symptoms in military personnel: self-reported and autonomic improvements. Mil Med Res. 2017;4(1). doi:10.1186/s40779-017-0147-0
125. Inslicht SS, Rao MN, Richards A, et al. Sleep and hypothalamic pituitary adrenal axis responses to metyrapone in posttraumatic stress disorder. Psychoneuroendocrinology. 2018;88:136–143. doi:10.1016/j.psyneuen.2017.12.002
126. Ulmer CS, Bosworth HB, Germain A, et al. Associations between sleep difficulties and risk factors for cardiovascular disease in veterans and active duty military personnel of the Iraq and Afghanistan conflicts. J Behav Med. 2015;38(3):544–555. doi:10.1007/s10865-015-9627-4
127. Phelps AJ, Kanaan RAA, Worsnop C, Redston S, Ralph N, Forbes D. An ambulatory polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep. 2018;41(1). doi:10.1093/sleep/zsx188
128. Liaveri PG, Dikeos D, Ilias I, et al. Quality of sleep in renal transplant recipients and patients on hemodialysis. J Psychosom Res. 2017;93:96–101. doi:10.1016/j.jpsychores.2016.12.013
129. Van Liempt S, Arends J, Cluitmans PJM, Westenberg HGM, Kahn RS, Vermetten E, Sympathetic activity and hypothalamo-pituitary-adrenal axis activity during sleep in post-traumatic stress disorder: a study assessing polysomnography with simultaneous blood sampling. Psychoneuroendocrinology. 2013;38(1):155–165. doi:10.1016/j.psyneuen.2012.05.015
130. Miller KE, Jamison AL, Gala S, Woodward SH. Two independent predictors of nightmares in posttraumatic stress disorder. J Clin Sleep Med. 2018;14(11):1921–1927. doi:10.5664/jcsm.7494
131. Imai R, Hori H, Itoh M, et al. Inflammatory markers and their possible effects on cognitive function in women with posttraumatic stress disorder. J Psychiatr Res. 2018;102:192–200. doi:10.1016/j.jpsychires.2018.04.009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.