Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Skull Base Metastasis from Hepatocellular Carcinoma: Clinical Presentation and Efficacy of Radiotherapy
Received 3 February 2022
Accepted for publication 21 April 2022
Published 29 April 2022 Volume 2022:9 Pages 357—366
DOI https://doi.org/10.2147/JHC.S361045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Laura A. Dawson
Kangpyo Kim, Joongyo Lee, Jinsil Seong
Department of Radiation Oncology, Yonsei University College of Medicine, Seoul, Republic of Korea
Correspondence: Jinsil Seong, Department of Radiation Oncology, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul, 03722, Republic of Korea, Tel +82-2-2228-8095, Fax +82-2-2227-7823, Email [email protected]
Purpose: Skull base metastasis (SBM) from hepatocellular carcinoma (HCC) presents detrimental survival outcomes with cranial nerve symptoms; however, they have received little attention. This study aimed to investigate the clinical presentation and efficacy of radiation therapy (RT) in patients with SBM from HCC.
Patients and Methods: We identified patients with SBM from HCC in Yonsei Cancer Center from 2005 to 2019. Image evaluations and SBM-related symptoms were reviewed. Overall survival was calculated using the Kaplan–Meier method and compared through the Log rank test. The oligometastasis group included patients with less than five foci of tumors, while the extensive metastasis group presented five or more sites.
Results: The incidence of SBM from HCC was 1.5% (58/3793 patients), commonly found in the middle cranial fossa. SBM associated symptoms presented in 51 patients, and the most common were head and neck area pain, and orbital symptoms, The palliation rate after RT was 65% (24/39 patients) for overall symptoms and 83.3% (20/24 patients) for cranial nerve symptoms. In whole cohort, overall survival was analyzed, and the median overall survival of patients with oligometastasis was better than extensive metastasis (23.7 months vs 1.8 months, p < 0.001). In subgroup who received RT (39 patients), the median overall survival was 23.7 and 2.7 months for patients with oligo and extensive metastasis, respectively (p < 0.001).
Conclusion: This study confirmed clinical features of SBM from HCC. Overall survival was generally poor, but patients presenting oligometastasis seemed to have possibility of relative long-term survival. Although radiation was effective in SBM-induced symptom relief, dose–response relationship in local control rate and overall survival needs further studies with larger number of patients.
Keywords: hepatocellular carcinoma, skull base metastasis, oligometastasis, radiotherapy
Introduction
Recent advances in treatment and diagnostic methods have made a substantial improvement in the survival outcomes of patients with hepatocellular carcinoma (HCC), consequently increasing the incidence of metastatic HCC.1,2 Systemic treatment is still the standard of care in patients with extrahepatic metastasis regardless of tumor burden.3,4 However, there are evidences that local treatment is beneficial for symptom palliation and survival outcome, especially among those with oligometastasis.5–8
Skull base metastasis (SBM) is one of the challenging situations as it often involves cranial nerves.9 It frequently presents detrimental symptoms such as pain, facial sensory changes, visual impairments, or dysphagia/dysarthria. Radiation therapy (RT) is an effective treatment often used for palliating these symptoms caused by SBM in other primary malignancies.10–12 However, the RT for SBM in HCC has received little attention due to its extremely low incidence (0.4–1.6%) and poor treatment outcome.13–15 As SBM from HCC is frequently associated with other osseous or multi-organ metastasis, it has been managed as a terminal disease.16,17 Furthermore, clinical presentations of SBM including the incidence, development time, and location of metastasis are also rare. Therefore, there is no proper management guideline for SBM in HCC.
In this study, we retrospectively analyzed a cohort of HCC patients with SBM to understand the clinical features of the disease and the efficacy of RT for SBM in oncologic outcomes.
Patients and Methods
Study Population
From 2005 to 2019, patients with SBM from HCC were identified in Yonsei Cancer Center database. If judged as an evident metastasis based on the patient’s medical history and findings of magnetic resonance imaging (MRI), biopsy was not mandatory. Patients who had a primary cancer other than HCC were excluded, unless SBM was pathologically confirmed as a metastasis from HCC. Oligometastatic disease was defined when there were less than five foci of viable metastatic tumors at the time of diagnosis of SBM. Viability of the primary tumor and development time of SBM were not considered in defining the oligometastasis. When there was a history of diagnosis and treatment for extrahepatic metastasis other than SBM, radiologic examinations were used to judge whether the lesion is viable or not. When there was no radiologic evidence of progression of the lesion, it was not counted.
Radiation Therapy for SBM
The application of RT for SBM was decided by a multidisciplinary discussion that considers the patient’s general condition and disease status. Fractionated conventional RT, hypofractionated RT, or stereotactic radiosurgery were performed by radiation oncologists, and gamma knife surgery was done by neurosurgeons.
Thermoplastic head-neck-shoulder devices were applied individually for accurate delivery of radiation. A daily patient alignment using kilo-voltage or megavoltage computed tomography was performed for hypofractionated RT or stereotactic radiosurgery cases. A skull tracking system was used for real-time alignment when SBM was treated with CyberKnife. For gamma knife surgery, stereotactic frame was applied. High-resolution contrast enhanced MRI and computed tomography (CT) images were acquired and MRI-CT image match was performed to reduce image distortion.
Target delineation of SBM for RT was conducted through fusioning simulation CT and diagnostic MRI for exact localization. Additional margins of 0–3 mm were applied to compensate for the set-up error. Dose regimens of RT were decided considering the metastatic burden and location and size of the SBMs. Fractionated RT regimens were preferred when critical normal organs including the brain stem and cranial nerves were located near the site of SBM. Hypofractionated RT with higher total radiation dose was prioritized in oligometastatic patients. Radiation dose was prescribed to the 50–90% isodose line in stereotactic radiosurgery and gamma knife surgery, which encompass the entire target volume.
Radiation dose to SBM was converted to biologically effective dose (BED) to compensate the difference of total and per fraction radiation dose. A BED is a measure of the true biological dose delivered by a combination of the dose per fraction and total dose to a particular tissue characterized by a specific α/β ratio. An α/β ratio of 10 was used for tumor control in this study.
Follow-Up Image Evaluation, Symptom Evaluation, and Toxicity
Brain MRI or CT scan was performed 1 month after RT and SBM lesions were evaluated with Response Evaluation Criteria in Solid Tumors (RECIST) (1.1). Thereafter, patients were suggested to undergo an MRI done once every 3 months for the first year of follow-up.
Status of primary liver tumor was evaluated with eighth edition of American Joint Committee on Cancer staging system through abdominal CT or liver MR images. Image evaluations of were performed every 2–3 months and the status of liver tumor was also evaluated with RECIST criteria.
All patients were thoroughly investigated by neurologists to check for undetected cranial nerve–related symptoms when SBM was initially diagnosed. Patient-reported pain scoring was conducted when patients could cooperate. Palliation of each symptom after RT was assessed based on the outpatient medical records of radiation oncologists or neurosurgeons.
RT-related toxicity at the time of follow-up was graded according to the Common Terminology Criteria for Adverse Events (version 5.0).18
Statistical Analysis
Statistical analyses were conducted using the IBM SPSS, version 25.0 (IBM Corp., Armonk, NY) and R (version 3.6.1; R Development Core Team 2009, Vienna, Austria). Overall survival was defined as the interval from the date of diagnosis of SBM to the date of death or the last visit. We used the Cox regression analysis to select the variables to be used for survival analyses. The cumulative survival probability was calculated by using the Kaplan–Meier method, and survival curves were compared using the Log rank test. Overall survival analysis is also conducted in patients who received RT with the same statistical methods as subgroup analysis. The differences in patient characteristics according to metastatic burden and radiation dose were compared using the chi-square tests. One-way analysis of variance (ANOVA) method was used to compare the patient characteristics across the three or more groups in case the characteristics are numerical variables. Statistical significance was set as P-values <0.05.
Results
Patient Characteristics
The number of patients who were diagnosed with SBM from HCC was 58 or 1.5% of the 3793 HCC patients. Patient characteristics of the entire study population at the time of SBM development are described in Table 1. The proportion of patients with Child-Pugh (CP) grades B or C was 39.7%. The median time from diagnosis of HCC to SBM was 16 months (range 0–140 months). Fifty-one patients had extensive metastatic status, which was over 85% of the whole cohort.
 |
Table 1 Patient Characteristics at the Time of SBM Development |
Clinical Presentation of SBM
The distribution and size of the SBMs in the study are shown in Figure 1. All except four SBMs presented as a single mass-forming like lesion. The middle cranial fossa was the most common region of metastasis, while the anterior SBM was rare. The size of the SBM was described as the longest diameter of the tumor observed in MRI, and tumors of 2.0–4.0 cm comprised 60% of the study population. There were six patients with a tumor size less than 2 cm, of which only two patients showed SBM-related symptoms. All patients, except for seven, experienced SBM-associated symptoms.
The most common symptom presentation was pain in the head and neck area (22 patients). Nausea and vomiting were accompanied with pain in nine patients, and increased intracranial pressure induced mental change in three patients. For cranial nerve-associated symptoms, orbital symptoms presented in 14 patients and their detailed presentation was as follows: eight diplopia, six ptosis, and five visual impairments. The vagus nerve and hypoglossal nerve-related symptoms were found in 13 patients, presenting as dysphagia or dysarthria often accompanied with swallowing difficulties or tongue deviation. Other detected symptoms were auditory function impairment (five patients), facial sensory changes (five patients), and facial pain (two patients).
Treatment
Characteristics of the treatment are shown in Table 2. Thirty-nine patients received RT, eight of whom received a combination of systemic agents. The median interval between symptom initiation and RT was 20 days (range, 0–96). The median radiation dose was 50.7 Gy in BED, and the most frequently used radiation dose schemes were 30 Gy in 10 fractions (BED 39 Gy) and 39 Gy in 13 fractions (BED 50.7 Gy). Fourteen patients received hypofractionated RT or stereotactic radiosurgery with a fraction size ≥5 Gy.
 |
Table 2 Treatment Characteristics and Response Rates |
Overall Survival
The survival curves are drawn and compared according to disease extent (oligo vs extensive metastasis), liver function (CP grade A vs CP grade B, C) and radiation dose (BED ≥ 50 Gy vs <50 Gy), which were selected as statistically significant in Cox regression analyses.
The median overall survival for entire cohort was 2.3 months (range, 0.1–46.7) with a median follow-up period of 2.3 months after diagnosis of SBM. The overall survival differed according to metastatic burden: the median of 23.7 months in seven patients with oligometastasis vs 1.8 months in 51 patients with extensive metastasis (Figure 2A, P<0.001). When stratified with liver function, patients with CP grade A showed better survival outcomes than patients with CP grades B or C (Figure 2B, median survival 4.3 months for CP grade A vs 1.0 months for CP grade B or C, P<0.001). The survival rate according to radiation dose was significantly different; The median was 4.7 months in BED ≥ 50 Gy vs 1.6 months in BED < 50 Gy, and 1.0 month in BED 0 Gy (Figure 2C, P=0.001).
 |
Figure 2 Survival analysis according to (A) the metastatic burden, (B) Child–Pugh grade, and (C) the BED. BED, biologically effective dose. |
In subgroup who received RT (39 patients), the median overall survival was 3.2 months (range, 0.5–46.7) with a median follow-up period of 3.1 months. The median overall survival in six patients of oligometastasis group was 23.7 months vs 2.7 months in 33 patients of extensive metastasis group (Figure 3A, P<0.001). There were also statistical significance in overall survival when stratified by liver function and BED. Twenty-two patients with CP grade A showed better survival outcomes than patients with CP grades B or C with the median overall survival 4.7 months vs 1.6 months (Figure 3B, p=0.022). The median survival was 4.7 months for BED>50 Gy group while 1.6 months for BED < 50 Gy (Figure 3C, P=0.001).
 |
Figure 3 Subgroup survival analysis in patients who received radiation therapy according to (A) the metastatic burden, (B) Child -Pugh grade, and (C) the biologically effective dose (BED). |
Patient groups according to metastatic burden and radiation dose in BED were compared to find any bias that could affect survival outcomes of the patients (Supplementary Tables 1 and 2); however, there were no significant differences (P>0.05). Treatment characteristics were well balanced between the oligo- and extensive metastasis group, and the results are presented in Supplementary Table 3.
Response Evaluation, Symptom Palliation, and Toxicity
Follow-up MRI or CT images to evaluate the local response of SBM were found only in 30 patients among the 39 who received RT. Local response according to the RECIST criteria after 1 month of RT was 2 (6.7%), 8 (26.8%), 9 (30%), and 11 (36.7%) patients with complete response, partial response, stable disease and progressive disease, respectively. Local progression-free rates after RT were similar between oligo and extensive metastasis groups with 66.7% and 62.5%, respectively. There was also no significant difference in the local progression-free rates between BED ≥ 50 Gy and BED < 50 Gy groups.
Symptom palliation was achieved in 24 patients (65% of the patients who received RT). Head and neck pain was relieved after RT in 17 patients (17/22 patients, 77.3%). Among 24 patients with cranial nerve-related symptoms, 83% experienced symptom relief. All patients with the vagal or hypoglossal nerve-related symptoms showed partial or complete remission of the symptoms, while three with diplopia and one with auditory function impairment were not relieved by RT. The median time for palliation after initiation of RT was 10 days (range, 4–94 days). More than 60% of patients (15/24 patients) experienced symptom palliation during or within 1 month of RT, while late symptom palliations over 3 months after RT were detected in two patients.
There was no grade 3 or more RT-related toxicity. Alopecia in the radiated area was the most common sign of toxicity (grade 1; nine patients, grade 2; three patients), and the other signs were headache (grade 1; five patients) and nausea/vomiting (grade 1; three patients). All signs of toxicity were self-limiting, or the patients died before the toxicity disappeared.
Discussion
In this study, we investigated the clinical features and oncologic outcome in patients with SBM from HCC with the largest number of patients ever reported. Patients presenting oligometastasis were expected to have long-term survival, and high dose RT (BED ≥ 50 Gy) was beneficial in their survival outcome.
Incidence of SBM in the study was 1.5% (58/3793 patients), which corresponds to previous studies.13,15,17,19,20 The reported incidence of SBM from HCC is approximately 0.4–1.6%; however, the rates are based on the data when accurate localization of SBM through MRI was not affordable. The finding of the middle cranial fossa as the most frequent SBM location and anterior SBM as rare seems natural considering the skull base anatomy. Only the olfactory nerve passes through anterior cranial fossa, while other cranial nerves pass through foramens located in middle and posterior cranial fossa. Since a symptom-based image evaluation is the general principle for the diagnosis of SBM in HCC, metastatic tumors at the middle and posterior fossa are found with a higher probability than tumors in anterior regions. Furthermore, patients with an SBM lesion of 2 cm or less were more likely to be asymptomatic when compared with patients with larger SBM. The ratio of asymptomatic SBM patients was 66.7% (4/6 patients) with 2 cm or less size of metastasis, while the ratio was 13.6% (8/59 patients) in whole patients. Therefore, the incidence of SBM was assumed to be higher than previously reported data when small size or anteriorly located asymptomatic metastases are considered.
As a rare disease entity, the effect of RT in SBM-related symptom palliation had often been analyzed with other primary malignancies, such as breast or prostate cancer. In 2010, a Japanese group reported that stereotactic RT with dose of 35 Gy/10 fractions for SBM was effective in cranial symptom palliation, while there was only 1 patient with HCC from among the 11 patients in total.9 A recent study dealing with SBM (or tumors with direct invasion to skull base) treated with gamma knife surgery on a dose of 15 Gy– 24 Gy/1 fraction showed effective local control rate, but there were only two patients with HCC in analysis.20 Discrepancies of pathologic features of each malignancy made it difficult to determine whether symptoms induced by SBM from HCC can also be relieved by RT. In our study, overall symptom palliation rates were over 60%, and cranial nerve–related symptom palliation rates were even higher with over 80%. In our study, the reasons we consider for substantial symptom palliation are radiosensitivity of the tumor with accurate localization of metastasis and soft tissue forming nature of HCC bone metastasis.17,21 Prompt application of RT for SBM can decrease the size of soft tissue forming SBM, and consequently, decompressed cranial nerves can often recover their original function. Several studies in regard to dose–response relationship in symptom palliation of patients with bone metastasis from HCC are reported, but SBM-specific data are nonexistent. Seong et al22 reported that BED of 43 Gy was a statistically significant cut off value for objective pain response, and Kaizu et al23 also reported that dose–response relationship exists in pain relief. However, other studies failed to show the correlations.24–26 In our study, it was also difficult to find out dose–response relationships in symptom palliation, as it was achieved in some patients within the period of RT.
Aside from symptom palliation, there were efforts to find out dose–response relationship in patients with oligometastatsis whether higher radiation dose for metastatic lesion can be beneficial in higher local control rate and prolonging patients’ survival. In 2015, Kamran et al reported that radiation sensitivity, which is analyzed through multigene expression model of tumor, between primary and metastatic tissues of colon cancer showed significant differences.27 The result infers that individual dose schemes are required for every single organs to control the metastatic lesion. A recent retrospective data reported that ablative dose of >60Gy to bone metastasis from HCC resulted in prolonged survival when patients had oligometastasis (≤5 lesions), while patients with BED ≥ 50 Gy survived longer compared to patient groups with BED <50 Gy group in our study. Although our data need caution in interpreting the result due to biased patient characteristics and small number of patients, the result supports that further efforts to find appropriate dose schemes according to the metastatic lesion should be conducted in future. In our study, we conducted further analyses to analyze whether a higher radiation dose in BED was beneficial in both oligo and extensive metastasis groups (Supplementary Figure 1). In the extensive metastasis group, the median survival for patients with BED ≥ 50 Gy was 4.5 months, while it was 1.6 months in patients with BED < 50 Gy (Supplementary Figure 1A, P=0.055). In the oligometastasis group, the median survival for patients with BED ≥ 50 Gy was 23.7 months, which was significantly better when compared with BED < 50 Gy group with a median survival of 3.8 months (Supplementary Figure 1B, P<0.001). For patients with SBM from HCC, palliation dose schemes with shorter fraction number would be appropriate for patients with extensive metastasis, as such patients showed detrimental survival outcomes with median survival of less than 5 months regardless of radiation dose. However, for patients with oligometastasis, application of higher radiation dose might be an option with possibility of better local control or survival rate.
It has been thought that the rates of neurological symptoms improvement are closely associated with the length of time to RT following the onset of symptoms. Vikram et al28 reported that RT within 1 month after the symptom onset showed improvement in 87% of the patients, which is three times more than in those with RT 3 months or more after the onset. However, our study may provide another opinion on cranial symptom palliation for patients whose symptoms persisted for several weeks prior to RT. There were eight patients in total who initiated RT after 30 days or more from symptom development. Among them, four patients showed improvement of cranial nerve symptoms such as ptosis, facial sensory change, or facial pain, and two patients showed alleviation of pain.
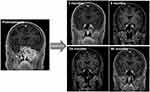
One of the representative cases in our study is shown in Figure 4. The solitary SBM located in pterygoid/infratemporal fossa was found with symptoms of left auditory pain, facial pain, and impaired visual/auditory functions. After 60 Gy/30 fractions of RT (BED 72 Gy), SBM showed no local progression within 40 months of follow-up. Initiation of RT was 58 days after symptom development, and facial pain decreased in the visual analog scale score from 8 to 4 after 16 days of RT (26 Gy/13 fractions). Visual and auditory impairment were partially recovered and facial pain completely disappeared after 3 months of RT. The case represents our study in several points; SBM was located in the middle cranial fossa with cranial nerve symptoms, long-term survival was achieved through RT with BED >50 Gy for oligometastatic SBM, and pain and cranial nerve symptoms were successfully palliated despite a delay of RT initiation.
This study has several limitations due to its retrospective nature. First, though there were no significant differences in patient and treatment characteristics between the oligometastasis and extensive metastasis groups, there could be a bias in the study population that affects oncologic outcome of this study. For example, patients presenting oligometastasis showed higher rate of CP grade A and complete response status of primary liver tumor, which could induce favorable survival outcomes of the patients. Second, the sample sizes for the study were too small to elicit a clear effect of high radiation dose on patients’ survival. Further multicenter researches with a larger number of patients are required to support our data whether higher radiation doses can contribute to the prolonged survival of patients with SBM.
Conclusion
This study confirmed clinical features of SBM from HCC; frequently accompanying extensive metastasis, location and physical characteristics of metastasis, and incidence and type of SBM-induced symptoms. Patients presenting oligometastasis with less than 5 foci of viable lesions can be expected for relatively long-term survival though overall survival was generally poor. SBM-induced symptoms including cranial nerve signs were relieved in considerable portion of patients promptly, but dose–response relationship in local control rate and overall survival needs to be studied with larger number of patients.
Ethics Approval and Informed Consent
All patients included in this study provided written informed consent and the study protocol was approved by the institutional review board of Severance Hospital, Yonsei University (IRB approval number 4-2021-1295). This study was performed in compliance with the Declaration of Helsinki.
Acknowledgments
This study was supported by the Dong-A research fund (Grant number: 2018-31-0904). Kangpyo Kim is now affiliated with the Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Uka K, Aikata H, Takaki S, et al. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13(3):414. doi:10.3748/wjg.v13.i3.414
2. Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20(11):1781–1787. doi:10.1111/j.1440-1746.2005.03919.x
3. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
4. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.2147/NDT.S58059
5. Kim TH, Park S, Rim CH, Choi C, Seong J. Improved oncologic outcomes by ablative radiotherapy in patients with bone metastasis from hepatocellular carcinoma. J Cancer Res Clin Oncol. 2021;147:1–8.
6. Kim K, Kim TH, Kim TH, Seong J. Efficacy of local therapy for oligometastatic hepatocellular carcinoma: a propensity score matched analysis. J Hepatocell Carcinoma. 2021;8:35. doi:10.2147/JHC.S290197
7. Park JS, Yoon DS, Kim KS, et al. What is the best treatment modality for adrenal metastasis from hepatocellular carcinoma? J Surg Oncol. 2007;96(1):32–36. doi:10.1002/jso.20773
8. Choi HJ, Cho BC, Sohn JH, et al. Brain metastases from hepatocellular carcinoma: prognostic factors and outcome. J Neurooncol. 2009;91(3):307–313. doi:10.1007/s11060-008-9713-3
9. Mori Y, Hashizume C, Kobayashi T, Shibamoto Y, Kosaki K, Nagai A. Stereotactic radiotherapy using Novalis for skull base metastases developing with cranial nerve symptoms. J Neurooncol. 2010;98(2):213–219. doi:10.1007/s11060-010-0179-8
10. Svare A, Fosså SD, Heier MS. Cranial nerve dysfunction in metastatic cancer of the prostate. Br J Urol. 1988;61(5):441–444. doi:10.1111/j.1464-410X.1988.tb06594.x
11. Moris G, Roig C, Misiego M, et al. The distinctive headache of the occipital condyle syndrome: a report of four cases. J Headache Pain. 1998;38(4):308–311. doi:10.1046/j.1526-4610.1998.3804308.x
12. McAvoy C, Kamalarajab S, Best R, Rankin S, Bryars J, Nelson KJE. Bilateral third and unilateral sixth nerve palsies as early presenting signs of metastatic prostatic carcinoma. Eye. 2002;16(6):749–753. doi:10.1038/sj.eye.6700210
13. Trivedi P, Gupta A, Pasricha S, Agrawal G, Shah MJ. Isolated skull base metastasis as the first manifestation of hepatocellular carcinoma—a rare case report with review of literature. J Gastrointest Cancer. 2009;40(1):10–14. doi:10.1007/s12029-009-9081-z
14. Guo X, Yin J, Jiang YJ. treatment. Solitary skull metastasis as the first symptom of hepatocellular carcinoma: case report and literature review. Neuropsychiatr Dis Treat. 2014;10:681. doi:10.2147/NDT.S58059
15. Nozaki I, Tsukada T, Nakamura Y, Takanaka T, Yamada MJ. Multiple skull metastases from hepatocellular carcinoma successfully treated with radiotherapy. Intern Med. 2010;49(23):2631–2634. doi:10.2169/internalmedicine.49.4236
16. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366–3375. doi:10.1200/JCO.2005.04.754
17. Hayashi S, Tanaka H, Hoshi H. Palliative external-beam radiotherapy for bone metastases from hepatocellular carcinoma. World J Hepatol. 2014;6(12):923. doi:10.4254/wjh.v6.i12.923
18. Health UDo, Services H. Common terminology criteria for adverse events (CTCAE) version 5.0; 2017. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
19. Hsieh C-T, Sun J-M, Tsai W-C, Tsai T-H, Chiang Y-H, Liu M-Y. Skull metastasis from hepatocellular carcinoma. Acta Neurochirurgica. 2007;149(2):185–190. doi:10.1007/s00701-006-1071-3
20. Kotecha R, Angelov L, Barnett GH, et al. Calvarial and skull base metastases: expanding the clinical utility of Gamma Knife surgery. J Neurosurg. 2014;121(Suppl_2):91–101. doi:10.3171/2014.7.GKS141272
21. Longo V, Brunetti O, D’Oronzo S, Ostuni C, Gatti P, Silvestris F. Bone metastases in hepatocellular carcinoma: an emerging issue. Cancer Metastasis Rev. 2014;33(1):333–342. doi:10.1007/s10555-013-9454-4
22. Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver International. 2005;25(2):261–265. doi:10.1111/j.1478-3231.2005.01094.x
23. Kaizu T, Karasawa K, Tanaka Y, et al. Radiotherapy for osseous metastases from hepatocellular carcinoma: a retrospective study of 57 patients. Am J Gastroenterol. 1998;93(11):2167–2171. doi:10.1111/j.1572-0241.1998.00614.x
24. Roca EL, Okazaki N, Okada S, et al. Radiotherapy for bone metastases of hepatocellular carcinoma. Jpn J Clin Oncol. 1992;22(2):113–116.
25. Matsuura M, Nakajima N, Ito K. Radiation therapy for bone metastasis of hepatocellular carcinoma. Int J Clin Oncol. 1998;3(1):31–35. doi:10.1007/BF02490099
26. He J, Zeng ZC, Tang ZY, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer. 2009;115(12):2710–2720. doi:10.1002/cncr.24300
27. Ahmed KA, Fulp WJ, Berglund AE, et al. Differences between colon cancer primaries and metastases using a molecular assay for tumor radiation sensitivity suggest implications for potential oligometastatic SBRT patient selection. Int J Radiat Oncol Biol Phys. 2015;92(4):837–842. doi:10.1016/j.ijrobp.2015.01.036
28. Vikram B, Chu FC. Radiation therapy for metastases to the base of the skull. Radiology. 1979;130(2):465–468. doi:10.1148/130.2.465
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.