Back to Journals » Infection and Drug Resistance » Volume 13
Six Years Trend Analysis of Tuberculosis in Northwestern Tigrai, Ethiopia; 2019: A Retrospective Study
Authors Negash H , Legese H , Adhanom G , Mardu F , Tesfay K, Gebreslasie Gebremeskel S , Berhe B
Received 22 November 2019
Accepted for publication 13 February 2020
Published 24 February 2020 Volume 2020:13 Pages 643—649
DOI https://doi.org/10.2147/IDR.S239717
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Hadush Negash,1 Haftom Legese,1 Gebre Adhanom,1 Fitsum Mardu,2 Kebede Tesfay,2 Senait Gebreslasie Gebremeskel,3 Brhane Berhe2
1Unit of Medical Microbiology, Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Adigrat University, Tigrai, Ethiopia; 2Unit of Medical Parasitology, Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Adigrat University, Tigrai, Ethiopia; 3Unit of Pediatrics and Child Health, Department of Midwifery, College of Medicine and Health Sciences, Adigrat University, Tigrai, Ethiopia
Correspondence: Hadush Negash Email [email protected]
Background: Ethiopia is one of the 22 high tuberculosis burden countries. In our country. there are limited published data to show the trend analysis of tuberculosis. Hence, we designed this trend analysis to fill the information gap in our study area. Institutional based retrospective cross-sectional study was employed from 2013 to 2018 to determine the trend analysis of tuberculosis among tuberculosis presumptive clients in Northwestern Tigrai. We have used a standard checklist to extract the data. There were some missing data from the logbooks which are then excluded from the analysis.
Results: A total of 7793 tuberculosis presumptive clients were requested for laboratory diagnosis of which about 7639 results had a valid result for X-pert MTB/Rif assay. The overall detection rate of tuberculosis was found to be 9.9% (756/7639). Of the total tuberculosis cases, 8.7 % (66/756) were rifampicin-resistant. The trend of tuberculosis across the six years was fluctuating with a declining trend in the recent three years. HIV infection and being presumptive to drug resistance were associated with tuberculosis detection.
Conclusion: Although there was a cumulative declining trend of tuberculosis within the last six years, prevention and control strategies still need to be improved to achieve the stop tuberculosis strategy. To create a world free of tuberculosis, there should be quality service provision regarding tuberculosis case detection and treatment.
Keywords: X-pert MTB/Rif assay, retrospective, Northwestern Tigrai, trend, tuberculosis
Introduction
Globally, tuberculosis (TB) is a major public health problem that is affecting 9 million people, leading to 1.3 million deaths with the majority of cases and deaths being in Sub-Saharan Africa.1 TB affects all age groups in a community.2,3 Access to treatment, poor socioeconomic status,4,5 health care service utilization and access, delays in seeking care and diagnosis6 and poor knowledge about the disease7 all contribute to the differences in TB case notifications.
Multi-drug resistant TB (MDR-TB) is a global public health challenge. The emerging drug-resistant TB increases the burden of communicable and non-communicable diseases. Sub-Saharan countries are highly affected by drug-resistant TB due to limited resources for early detection and treatment of TB. According to the WHO 2015 report, there were about 480,000 new MDR-TB and 100,000 Rifampicin resistant TB cases worldwide.8 Emerging of MDR-TB is the implication of poor TB prevention and control programs and sub-optimal TB management.9
Ethiopia ranked seventh among the 22 high burden countries of TB and third in Africa.10,11 Moreover, TB is the third cause of hospital admission and the second top cause of mortality in Ethiopia.10,12 Ethiopia is undergoing an accelerated decentralization of directly observed therapies (DOTs), which improved the case detection rate and treatment success.13,14 However, this improvement is not enough to achieve a stop TB strategy. In Ethiopia, TB detection using passive finding is missing some cases to be notified8 and is below the global target of 70% detection rate.1,13 In settings with limited resources, an active case finding is needed to improve early case finding and treatment at a reasonable cost.15–18 TB case detection varies among different regions of Ethiopia.13
Determining the trend and detection of TB helps to improve the performance of DOTs services and case notification rate. Few studies were reported on trends of TB detection and associated factors14,19,20 in Ethiopia. However, there is limited information regarding the trend analysis of TB in our study area. Hence, this study was aimed to assess the trend analysis of TB from January 2013 to December 2018 in Northwestern Tigrai, Ethiopia.
Materials and Methods
Study Area, Design and Period
An institutional-based retrospective cross-sectional study was conducted in Northwestern Tigrai from January 2013 to December 2018 to determine trend analysis of TB. Shire Enda-Silase is the zonal administrative city of Northwestern Tigrai. In Northwestern Tigrai there are 4 primary hospitals, 34 health centres and 1 teaching and general hospital. Based on the 2015 population projection of Ethiopian cities Shire Enda-Silase has a total population of 70,800 (35,000 males and 35,800 females). The city is located about 1084 s kilometres away from Addis Ababa, the capital city of Ethiopia, at a latitude and longitude of 14°6′N 38°17′E, with an elevation of 1953 meters (4017 feet) above sea level. A real-time Polymerase chain reaction (PCR) machine called X-pert MTB/Rif assay has been used for the diagnosis and drug resistance pattern of TB. Suhul General Hospital is serving more than 1,000,000 people as a teaching and general hospital for its catchment areas.
Sample Size Sampling Technique
A total of 7793 TB presumptive clients were examined for X-pert MTB/Rif assay from different areas of Northwestern Tigrai, in Suhul General Hospital. Socio-demographic data and laboratory results were extracted from registration books using structured questionnaire and checklist.
Population
All TB presumptive clients in Northwestern Tigrai.
Eligibility Criteria
Data were extracted on about 7639 (98%) clients who had complete information on laboratory results and patient records. Additionally, clients who were requested only for microscopic examination were excluded from the study. From the total number of clients examined, about 154 (2%) were excluded from the analysis due to insufficient sputum sample, error and invalid results of the X-pert MTB/Rif assay machine.
Laboratory Diagnosis of Tuberculosis
Presumptive clients were being diagnosed using X-pert MTB/Rif assay and microscopic examination in Suhul General Hospital. However, we preferred to include results of X-pert MTB/Rif assay for its increased sensitivity and ability to detect drug-resistant TB. There is no TB culture activity in the hospital. Hence, resistance to other anti-tuberculosis drugs was not screened.
HIV Screening
HIV screening was being performed at the voluntary counselling (VCT) and testing outpatient department of Suhul General Hospital.
Handling and Tracking of Missing (Incomplete) Data
Incomplete data in the laboratory registration books were extracted from patient records and charts of Suhul General Hospital. For the missing data, patients’ medical record numbers were used to get the charts and have the data.
Data Collection and Quality Assurance
Data were collected with a data collection format adopted from the standard checklist of world health organization (WHO). The checklist contains unique code (client’s medical record number to keep confidentiality of patient results), age and age categories (using the formula: K= 1+ 3.322 log (n=7639), minimum (4 years of age), maximum (90 years of age). Hence, < 15 years, 15–30 years, 30–45 years, 45–60 years and > 60 years), sex (male/female), residence (urban/rural), HIV status (positive/negative/unknown), year of diagnosis, season (autumn/winter/spring/summer), clinical diagnosis (PTB/MDR-PTB) and result of X-pert MTB/Rif assay (MTB not detected/MTB detected, Rifampicin resistant not detected, MTB detected Rifampicin resistant not detected). All the above data were collected from Suhul General Hospital laboratory department TB registration books (from the archived log-books in laboratory store) and client charts and records. Data were extracted by trained data collectors. The collected data were cleaned and edited using EPI Info version 7 software and checked for completeness, clarity and consistency by the principal investigator on a daily basis.
Statistical Analysis
We used STATA version 13 software for data checking and analysis. Bi–variate and multi–variate regression analysis were employed to measure the association between dependent and independent variables. Variables with P < 0.25 in the bivariate logistic regression were entered to multivariate regression analysis to compute AOR using backward: conditional method. We set reference categories for each variable accordingly. A p-value less than 0.05 with corresponding 95% confidence interval (CI) was considered as statistically significant. Data for categorical variables were summarized and organized using graphs and tables. Mean and standard deviation were used to summarize continuous variables.
Ethical Consideration
We obtained ethical clearance from research and community service counsel of Adigrat University with a reference number of AGU/CMHS/098/11. Written permission was obtained from the chief executive officer, department of patient cards and records and laboratory head of Suhul General Hospital prior to the actual data collection. To keep the privacy of the clients’ medical data, we obtained a consent waived by the ethical review committee confirming data were anonymized and maintained with confidentiality.
Operational Definition
Presumptive TB: An individual who presents with symptoms or signs suggestive of TB like sweating, coughing more than two weeks, loss of appetite, weight loss and weakness.
MDR presumptive patient: Is a patient who relapses for TB, lost to follow up patients and having close contact with drug-resistant TB infected persons.
Results
Socio-Demographic Characteristics and Detection of TB Cases
A total of 7793 sputum samples were examined using X-pert MTB/Rif assay in Northwestern Tigrai within the six study years. The mean age of the study participants was 38.5 ± 16.6 years, ranged from 4 to 90 years old. Of the total study participants, 62% (4736/7639) were males. Only 26.5 % (2022/7639) of the patients were screened for HIV (1423 were negative and 599 were positive) during TB diagnosis (Table 1).
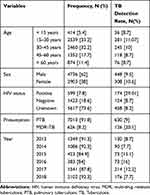 |
Table 1 Distribution of Tuberculosis with Socio-Demographic and Clinical Variables in Northwestern Tigrai, Ethiopia from January 2013 to December 2018 (n = 7639) |
Detection of TB and Rifampicin-Resistant TB
In the six study years, the overall detection of TB was observed to be 9.9% (756/7639). The prevalence of rifampicin-resistant TB among the confirmed cases was reported to be 8.7% (66/756). Although most of the TB types were pulmonary TB, there were also few extrapulmonary TB as far as we have included different specimens like sputum, CSF, Pleural and other body fluids.
The Trend of TB Detection
With a considerable fluctuation, detection of TB was elevated from 2013 to 2016 (8.7% in 2013 to 16% in 2016) and then declined from 2016 to 2018 (16% in 2016 to 7.73% in 2018) (Figure 1).
 |
Figure 1 Trend of tuberculosis in Northwestern Tigrai, Ethiopia from January 2013 to December 2018. Abbreviation: TB, Tuberculosis. |
Distribution of TB Cases by Age Group
High detection of TB was observed among individuals of 15–30 years of age (11.1%), followed by 30–45 years (9.96%) (Figure 2).
 |
Figure 2 Distribution of tuberculosis among different age groups in Northwestern Tigrai, Ethiopia from January 2013 to December 2018. |
Associated Factors for TB
HIV infection (AOR = 4.04, CI: 3.29–4.968, p = 0.001) and being presumptive to MDR-TB (AOR = 1.9, CI: 0.374–1.167, p = 0.001) were significantly associated with presence of TB among the study participants (Table 2).
 |
Table 2 Bivariate and Multivariate Analysis of Factors Associated with Tuberculosis Among Presumptive Clients in Northwestern Tigrai, Ethiopia from January 2013 to December 2018 (n = 7639) |
Discussion
TB remains a major health problem in developing countries including Ethiopia.21 Six years of retrospective data were collected to analyze the trend of TB in Northwestern Tigrai, Ethiopia. The overall detection of TB was 9.9% (756/7639). This finding is higher than previous studies reported from Thailand, 0.215%22 and Ethiopia, 6%.23 In contrast, it is lower than the study reported from Vietnam, 15.57%.24 This variation might be due to sample size differences, study period, the burden of TB and the double burden of TB and HIV. The possible explanation for the difference in the detection rate of TB among countries similar to Ethiopia might also be attributed by HIV epidemicity, overcrowding, sensitivity difference in laboratory diagnostic techniques and variation in the effectiveness of prevention strategies. Higher prevalence of TB was reported among individuals aged 15–30 years (11.10%, 281/2539) followed by 30–45 years (10%, 245/2460). This might be due to the higher incidence of HIV within this age category (63.3%, 378/599) and the biology of TB compared with the elderly and children.
In this study, the detection of rifampicin-resistance, the most potent anti-TB drug,25 was 8.73% (66/756). The rate of rifampicin-resistance from our study is consistent with previous studies reported from Ethiopia, 9.9%,26 9%27 and 10.3%.28 However, other studies reported higher detection of rifampicin-resistant TB from India, 28.1%,19 15.8%29 and 33.3%.30 This might be due to the differences in client selection and laboratory techniques that are used to diagnose the resistance pattern of TB.
This study revealed that the trend of TB was increased from 2013 (8.7%) to 2016 (16%). This increment of trend might be due to the emerging triple burden of TB in the nation, improved case detection and notification rate. In the last three years of study, declined trend of TB was observed from 2016 (16%) to 2017 (12.2%) and 2018 (7.73%). The declining trend of TB within the recent three years is promising to create a world free of TB. 23.75% and 36.3% annual declining trend of TB was assessed from 2016 to 2017 and 2017 to 2018 respectively. This is even better than the 20% reduction rate targeted by WHO in 2015 and to be achieved in 2020.31 The overall declining trend of TB within the recent three years is comparable to the national level, 210/100,000 in 2014,32,200/100,000 in 2015,33,196/100,000 in 201734 and 172/100,000 in 2018.32 Similar studies from Zambia,35 Zimbabwe,36 Thailand,37 Saudi Arabia38 and United Kingdom24 reported an increasing trend of TB. However, studies from Saudi Arabia39,40 reported a decreasing trend of TB over the years. These variations may be explained by national TB control programs and HIV endemicity in the different regions.
In this study, participants who had been infected with HIV were more likely (AOR: 4.04, CI: 3.29–4.968) to have TB than those who were not infected with HIV. This finding was in line with previous studies from India41 Kenya42 and Ethiopia.43 The possible reason for this difference might be; HIV infection enhances TB progression and activation of latent TB to active TB. Being presumptive to MDR-TB was also statistically associated with having TB (AOR = 1.9, CI: 0.374–1.167). This might be due to their close contacts and history previous exposure to individuals with drug-resistant TB.
Limitation of the Study
As we collected retrospective data from logbooks, we encountered data missing and incompleteness. Variables included for associated factors were also limited.
Conclusion
The overall trend of TB within the six study years showed a decline within recent years in the study area. HIV infection and being presumptive for MDR-TB were statistically associated with the presence of TB. TB detection was higher among productive age groups (15 to 45 years), which affects the socioeconomic status of individuals and the country as a whole. Hence, national health system policy and federal ministry of health should consider introducing regular screening strategy for TB with X-pert MTB/Rif for early diagnosis and treatment to enhance the prevention and control strategies.
Abbreviations
AOR, adjusted odds ratio; COR, crude odds ratio; CI, confidence interval; DOTs, directly observed therapies; MDR-TB, multi-drug resistant TB; TB, tuberculosis.
Ethics Statement
We obtained ethical clearance from research and community service counsel of Adigrat University with a reference number of AGU/CMHS/098/11. Written permission was obtained from the chief executive officer, department of patient cards and records and laboratory head of Suhul General Hospital prior to the actual data collection. To keep the privacy of the clients’ medical data, we obtained a consent waived by the research and community service counsel confirming data were anonymized and maintained with confidentiality.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are very thankful to Suhul General Hospital Laboratory Department, chief executive officer and the data collectors for their valuable contribution in this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, drafting the manuscript, revising the manuscript critically, read and approve the final draft of the manuscript for submission, gave final approval of the manuscript version to be published and agreed to be accountable for every step of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global TB Report. Geneva: WHO; 2013.
2. Thorson A, Diwan VK. Gender inequalities in TB: aspects of infection, notification rates, and compliance. Curr Opin Pulm Med. 2001;7:165–169. doi:10.1097/00063198-200105000-00009
3. Mukherjee A, Saha I, Sarkar A, Chowdhury R. Gender differences in notification rates, clinical forms and treatment outcome of TB patients under the RNTCP. Lung India. 2012;29:120–122. doi:10.4103/0970-2113.95302
4. Marais BJ, Gupta A, Starke JR, El Sony A. TB in women and children. Lancet. 2010;375:2057–2059. doi:10.1016/S0140-6736(10)60579-X
5. Diwan VK, Thorson A. Sex, gender, and TB. Lancet. 1999;353:1000–1001. doi:10.1016/S0140-6736(99)01318-5
6. Oxlade O, and Murray M. TB and poverty: why are the poor at greater risk in India? PLoS One. 2012;7:e47533. doi:10.1371/journal.pone.0047533
7. Boccia D, Hargreaves J, De Stavola BL, Fielding K, Schaap A. The association between household socioeconomic position and prevalent TB in Zambia: a case-control study. PLoS One. 2011;6:e20824. doi:10.1371/journal.pone.0020824
8. Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of TB. BMC Pub Heal. 2008;8:15. doi:10.1186/1471-2458-8-15
9. Wang J, Fei Y, Shen H, Xu B. Gender difference in knowledge of TB and associated health-care seeking behaviours: a cross-sectional study in a rural area of China. BMC Pub Heal. 2008;8:354. doi:10.1186/1471-2458-8-354
10. WHO. Global TB Report. WHO; 2016. Available from: http://www.who.int/tb/publications/global_report.
11. Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K. Surveillance of anti-TB drug resistance in the world: an updated analysis, 2007±2010. Bull World Health Organ. 2012;90:
12. World Health Organization. Global TB Report. Geneva: WHO; 2012.
13. World Health Organization. Global TB Report. Geneva: WHO; 2009.
14. Addis Ababa TB. Leprosy and TB/HIV Prevention and Control Program Manual. Ministry of Health of Ethiopia (EMOH); 2008.
15. Federal Ministry of Health. TB Prevention and Control Program: Special Issue for World TB Day. Addis Ababa, Ethiopia; 2011.
16. Yassin MA, Datiko DG, Shargie EB. Ten-year experiences of the TB control program in the southern region of Ethiopia. Int J Tuberc Lung Dis. 2006;10:1166–1171.
17. Yassin MA, Datiko DG, Tulloch O, Markos P, Aschalew M. Innovative community-based approaches doubled TB case notification and improve treatment outcome in Southern Ethiopia. PLoS One. 2013;8:e63174. doi:10.1371/journal.pone.0063174
18. Datiko DG, Lindtjorn B. Health extension workers improve TB case detection and treatment success in southern Ethiopia: a community randomized trial. PLoS One. 2009;4:e5443. doi:10.1371/journal.pone.0005443
19. Datiko DG, Lindtjorn B. Cost and cost-effectiveness of smear-positive TB treatment by Health Extension Workers in Southern Ethiopia: a community randomized trial. PLoS One. 2010;5:e9158. doi:10.1371/journal.pone.0009158
20. Getahun H, Raviglione M. Active case-finding for TB in the community: time to act. Lancet. 2010;376:1205–1206. doi:10.1016/S0140-6736(10)61503-6
21. Shargie EB, Lindtjorn B. DOTs improve treatment outcomes and service coverage for TB in South Ethiopia: a retrospective trend analysis. BMC Pub Heal. 2005;5:62. doi:10.1186/1471-2458-5-62
22. Wolde Yohannes D, Kebede N, Erku W, Tadesse Z. Ten years’ experience of DOTs therapy for TB in Addis Ababa, Ethiopia. Ethiop Med J. 2011;49:221–229.
23. World Health Organization. Global TB Control. Surveillance, Planning, Financing. WHO Report 2005. Geneva, Switzerland: WHO/HTM/TB; 2005:349.
24. Pongwittayapanu P, Anothaisintawee T, Malathum K, Wongrathanandha C. Incidence of newly diagnosed TB among healthcare workers in a teaching hospital, Thailand: 2011–2015. Ann Global Health. 2018;84(3):342–347. doi:10.29024/aogh.2304
25. Bikila D, Yohannes W, Abdeta A, et al. Trend analysis of pulmonary TB at St. Paul hospital Millennium medical college, Addis Ababa, Ethiopia. Austin J Pulm Respir Med. 2017;4(2):02–10.
26. John M-A, Menezes CN, Chita G, Sanne I, Grobusch MP. Trend of TB in Vietnam, 1997–2004. Emerg Infec Dise. 2007;13(5):796–797.
27. Wright A, Zignol M, Van Deun A. Epidemiology of anti-TB drug resistance 2002-07: an updated analysis of the global project on anti-TB drug resistance surveillance. Lancet. 2009;373:1861–1873. doi:10.1016/S0140-6736(09)60331-7
28. Arega B, Menbere F, Getachew Y. Prevalence of rifampicin-resistant Mycobacterium tuberculosis among presumptive tuberculosis patients in selected governmental hospitals in Addis Ababa, Ethiopia. BMC Infect Dis. 2019;19:307. doi:10.1186/s12879-019-3943-1
29. Mulu W, Abera B, Yimer M. Rifampicin-resistance pattern of Mycobacterium TB and associated factors among presumptive TB patients referred to Debre Markos referral hospital, Ethiopia: a cross-sectional study. BMC Res Notes. 2017;10:8. doi:10.1186/s13104-016-2328-4
30. Singh AK, Maurya AK, Kumar M, et al. Resistance patterns and trends of extensively drug-resistant TB: 5-year experience. Microbio Infec Dis. 2013;3(4):169–175. doi:10.5799/ahinjs.02.2013.04.0103
31. Mulisaa G, Workneha T, Hordofa N, et al. Multidrug-resistant mycobacterium tuberculosis and associated risk factors in Oromia Region of Ethiopia. Int J Infect Dis. 2015;39:57–61. doi:10.1016/j.ijid.2015.08.013
32. Nigus DM, Lingerew WM, Beyene BA. Prevalence of multidrug-resistant tuberculosis among presumptive multidrug-resistant tuberculosis cases in Amhara National Regional State, Ethiopia. J Mycobac Dis. 2014;4:152. doi:10.4172/2161-1068.1000152
33. World Health Organization. 2018. Global TB Report. United to End TB: An Urgent Global Response to a Global Epidemic. ISBN 978-92-4-156564-6 Geneva WHO.
34. World Health Organization. Global TB Report. Geneva: WHO; 2014.
35. World Health Organization. Global TB Report. Geneva: WHO; 2015.
36. World Health Organization. Global TB Report. Geneva: WHO; 2017.
37. Kapata N, Chanda-Kapata P, O’Grady J, et al. Trends of Zambia’s TB burden over the past two decades (1990–2010). Trop Med Int Heal. 2011;2(2). doi:10.1111/j.1365-3156.2011.02849
38. Muringazuva C, Owiti P, Edwards J, Mutetse GN, Dobbie M, Chimedza J. TB in the military: trends in notification and treatment outcomes within the Zimbabwe defence forces, defendants and communities 2010–2015. BioRxiv. 2018. doi:10.1101/382325
39. Elsadig Y, Muraleedharan G, Medan KA, et al. TB trend in Majmaah, Saudi Arabia: a 10 year retrospective study (2005–2014). Majmaah J Heal Sci. 2016;4(2):1–10.
40. Glaziou P, Floyd K, Raviglione M. Trends in TB in the UK: 1913–2016. Thorax Month. 2018;(1):1–2.
41. Al-Hajoj SA. Tuberculosis in Saudi Arabia: can we change the way we deal with the disease? J Infect Public Health. 2010;1–8. doi:10/1016/jjiph.2009.12.001
42. Al-Hajoj S, Varghese B. TB in Saudi Arabia: the journey across time. J Infect Dev Ctries. 2015;9(3):222–231. doi:10.3855/jidc.5296
43. Zumla A, Malon P, Henderson J, Grange JM. Impact of HIV infection on TB. Postgrad Med J. 2000;76:259–268. doi:10.1136/pmj.76.895.259
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
