Back to Journals » Clinical Ophthalmology » Volume 12
Six-month results of intravitreal ranibizumab for macular edema after branch retinal vein occlusion in a single-center prospective study: visual outcomes and microaneurysm formation
Authors Kawamura M , Hirano Y , Yoshida M , Mizutani T, Sugitani K, Yasukawa T , Ogura Y
Received 10 April 2018
Accepted for publication 30 May 2018
Published 20 August 2018 Volume 2018:12 Pages 1487—1494
DOI https://doi.org/10.2147/OPTH.S170698
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Mihoko Kawamura.
Views: 366
Mihoko Kawamura, Yoshio Hirano, Munenori Yoshida, Takeshi Mizutani, Kazuhiko Sugitani, Tsutomu Yasukawa, Yuichiro Ogura
Department of Ophthalmology & Visual Science, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan
Purpose: The aim of this study is to report the 6-month results after one intravitreal ranibizumab (IVR) injection followed by pro re nata dosing for macular edema (ME) after branch retinal vein occlusion.
Patients and methods: The inclusion criteria included a minimal patient age of 18 years, 20 letters or more best-corrected visual acuity (BCVA) (Early Treatment Diabetic Retinopathy Study [ETDRS] score, 77 letters or less), and central retinal thickness (CRT) of 250 microns or more. The primary outcome measure was the mean BCVA change from baseline at month 6; the secondary outcomes were mean changes in CRT, residual ME, and microaneurysm formation.
Results: Twenty patients were enrolled from March 2014 through October 2016 at Nagoya City University Hospital. The baseline mean ETDRS letters and CRT were 63.1 and 500 microns, respectively; mean time from symptom onset to initial therapy was 1.80 months; and mean ETDRS gain and CRT reduction were 15.2 letters and 230 microns, respectively. The percentages of patients with Snellen equivalent BCVAs of 20/40 (70 ETDRS letters) or better and 20/20 (85 ETDRS letters) were 90% and 15%, respectively. Residual ME and microaneurysms were observed in 85% and 35% of patients. Microaneurysm formation was associated with delayed initial therapy.
Conclusion: Prompt initiation of IVR injection provided a better visual prognosis at month 6 and suppressed the microaneurysm formation.
Keywords: branch retinal vein occlusion, macular edema, microaneurysm, ranibizumab, prompt treatment
Introduction
The main cause of visual loss in patients with branch retinal vein occlusion (BRVO) is macular edema (ME).1,2 The availability of anti-vascular endothelial growth factor (VEGF) therapy has revolutionized the treatment of ME in patients with retinal diseases.3–5 Currently, anti-VEGF therapy is the first-line therapy for ME in patients with BRVO. However, the time of the administration of the initial therapy after the disease onset, the inclusion criteria such as the best-corrected visual acuity (BCVA) and central retinal thickness (CRT), and the treatment regimen have not been established fully.
Regarding the time of the initial therapy, previous reports6,7 have shown that early treatment with the anti-VEGF drug bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA, USA) achieved visual improvements. Moreover, we previously reported the results of a retrospective study showing that early administration of intravitreal ranibizumab (IVR, Lucentis®; Genentech, Inc.) injections suppressed microaneurysm formation and refractory ME,8 although no improvement in the visual outcomes was seen.
Regarding the treatment regimen, the BRAnch Retinal Vein Occlusion (BRAVO) trial,5 the first prospective, randomized, multicenter study, used monthly IVR through month 6. Although the BRAVO trial reported substantial visual outcomes, such frequent injections might be excessive. The BRIGHTER study9 reported the efficacy of IVR injections that were administered according to the three plus pro re nata (PRN) regimen. Moreover, Miwa et al10 reported that the efficacy of IVR injections administered according to the one plus PRN regimen was comparable to that of the three plus PRN regimen in visual functional outcomes and the effectiveness for minimizing the number of injections. Moreover, the retreatment criteria have not been well established. For example, Snellen equivalent BCVA of 20/40 or better or 250 microns or more of mean central subfield thickness on time-domain optical coherence tomography (OCT) in the BRAVO and just loss of visual acuity (VA) in the BRIGHTER were used as the criteria for retreatment.
In the current study, relatively early administration of IVR, followed by the one plus PRN regimen with strict retreatment criteria, was examined prospectively to evaluate the efficacy and safety in patients with BRVO. The primary efficacy outcome measure was the mean change in the BCVA from baseline at month 6 after the initial injection, and the secondary outcomes included other parameters of the visual outcomes, the mean change in the CRT from baseline, residual ME, and microaneurysm formation.
Patients and methods
Patients
This was a prospective, interventional study conducted at Nagoya City University Hospital from March 2014 to October 2016 to evaluate the efficacy and safety of IVR injections for ME in patients with BRVO who completed 6 months of follow-up (total 24 months). The institutional review board of the Nagoya City University Graduate School of Medical Sciences approved the study protocol (no. 00000891-3). The clinical trial was registered in ClinicalTrials.gov (ID: NCT02478515). All patients provided written informed consent for participation in the study. The research methods and analyses adhered to the tenets of the Declaration of Helsinki.
Methods and study criteria
All patients underwent a complete ophthalmic examination including measurement of the BCVA using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, intraocular pressure, and CRT using an OCT (Cirrus HD-OCT; Carl Zeiss Meditec, Oberkochen, Germany) retinal color map, indirect ophthalmoscopy, fundus photography, and fluorescein angiography (FA) using the Optos200Tx imaging system (Optos plc, Dunfermline, UK). The patients provided a medical history that included the concomitant medications and measurement of the vital signs. Two masked retinal specialists diagnosed BRVO based on fundus examinations and FA images and determined the eligibility for study participation. Eyes with hemi-central retinal vein occlusion (RVO) or central RVO were excluded. Patients who had ME after BRVO and met the following criteria were enrolled: patient age of 18 years or older, 20 letters or more of BCVA in ETDRS letter score (77 letters or less), and 250 microns or more of CRT on OCT. The exclusion criteria were a history of previous anti-VEGF treatment and/or vitrectomy, intraocular corticosteroid use within 3 months before participation in the current study, application of panretinal laser photocoagulation or scatter laser photocoagulation to treat the areas of nonperfusion, application of laser photocoagulation for ME within 3 months before participation in the current study, evidence of any other retinal diseases, and cataracts causing visual loss or poor image quality.
Treatment regimen and assessments
The eligible patients received an IVR injection followed by monthly visits through month 6. At each visit, patients underwent a complete ophthalmic examination that was the same as the screening examination except for FA, which was performed at baseline and month 6. Additional IVR injections were administered according to a PRN dosing regimen. The retreatment criteria included visual loss of five or more ETDRS letters compared with the previous visit, 250 microns or more of CRT on OCT images, or evidence on an OCT color map of residual or recurrent ME including parafoveal lesions. Two retina specialists determined the BRVO subtype in each eye, that is, major and macular or ischemic and nonischemic based on the baseline FA images, and microaneurysm formation based on the FA images at month 6. Any new symptoms or signs, illness, or worsening of preexisting diseases were considered adverse events.
Outcome measures
The primary efficacy outcome measure was the mean change in the BCVA from baseline at month 6. The secondary efficacy outcome measures included the percentage of patients who gained 15 ETDRS letters or more, with a Snellen equivalent BCVA of 20/40 or better or 20/20 (70 or 85, ETDRS equivalent) or better, and the mean change in the CRT from baseline. Residual ME was defined as 250 microns or more of CRT on OCT images, or 350 microns or more of retinal thickness on an OCT color map including parafoveal lesions. The presence or absence of microaneurysms was determined by two masked retinal specialists based on FA images at month 6. The percentages of patients with residual ME or microaneurysm formation at month 6 were also evaluated. Moreover, the prognostic factors for BCVA (ETDRS score), residual ME, and microaneurysm formation at month 6, were examined.
Statistical analysis
Analysis of variance with the Bonferroni correction was used to estimate the continuous outcome measures: baseline and posttreatment ETDRS letters, changes in the ETDRS letters from baseline, baseline and posttreatment CRTs, and the change in the CRT from baseline. Pearson’s correlation coefficient was used to analyze the bivariate relationships between factors and BCVA (ETDRS letters) at month 6. Logistic regression analysis was used to detect prognostic factors associated with residual ME and microaneurysm formation at month 6; then, multivariate analyses were performed if the multiple factors were identified. A P-value <0.05 was considered statistically significant.
Results
Baseline characteristics
Twenty eyes of 20 patients (six men, 14 women; mean age, 67.7±10.1 years; range, 42–82) were enrolled. The patient baseline characteristics are shown in Table 1. The mean baseline ETDRS letter score was 63.1±13.1 (range, 32–82). The mean baseline CRT was 500±105 microns (range, 336–699). The mean time from symptoms of visual loss to the initial injection was 1.80±1.1 months (range, 0.5–4.5). All patients completed the study through month 6.
  | Table 1 Baseline characteristics |
Functional and anatomic outcomes at month 6
The BCVA improved significantly after the IVR injection at month 1 (Figure 1A); thereafter, the improvement continued through month 6, at which time, the ETDRS letters score was 78.3±7.1 and the gain from baseline was 15.2±10.3 letters (Figure 1B).
At month 6, 50% of patients had gained a mean of 15 ETDRS letters or more compared with the baseline BCVA letter score (Table 2). The percentages of patients with a Snellen equivalent BCVA of 20/40 or better or 20/20 or better at 6 months were 90% and 15%, respectively (Table 2). Baseline BCVA (ETDRS letters) (P=0.00342) and number of IVR (P=0.0195) were the positive predictors for BCVA (ETDRS letters) at month 6 (Table 3).
  | Table 2 Change from baseline best-corrected visual acuity at month 6 |
Concomitant with the improvement in the BCVA, the CRT decreased significantly after the IVR injection (Figure 2A). This significant improvement was observed even at month 1 and kept on through month 6 (Figure 2A and B). The mean CRT reduction at month 6 compared with baseline was 44.0%±16.8%.
Mean number of IVR injections
The mean number of IVR injections was 4.05±1.05 (Table 4). Eight eyes required three injections, five eyes four injections, five eyes five injections, and two eyes six injections (Table 4). No eyes received only one or two injections.
  | Table 4 Residual macular edema, microaneurysm, and total number of injections at month 6 |
Residual ME
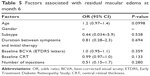
The percentages of patients with a CRT of 250 microns or more and 300 microns or more were 50% and 15%, respectively (Table 4). In three (15%) eyes, the ME resolved completely at month 6 (Table 4). The other 17 (85%) eyes received additional IVR injections according to the retreatment regimen in this study. No predictive factors for residual ME were identified (Table 5).
Microaneurysm formation
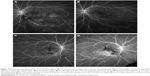
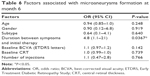
In seven (35%) eyes, microaneurysms were seen on the FA images at month 6 (Table 4). Six of the seven eyes had leaky microaneurysms, which resulted in ME around the microaneurysms. Laser to treat the leaky microaneurysms was not applied through month 6. Representative images with and without microaneurysms at month 6 are shown in Figure 3. The time of the initial therapy from disease onset was predictive of microaneurysm formation (P=0.0367; Table 6).
Safety outcomes through month 6
No serious ocular or nonocular adverse events associated with IVR injections developed in any patients through month 6.
Discussion
In the current prospective study, we evaluated the 6-month results of ranibizumab for ME associated with BRVO. The characteristic features of this study were that patients with relatively good baseline BCVA were included; the duration between disease onset and initial therapy was relatively short; the treatment regimen was one plus PRN; retreatment criteria were strict to keep dry macula; and not only the primary efficacy outcome (mean change from the baseline BCVA at month 6) but also the secondary outcomes including other parameters of visual function, mean changes in the CRT from baseline, residual ME, and microaneurysm formation were assessed.
Importantly, the current study found that the ETDRS letter score at month 6 was 78.3 letters, which was relatively better than those of previous prospective studies: 71.3 letters in the BRAVO trial5 and 74.3 in the BRIGHTER study,9 although the inclusion criteria and treatment regimens of those studies differed from those of the current study. Further, a benefit of the treatment in the current study was the high percentage of patients who had good Snellen equivalent BCVA at month 6. The percentages of patients who had Snellen BCVA of 20/40 or better and 20/20 or better were 90% and 15%, respectively, which were comparable or better than the respective 64.8% and 19.8% in the BRAVO trial. The mean baseline ETDRS letter scores were 63.1, 53.0, and 59.5 letters in the current study, BRAVO trial, and BRIGHTER study, respectively. Because patients with relatively good baseline BCVA (exceeding 20/40 Snellen equivalent) were also included in the current study, the mean baseline BCVA was naturally better than the other studies. However, it is possibly effective in order to obtain good visual outcomes in the treatment for BRVO before the damage reaches the irreversible stage. It is true that a small percentage of cases with BRVO resolve spontaneously without treatment. Therefore, some physicians might hesitate to start treating ME associated with BRVO if the BCVA is relatively maintained. However, it is impossible to know in which cases ME associated with BRVO will resolve spontaneously. Moreover, Rogers et al11 reported that a clinically significant improvement higher than 20/40 in Snellen BCVA was uncommon without intervention in eyes with BRVO. Thus, longer observations in such cases might sometimes cause the failure of treatment.
Previous reports6,7 have found that early treatment with another anti-VEGF agent (bevacizumab) was effective for achieving functional and visual improvements in BRVO. We can see the same tendency in the 24-month BRIGHTER trial.12 In fact, much earlier administration of IVR (1.8 months) in the current study than at 3.3 months in the BRAVO trial and 10.3 months in the BRIGHTER study resulted in better visual prognoses at month 6, although the duration between symptoms and initial therapy was not a predictive factor for BCVA at month 6 (P=0.0793; Table 3).
In the current study, strict retreatment criteria were adopted; that is, visual loss of five or more ETDRS letters compared with the previous visit, 250 microns or more of CRT on OCT images, or evidence on an OCT color map of residual or recurrent ME including parafoveal lesions. On the other hand, both the BRAVO and BRIGHTER trials did not have strict reinjection criteria: that is, Snellen equivalent BCVA of 20/40 or better or 250 microns or more of mean central subfield thickness on time-domain OCT in BRAVO and just loss of VA in BRIGHTER. The stricter retreatment criteria might provide the better visual prognosis.
Baseline BCVA and number of IVR were identified as positive predictors for better BCVA at month 6 (Table 3). Several previous reports8,13,14 showed that a better baseline BCVA was associated with a better visual outcome, which was consistent with the current result. It is interesting that more frequent IVR was associated with a better visual outcome in the current study. However, these were just 6-month results and, therefore, we have to undergo long-term studies to determine the effectiveness for better visual outcomes.
Another important finding in the current study was that the mean CRT decreased significantly at month 6, and the reduction from baseline was 230 microns (44% reduction from baseline). However, the efficacy of the CRT reduction was inferior to that in the BRAVO trial (345 microns, 62.6%) and comparable to that in the BRIGHTER study (217 microns, 42.0%). This difference was evident because the baseline CRTs differed as following: 500 microns in the current study, 552 microns in the BRAVO trial, and 530 microns in the BRIGHTER study. In the BRAVO trial, the mean CRT at month 6 was 207 microns, which was thinner than the 270 microns in the current study, whereas the BCVA (ETDRS letters) at month 6 in the BRAVO trial was lower than in the current study. Considering the results, the other factors might be related to the BCVA at month 6. One possible factor, retinal damage, that is, photoreceptor apoptosis, was considered to be related to prolonged ME because of delayed administration of anti-VEGF agents.
Our third important finding was related to microaneurysm formation. We previously reported10 in a retrospective study that microaneurysms caused refractory ME in BRVO and early administration of ranibizumab suppressed microaneurysm formation, leading to less refractory ME. In the current prospective study, microaneurysm formation was evaluated with FA images at month 6. We found that a longer duration between disease onset and initial therapy was associated with microaneurysm formation, which is consistent with our previous reports. Therefore, the microaneurysms that were already formed in the patients in the current study might cause refractory ME in the future. Because the current study was designed with a 24-month observation period, only a continued follow-up of the patients might help to identify a relationship between microaneurysm formation and refractory ME.
The mean number of IVR injections in the current study was relatively less (4.1) compared with that in the BRAVO trial (5.7) and BRIGHTER study (4.8), because in the BRAVO trial, injections were administered monthly through month 6 and in the BRIGHTER study, the three plus PRN regimen was followed. The current study used the one plus PRN regimen to reduce the number of injections and the goal was achieved, but the mean number of IVR injections in the prospective study conducted by Miwa et al,10 in which the one plus PRN regimen was used, was 3.8 through 12 months, which suggested that excessive injections were administered in the current study. However, a comparison of the effectiveness in the visual outcomes based on the number of injections was impossible because the retreatment regimen also differed. Some patients in the current study received additional IVR injections because the CRT was 250 microns or more despite a dry macula, which might result in the need for more IVR injections. Consideration should be given to modifying the treatment regimen, such as no retreatment for patients with a dry macula on OCT even if the CRT exceeds 250 microns.
Although the current study showed that an earlier administration of ranibizumab with the one plus PRN regimen provided benefits to the physicians and the patients with ME after BRVO, some questions still remain. The appropriate treatment regimen should be determined in order to achieve more efficacious outcomes in BCVA with fewer injections and the fewer patient visits. Currently, anti-VEGF therapy is the first-line therapy for ME in BRVO; however, the following need to be determined: the duration of the anti-VEGF therapy, an alternative therapy for persistent ME, and when the treatment should be switched.
Limitations
The current study had several limitations. The sample size was small, only one center was involved, and the study design was not randomized. A continued follow-up with more patients is needed to confirm the current results and provide more benefits and lessen the treatment burden for patients with ME associated with BRVO.
Acknowledgments
The authors thank Fumie Shibuya, Yasuyo Matsuda, Sayaka Oshio, and Soushi Shimizu from Nagoya City University Graduate School of Medical Sciences for their help with data collection. This study was partially supported by Novartis Pharma K.K. (Tokyo, Japan) and was presented at the 121st Annual Meeting of the Japanese Ophthalmological Society (Tokyo, Japan) on April 8, 2017.
Disclosure
The financial support was received from Novartis Pharma K.K. (Tokyo, Japan), a Grants-in-Aid for Scientific Research (C) 15K10875 (Y.H.), 60273447 (M.Y.), and 25462758 (T.Y.), and Scientific Research (B) 15H04997 (Y.O.) from the Japan Society for the Promotion of Science (Tokyo, Japan). Y.H. was also supported by the Mochida Memorial Foundation (Tokyo, Japan), Takeda Science Foundation (Osaka, Japan), Suzuken Memorial Foundation (Nagoya, Japan), and Santen Pharmaceutical’s Founder (Tokyo, Japan). The other authors report no conflicts of interest in this work.
References
Wong TY, Scott IU. Clinical practice. Retinal-vein occlusion. N Engl J Med. 2010;363:2135–2144. | ||
Hayreh SS. Ocular vascular occlusive disorders: natural history of visual outcome. Prog Retin Eye Res. 2014;41:1–25. | ||
Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. | ||
Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology. 2006;113:1706–1712. | ||
Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102.e1–1112.e1. | ||
Chung EJ, Hong YT, Lee SC, et al. Prognostic factors for visual outcome after intravitreal bevacizumab for macular edema due to branch retinal vein occlusion. Graefes Arch Exp Ophthalmol. 2008;246:1241–1247. | ||
Jaissle GB, Szurman P, Feltgen N, et al. Predictive factors for functional improvement after intravitreal bevacizumab therapy for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2011;249:183–192. | ||
Tomiyasu T, Hirano Y, Yoshida M, et al. Microaneurysms cause refractory macular edema in branch retinal vein occlusion. Sci Rep. 2016;6:29445. | ||
Tadayoni R, Waldstein SM, Boscia F, et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: six-month results of BRIGHTER. Ophthalmology. 2016;123:1332–1344. | ||
Miwa Y, Muraoka Y, Osaka R, et al. Ranibizumab for macular edema after branch retinal vein occlusion: one initial injection versus three monthly injections. Retina. 2017;37:702–709. | ||
Rogers SL, McIntosh RL, Lim L, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1094–1101. | ||
Tadayoni R, Waldstein SM, Boscia F, et al. Sustained benefits of ranibizumab with or without laser in branch retinal vein occlusion: 24-month results of BRIGHTER study. Ophthalmology. 2017;124:1778–1787. | ||
Kondo M, Kondo N, Ito Y, et al. Intravitreal injection of bevacizumab for macular edema secondary to branch retinal vein occlusion: results after 12 months and multiple regression analysis. Retina. 2009;29:1242–1248. | ||
Rehak J, Dusek I, Chrapek O, et al. Initial visual acuity is an important prognostic factor in patients with branch retinal vein occlusion. Ophthalmic Res. 2011;45:204–209. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.