Back to Journals » Cancer Management and Research » Volume 12
Siva 1 Inhibits Cervical Cancer Progression and Its Clinical Prognosis Significance
Authors Liu T , Ma Y, Wang Z, Zhang W, Yang X
Received 30 September 2019
Accepted for publication 5 December 2019
Published 15 January 2020 Volume 2020:12 Pages 303—311
DOI https://doi.org/10.2147/CMAR.S232994
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Rudolph Navari
Ting Liu, 1 Yifei Ma, 2 Zhiling Wang, 1 Wenjing Zhang, 1 Xingsheng Yang 1
1Department of Gynecology, Qilu Hospital of Shandong University, Jinan, Shandong 250012, People’s Republic of China; 2Department of Obstetrics and Gynecology, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong 250013, People’s Republic of China
Correspondence: Xingsheng Yang
Department of Gynecology, Qilu Hospital of Shandong University, 107 Wenhua Xi Road, Jinan, Shandong 250012, People’s Republic of China
Email [email protected]
Background: Cervical cancer is the second most common female malignancies. But the exact etiology of cervical cancer is still under investigation. Recent observations revealed that the loss expression of Siva 1 was related to several different types of tumors. It could play an indispensable role in both exogenous and endogenous apoptotic signaling pathways. Nevertheless, the relationship between Siva 1 expression and cervical cancer progression has not yet been fully clarified. This study aimed to explore the functional role of Siva1 in cervical cancer.
Materials and Methods: In this present experiment, expression of Siva 1 was detected in 87 cervical cancer, 34 CIN and 20 normal samples by immunohistochemistry. The correlation of Siva 1 expression and overall survival times (OS) was analyzed by Kaplan–Meier analysis. We up-regulated the expression of Siva 1 by plasmid pCMV3-Siva 1 in C33A cells. CCK8, flow cytometry, wound-healing, and transwell assays were performed to examine the influences of Siva 1 expression on cell proliferation, apoptosis, migration and invasion.
Results: The expression of Siva 1 was decreased in cervical cancer tissues compared with CIN and normal tissues. In addition, the Siva 1 immunoreactivity was significantly associated with tumor differentiation. Patients with Siva 1 negative staining exhibited a significantly decreased overall survival. Then, we established stable Siva 1 ectopic expression cells, and we found that elevated expression of Siva 1 promoted apoptosis, inhibited proliferation, and suppressed migration and invasion of cervical cancer cells.
Conclusion: The present study revealed a crucial role of Siva 1 in tumor progression and it may be a valuable prognostic indicator of cervical cancer.
Keywords: cervical cancer, Siva 1, prognosis, proliferation, apoptosis
Introduction
Cervical cancer is the second most common female malignancies, especially in developing countries. There are about 570,000 new cases worldwide each year, accounting for 5% of all new cancer cases,1 and more than 311,000 patients died of cervical cancer. China is the largest developing country, with nearly 98,900 incident cases and 30,500 mortalities reported in 2018.2 However, the incidence and mortality are increasing year by year. Current research indicates that persistent high-risk HPV infection is a necessary cause of cervical cancer. However, the exact etiology of cervical cancer is still under investigation.
Despite the improvements in surgical skills and radiotherapy method, the prognosis is still poor in patients with advanced stage. Unfortunately, the five-year survival rate of FIGO stage III patients is 40–43%, and stage IV is 15–20%. Therefore, it is critical to explore pathogenesis and identify efficient molecular biomarkers to ameliorate the diagnosis and treatment of cervical cancer.
Siva 1 was originally identified as a proapoptotic protein that bound to the cytoplasmic tail of CD27.3 The structure of Siva 1 protein contains an amphipathic helical region (SAH) at the amine-terminus, a death domain homology region in the internal sequences, a box-B-like ring finger and a zinc ring finger-like domain in the carboxyl terminus.4,5 Lots of convincing evidence suggest that Siva 1 induced apoptosis in several different malignant tumors. It can participate in both exogenous and endogenous apoptotic signaling pathways. Siva 1 was found to be a transcriptional target of two transcription factors, p53 and E2F1, and it plays an indispensable role in 53-dependent apoptosis and DNA repair.6,7 It could interact with some TNFR receptor family members, including CD27,1 GITR (glucocorticoid-induced), OX40 (CD134), 4-1BB (CD137) and CD40.8 Siva 1 was demonstrated to induce T lymphocyte apoptosis via a caspase-dependent mitochondrial pathway,9 it could also negatively regulate NF-κB signaling pathway through interaction with FOXP3 in T-cells. Because of the unique SAH region, Siva 1 binds to Bcl-xL and inhibits its antiapoptotic potency to protect breast cancer cells from the ultraviolet radiation.10 Whatsmore, His-Siva 1 recombinant protein was found to inhibit migration and invasion of HCT116 cells.11 And our previous study found that Siva 1 plays a role in restricting EMT and inducing apoptosis by phosphorylation of stathmin and polymerization of α-tubulin.12
Despite these studies, the expression of Siva 1 in cervical cancer and its biological function are still unclear. In this study, we examined the expression of Siva 1 in cervical cancer tissues and evaluate the prognostic value of it. Moreover, we explored the association of Siva 1 expression with several clinicopathological parameters. Some functional experiments were performed to explore the underlying molecular mechanism by up-regulation of Siva 1 in C33A cells. In addition, our study indicated that Siva 1 played a significant role in tumor progression and it might be a prospective therapeutic target and prognostic marker for cervical cancer.
Materials and Methods Tissue Collection
In this study, 87 cervical squamous cancer samples, 34 cervical intraepithelial neoplasia (CIN) tissues and 20 normal cervical tissues were collected from the Qilu Hospital of Shandong University between April 2005 to October 2007. The clinical stages were classified according to the FIGO staging criteria[12]. All of the enrolled participants had not received radiotherapy or chemotherapy before surgery. The pathological diagnosis of specimens was confirmed by three pathologists, respectively. All patients have provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki. The present study obtained the permission by Ethics Committee of Qilu Hospital and the approval number is KYLL-2018–372.
Immunohistochemistry Staining
Immunohistochemical staining for Siva 1 was conducted according to the PV-9000 kit protocol. Rabbit anti-human anti-Siva 1 polyclonal antibody (sc-48767, Santa Cruz Biotechnology) was diluted 1:200. The specificity of the antibody was determined with matched IgG isotype antibody as a negative control. Protein expression of all slides was evaluated by three pathologists, respectively. Each sample should be evaluated including the staining intensity and staining area. Immunohistochemical staining of Siva 1 was scored in accordance with the intensity (0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining) and the percentage of positive cells (0, no stained; 1, <10% tissue stained positive; 2, 10–50% stained positive; 3, >50% stained positive; 4, >75% stained positive). The expression result was scored based on the intensity score×percentage staining. If the data ≥2, it is regarded as positive (+).13
Cell Culture and Establishment of Stable Transfected Cell Line
The human cervical cancer cells, C33A cells, Hela cells and Siha cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Hyclone; GE Healthcare; Logan, UT, USA) at 37°C in 5% CO2. Siva 1 overexpression plasmid pCMV3-Siva 1 was designed by China National Pharmaceutical Group Co., Ltd. C33A cells transfected with plasmids using lipofectamine reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Two hundred micrograms per milliliter G418 (Invitrogen; Thermo Fisher Scientific, Inc.) was contained in the medium during the whole process of cell culture. The transfection efficiency was determined by both Western blot and RT-qPCR. Then, the stable over-expressing Siva 1 C33A cells were established for subsequent experiments.
RNA Isolation and Real-Time Polymerase Chain Reaction (RT-PCR)
The RNA of C33A cells was extracted by TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and then converted to cDNA using the miScript Reverse Transcription kit (Qiagen GmbH). Quantitative real-time PCR was performed by SYBR ExScript RT-PCR kit (Takara Biotechnology, Co., Ltd., Dalian, China) according to the manufacturer’s protocol. β-Actin was selected as the reference gene to normalize the mRNA expression. The primers used in this study were as follows: Siva 1-F: CCAAGCGACTCCTGTTCCTC; Siva 1-R: CCAATCAGCATCTGCCCAC. β-Actin- F: CTTAGTTGCGTTACACCCTTTCTTG; β-actin- R: TGTCACCTTCACCGTTCCAGTTT.
Western Blot Analysis
Cells were lysed and the proteins were separated in 10% SDS-PAGE, then transferred to a polyvinylidene difluoride membrane. After blocked with 5% non-fat milk the membranes were incubated with the following primary antibodies at 4°C overnight. Anti- Siva 1 antibody (sc-48767, Santa Cruz Biotechnology), anti- cleaved caspase-3 polyclonal antibody (ab2302, Abcam, Cambridge, UK), anti-Bax antibody (BA0315, Boster, Hubei, Wuhan, China), anti-Bcl-2 polyclonal antibody (BA0412, Boster, Hubei, Wuhan, China) were used. After washing with TBST 4 times for 5 min, the membranes were incubated with anti-rabbit or anti-mouse or anti-goat HRP-conjugated secondary antibody. The protein bands were visualized with a ECL reagent (Wanleibio Co., Ltd.) by the ImageQuant LAS 4000 system (GE Healthcare Life Sciences, Logan, UT, USA). β-Actin (A5316, Sigma) was used for equal loading.
CCK-8 Assay
The cell proliferation was performed by a Cell Counting Kit-8 (Sigma-Aldrich Co., St Louis, MO, USA) assay. In detail, 2x103 stable transfected cells and control cells were seeded into 96-well plates. At specific time points (0, 12, 24, 48, 72 h), 10 ul of CCK-8 working solution was added into each well and incubated at 37°C for 90 mins. The absorbance at 450 nm was detected using a microplate reader (Bio-Rad Laboratories, Inc.). The experiment was repeated three times.
Flow Cytometry Analysis of Cell Apoptosis
Stable transfected cells (5x105/well) and control cells were seeded in 6-well cell culture plates and collected with 0.5% trypsin. Then, the cells washed twice with cold PBS and resuspended in 400 ul binding buffer, and stained with Annexin V-FITC and PI in the dark according to the manufacturer’s protocol (Wanleibio Co., Ltd.). Analysis was performed using flow cytometry (BD, San Jose, CA, USA). This experiment was performed three times.
Transwell Migration Assays
For cell invasion assay, Corning Matrigel Invasion Chamber in 24-well plate (Corning Life Sciences, Lowell, MA, USA) was used. Briefly, 20 ul matrigel (BD) was added into the polycarbonate surface of each chamber to create an artificial basement membrane. Stable transfected cells and control cells were plated in the upper chamber in medium without serum. Eight hundred microliters DMEM media with 10% FBS was added to the lower chamber. After 24 hrs incubation, the cells of the chamber bottom were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet solution. The numbers of cells were calculated and images were captured under a microscope from five randomly fields in each well. Each transwell assay was carried out in biological triplicates.
In vitro Wound-Healing Assay
Transfected cells and control cells were seeded in a 6-well plate to reach confluence. The wound was scratched using a sterile pipette tip. Images were taken at a specific time after wounding with an inverted phase-contrast microscope (Motic, Xiamen, Fujian, China) at magnification x100. The cell migration rate was calculated as relative scratch width/the original scratched distance.
Statistical Analysis
All the data were analyzed using SPSS software version 22.0 (SPSS Inc., Chicago, IL). Differences between groups were analyzed by the Student’s t-test and one-way ANOVA. χ2 test was used to evaluate the correlation of Siva 1 expression with clinicopathological parameters. The Kaplan–Meier survival analysis was used to analyze the survival results. P < 0.05 was considered statistically significant.
Results
Downregulation of Siva 1 in Cervical Cancer
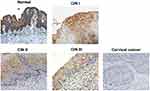
Former studies revealed that Siva 1 might have different roles in some cancers. However, the biological function of Siva 1 expression in cervical cancer was still unknown. Thus, immunohistochemistry (IHC) was carried out in 20 normal cervical tissues, 34 CIN tissues and 87 cervical cancer tissues. We found that the staining of Siva 1 was mainly located in the cell cytoplasm and nuclei (Figure 1). And Siva 1 expression was obviously down-regulated in cervical cancer tissues compared with normal and CIN tissues (P < 0.001). The percentages of Siva 1 positive expression in normal tissues, CIN and cervical cancer tissues were 90% (18/20) 61.7% (21/34) and 29.9% (26/87), respectively (P < 0.01) (Table 1). Moreover, the expression of Siva 1 was gradually decreased from CIN I to CIN III (P = 0.013). These results identified that the loss of Siva 1 expression may play a role in the progression of cervical cancer.
 |
Table 1 Detection of Siva 1 in Normal Cervix, CIN and Cervical Cancer Tissues |
Correlation Between Siva 1 Expression and Clinical Pathological Features
The association between Siva 1 expression and patients’ clinical parameters is shown in Table 2. Statistical analysis demonstrates that Siva 1 immunoreactivity was markedly associated with tumor differentiation (P=0.0204). However, we did not find statistical correlations between the expression level of Siva 1 with age (P=0.7183), tumor size (P=0.7844), FIGO stage (P=0.5361), lymph node metastasis (P=0.3414) and stromal invasion (P=0.9465).
 |
Table 2 Correlation of Siva 1 Expression with Clinicopathological Parameters of Cervical Cancer |
The Prognostic Significance of Siva 1 in Cervical Cancer
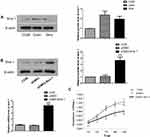
To further evaluate the relationship between Siva 1 expression and prognosis of cervical cancer, we used Kaplan–Meier curves and log-rank test to analyze the survival data based on follow-up. Decreased overall survival (OS) time was observed in cervical cancer patients with Siva 1-negative expression compared with those with Siva 1-positive expression (P=0.0336, Figure 2).
 |
Figure 2 Kaplan–Meier curves based on Siva 1 expression. |
Siva 1 Inhibit the Proliferation of Cervical Cancer Cells
C33A, Siha and CaSki cells were used to investigate the protein expression level of Siva 1 and potential biological function. We found that Siva 1 was expressed at a low level in C33A cells compared with other cells (Figure 3A). Then, C33A cells were transfected with the plasmid pCMV3-Siva 1 to generate stable overexpression transfected cells. The efficiency of over-expression of Siva 1 in pCMV3-Siva 1 was confirmed by Western blot and qRT-PCR, and it was about 3.68- fold compared with vector cells (Figure 3B). CCK-8 assay was performed to determine the influences of Siva 1 on cervical cancer cell viability. Figure 3C showed that up-regulation of Siva 1 significantly decreased the proliferation of C33A cells. The cell viability was decreased by 22.7% (P=0.006), 28.7% (P=0.001), 35.9% (P<0.0001), 35.9% (P<0.0001) and 34.4% (P=0.001) in 24, 48, 72, 96 and 120 h, respectively.
Siva 1 Overexpression Promotes Apoptosis of Cervical Cancer Cells
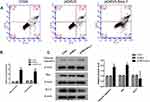
Cell apoptosis plays a significant role in tumor development, flow cytometry was used to explore the apoptotic effect of Siva 1 expression on cervical cancer. The results indicated that up-regulation of Siva 1 significantly promotes cell apoptosis, the rate of total apoptosis was 32.8% and 4.1% for pCMV3-Siva 1 transfected cells and vector cells, respectively (p< 0.01; Figure 4A). Figure 4B shows that there was a distinct increase in the number of cells in both early and late apoptosis (p<0.01).
The Effect of Siva 1 Expression on Cell Apoptosis-Related Proteins
To uncover the underlying molecular mechanisms by which Siva 1 promotes cell apoptosis of cervical cancer. The apoptosis-related proteins including cleaved caspase-3, Bax and Bcl-2 were detected by Western blot. We found that the overexpression of Siva 1 significantly increased the expression of cleaved caspase-3 by 1.61-fold, increased the expression of Bax by 1.95-fold and decreased the expression level Bcl-2 by 41% (Figure 4C).
Effects of Siva 1 Overexpression on Cell Migration and Invasion
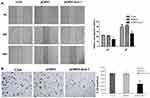
Cell invasion and migration is a vital process during tumor metastasis. We explored the migration and invasion ability by wound healing and transwell invasion assay after Siva 1 overexpression. The scratched cells were photographed at 24 h and 48h. The results showed that the capability of wound-healing was obviously decreased in Siva 1-overexpressed C33A cells (p<0.05; Figure 5A). For invasion assay, cells invaded into the downside of the chamber in the pCMV3-Siva 1 cells were significantly less than that of the control cells (p<0.01; Figure 5B).
Discussion
Cervical cancer affects the health and safety of more than 500,000 people worldwide each year. It is also the fourth leading cause of female-related death. Accurate diagnosis and effective treatment are essential for cervical cancer. Several former studies showed that Siva 1 played a potential apoptotic role in various receptor and nonreceptor mediated cell death pathways.14–19 In our previous study, Siva 1 was reported to be involved in the proliferation, apoptosis, migration and invasiveness of ovarian cancer. However, little is known about the exact mechanisms of the altered expression of Siva 1 during cervical cancer progression.
In the present study, we analyzed the expression of Siva 1 by immunohistochemistry in 87 cervical cancer samples, 34 CIN samples and 20 normal samples. The results indicated that Siva 1 expression was down-regulated in cervical cancer compared with normal tissues and CIN tissues. Notably, the loss expression of Siva 1 indicated its important role in cervical tumor progression. The relationships between Siva 1 and clinical variables were analyzed, and we found that Siva 1 was significantly associated with tumor differentiation (Table 2). The survival analysis demonstrated that the loss expression of Siva 1 significantly predicted worse clinical outcome in cervical cancer patients, which suggested Siva 1 might be a credible prognostic predictor for cervical cancer. According to the presented data, we assumed that the loss of Siva 1 might play a vital role in tumorigenesis and progression in cervical cancer.
To further investigate the biological function of Siva 1 in cervical cancer, we established stable over-expressed Siva 1 C33A cells. The CCK-8 and flow cytometry analysis demonstrated that up-regulation of Siva 1 could dramatically inhibit cell proliferation and induced cell apoptosis, the results were consistent with the study by Machado-Neto in leukemia Cells.20 A recent study showed that the unique 20-amino-acid amphipathic helical region (SAH) could bind to BCL-2 or BCL-XL and abolish their antiapoptotic activity.
Furthermore, downregulation of Siva 1 in U937 cells leads to an elevated expression of Bcl-xl, and decreased expression of Bax by suppressing the phosphorylation of JNK.12,18 A study also found that Siva 1 suppressed the XIAP-TAK1-mediated NF-κB signaling pathway, but favored the JNK signaling and caspase-3-dependent apoptosis.21 In our research, we found that Siva 1 upregulated the expression of caspase-3 and Bax, downregulated the expression of Bcl-2. Combining the former research and our study, it is confirmed that Siva 1 inhibits cell proliferation by inducing cell apoptosis in cervical cancer.
Tumor metastasis is a complex and multi-step process, which is the leading cause of cancer treatment failure and high mortality. Previous research found that bacterially expressed Siva 1 performed anticancer activities: suppressed the invasion, migration and induced apoptosis of colon cancer cells.11 In addition, Siva 1 protein also found significantly inhibited the tumor metastasis of the nasopharyngeal carcinoma tumor-bearing mice.22 Based on the former study, we hypothesized that Siva 1 may play a role in the migration and invasion of cervical cancer. And our results were consistent with the former research, by wound healing and transwell assays, C33A cell migration and invasion ability were significantly decreased when Siva 1 was up-regulated. Given the above conclusions, these data improve the understanding of Siva 1 biological function in cervical cancer.
In summary, the results of our study indicated that Siva 1 was downregulated in cervical cancer, and the loss expression of Siva 1 related with the poor prognosis of cervical cancer patients. For molecular mechanism analysis, we found that Siva 1 may play a vital role in cell proliferation, apoptosis, migration and invasion. The present study revealed a crucial role for Siva 1 in tumor progression and it may be a valuable prognostic indicator of cervical cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Prasad KV, Ao Z, Yoon Y, et al. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci U S A. 1997;94(12):6346–6351. doi:10.1073/pnas.94.12.6346
4. Gravestein LA, Blom B, Nolten LA, et al. Cloning and expression of murine CD27: comparison with 4-1BB, another lymphocyte-specific member of the nerve growth factor receptor family. Eur J Immunol. 1993;23(4):943–950. doi:10.1002/eji.1830230427
5. Mei Y, Wu M. Multifaceted functions of Siva 1: more than an Indian god of destruction. Protein Cell. 2012;3(2):117–122. doi:10.1007/s13238-012-2018-5
6. Jacobs SB, Basak S, Murray JI, Pathak N, Attardi LD. Siva is an apoptosis-selective p53 target gene important for neuronal cell death. Cell Death Differ. 2007;14(7):1374–1385. doi:10.1038/sj.cdd.4402128
7. Fortin A, MacLaurin JG, Arbour N, et al. The proapoptotic gene Siva is a direct transcriptional target for the tumor suppressors p53 and E2F1. J Biol Chem. 2004;279(27):28706–28714. doi:10.1074/jbc.M400376200
8. Spinicelli S, Nocentini G, Ronchetti S, Krausz LT, Bianchini R, Riccardi C. GITR interacts with the pro-apoptotic protein Siva and induces apoptosis. Cell Death Differ. 2002;9(12):1382–1384. doi:10.1038/sj.cdd.4401140
9. Py B, Slomianny C, Auberger P, Petit PX, Benichou S. Siva 1 and an alternative splice form lacking the death domain, Siva-2, similarly induce apoptosis in T lymphocytes via a caspase-dependent mitochondrial pathway. J Immunol. 2004;172(7):4008–4017. doi:10.4049/jimmunol.172.7.4008
10. Gudi R, Barkinge J, Hawkins S, et al. Siva 1 negatively regulates NF-kappaB activity: effect on T-cell receptor-mediated activation-induced cell death (AICD). Oncogene. 2006;25(24):3458–3462.
11. Zhang YH, Yu LG, Zhu WZ, et al. Preliminary research on the expression, purification and function of the apoptotic fusion protein, sival. Asian Pac J Cancer Prev. 2014;15(20):8685–8688.
12. Ma Y, Liu T, Song X, et al. Siva 1 inhibits proliferation, migration and invasion by phosphorylating stathmin in ovarian cancer cells. Oncol Lett. 2017;14(2):1512–1518.
13. Tang J, Yang Z, Wang Z, et al. Foxp3 is correlated with VEGF-C expression and lymphangiogenesis in cervical cancer. World J Surg Oncol. 2017;15(1):173.
14. Henke A, Launhardt H, Klement K, Stelzner A, Zell R, Munder T. Apoptosis in coxsackievirus B3-caused diseases: interaction between the capsid protein VP2 and the proapoptotic protein Siva. J Virol. 2000;74(9):4284–4290.
15. Cao C, Ren X, Kharbanda S, Koleske AJ, Prasad KV, Kufe D. The ARG tyrosine kinase interacts with Siva 1 in the apoptotic response to oxidative stress. J Biol Chem. 2001;276(15):11465–11468.
16. Xue L, Chu F, Cheng Y, et al. Siva 1 binds to and inhibits BCL-X(L)-mediated protection against UV radiation-induced apoptosis. Proc Natl Acad Sci U S A. 2002;99(10):6925–6930. doi:10.1073/pnas.102182299
17. Seseke F, Thelen P, Ringert RH. Characterization of an animal model of spontaneous congenital unilateral obstructive uropathy by cDNA microarray analysis. Eur Urol. 2004;45(3):374–381.
18. Chu F, Borthakur A, Sun X, et al. The Siva 1 putative amphipathic helical region (SAH) is sufficient to bind to BCL-XL and sensitize cells to UV radiation induced apoptosis. Apoptosis. 2004;9(1):83–95.
19. Chu F, Barkinge J, Hawkins S, Gudi R, Salgia R, Kanteti PV. Expression of Siva 1 protein or its putative amphipathic helical region enhances cisplatin-induced apoptosis in breast cancer cells: effect of elevated levels of BCL-2. Cancer Res. 2005;65(12):5301–5309.
20. Machado-Neto JA, Lazarini M, Favaro P, et al. ANKHD1 silencing inhibits Stathmin 1 activity, cell proliferation and migration of leukemia cells. Biochim Biophys Acta. 2015;1853(3):583–593. doi:10.1016/j.bbamcr.2014.12.012
21. Resch U, Schichl YM, Winsauer G, Gudi R, Prasad K, de Martin R. Siva1 is a XIAP-interacting protein that balances NFkappaB and JNK signalling to promote apoptosis. J Cell Sci. 2009;122(Pt 15):2651–2661. doi:10.1242/jcs.049940
22. Chen GH, Xue QQ, Li J, Gao TL, Sun QS, Bai GP. Anticancer activity of recombinant Siva1 protein in human nasopharyngeal carcinoma cell line CNE-2. Cancer Biomark. 2015;15(6):833–841. doi:10.3233/CBM-150527
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.