Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Sitagliptin Increases Beta-Cell Function and Decreases Insulin Resistance in Newly Diagnosed Vietnamese Patients with Type 2 Diabetes Mellitus
Authors Le TD , Nguyen NTP, Nguyen ST , Tran HTT , Nguyen LTH, Duong HH , Nguyen HM, Do BN
Received 23 March 2020
Accepted for publication 29 May 2020
Published 19 June 2020 Volume 2020:13 Pages 2119—2127
DOI https://doi.org/10.2147/DMSO.S255071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Tuan Dinh Le,1,* Nga Thi Phi Nguyen,2,3,* Son Tien Nguyen,2,3 Hoa Thi Thanh Tran,4 Lan Thi Ho Nguyen,4 Hoang Huy Duong ,1 Ha Manh Nguyen ,4 Binh Nhu Do5,*
1Department of Internal Medicine, Thai Binh University of Medicine and Pharmacy, Thai Binh, Vietnam; 2Department of Endocrinology, Military Hospital 103, Ha Noi, Vietnam; 3Department of Rheumatology and Endocrinology, Vietnam Military Medical University, Ha Noi, Vietnam; 4The National Endocrinology Hospital, Ha Noi, Vietnam; 5Division of Military Science, Military Hospital 103, Ha Noi, Vietnam
*These authors contributed equally to this work
Correspondence: Nga Thi Phi Nguyen; Binh Nhu Do Email [email protected]; [email protected]
Introduction: To investigate effects of Sitagliptin on the enhancement of beta-cell function, reducing insulin resistance, serum glucagon like peptide-1 (GLP-1) concentrations and blood glucose in patients with type 2 diabetes mellitus (T2D) and suggest one of the underlying mechanisms on beta-cell function and insulin resistance.
Patients and Methods: This was a cross-sectional and observational study in comparison to the control group. A study population of 44 newly diagnosed patients with T2D treated with Sitagliptin with a dose of 100 mg/day for 3 months was analyzed to compare 52 healthy participants. Indices for beta-cell function, peripheral insulin sensitivity, and insulin resistance were calculated with homeostasis model assessment 2 (HOMA2) calculator and compared. Serum GLP-1 concentrations were analyzed, and regression analysis was conducted to find the correlations between GLP-1 and beta-cell function and insulin resistance.
Results: Newly diagnosed patients with T2D witnessed a significant reduction in beta-cell function, serum GLP-1 concentrations at the time of diagnosis. After treatment with Sitagliptin 100 mg/day, they achieved significant improvements in beta-cell function, peripheral insulin sensitivity and insulin resistance. Serum GLP-1 concentrations were increased significantly to those levels in the control group and correlated with peripheral insulin sensitivity and insulin resistance in patients whose beta-cell functions improved.
Conclusion: Sitagliptin improved beta-cell function, insulin resistance and blood glucose in newly diagnosed patients with T2D. Meanwhile, Sitagliptin ameliorated serum GLP-1 concentrations, which contributed to the enhancement of beta-cell.
Keywords: Sitagliptin, serum GLP-1 concentrations, beta-cell function, insulin resistance, newly diagnosed patients with type 2 diabetes mellitus
Introduction
Diabetes which is a chronic metabolic disorder has recently sharply increased on a global scale. According to the International Diabetes Federation (IDF), there were 415 million patients diagnosed with T2D.1 Diabetes among Asian populations has some distinguishing characteristics from other races in the world, namely the early decrease in beta-cell function resulting in high postprandial blood glucose and the development to chronic diabetic complications occurs at an early stage of the disease.2 Hence, a therapeutic agent which increases beta-cell function plays an important role in antihyperglycemic protocols. Nowadays, anti-DPP4 antihyperglycemic agents have been widely used for patients with T2D under guidelines of diabetes associations and proved to be effective in the enhancement of beta-cell function via ameliorating serum incretin hormone concentrations (two major incretins, GLP-1 and glucose-dependent insulinotropic polypeptide [GIP]) - an anti-beta-cell apoptosis agent.3–5 There have been two incretin-related therapies for patients with T2D, namely glucagon-like peptide-1 agonists and dipeptidyl peptidase-IV inhibitors. The former was markedly known as Exedin-4 and the latter was well-known with Sitagliptin.6,7 In 2009, the American Association of Clinical Endocrinologists (AACE/ACE) issued the guideline for antihyperglycemic treatment protocol which mentioned about the usage of incretin therapies as the first-line drug for newly diagnosed patients with T2D (i.e, incretin therapies could be monotherapy or in combination with other antidiabetic drugs such as biguanide, sulfonylurea, or insulin).8 These days, incretin therapies regarding treatment for patients with T2D have been developed on a global scale and shown positive effects on not only glycemic control but prevention from chronic diabetic complications as well.9
Whilst anti-DPP4 agents have many effects on antihyperglycemic conditions, there have been little researches on the Asian population to investigate the role of these drugs on beta-cell function, peripheral insulin sensitivity, insulin resistance and serum GLP-1 concentrations in comparison to healthy subjects but results were controversial.
Regarding Vietnam, albeit Sitagliptin has been recruited as a clinical treatment therapy for patients with T2D for several years, there has been no research on the effect of Sitagliptin to beta-cell function as well as insulin resistance.
In the present study, we took advantages of those theories to investigate effects of Sitagliptin on beta-cell function, blood glucose, serum GLP-1 concentrations, and to further identify one potential mechanism in the enhancement of beta-cell function in Vietnamese population, which may contribute to the overall judgment of efficacy of DPP4-inhibitor agents.
Patients and Methods
Study Design and Patient Characteristics
This was a cross-sectional and observational, treatment-controlled study to compare the effects of 3-month treatment with Sitagliptin (with a dose of 100 mg, once daily) and control group (healthy subjects). There were 44 patients assigned to the Sitagliptin group (100 mg/day) and 52 healthy subjects assigned to the control group. Patients with T2D were outpatients of the Vietnam Endocrinology Hospital from 6/2014 to 10/2017. Patients with a mean age of 52.70 were newly diagnosed with T2D adhered to the ADA 2014 criteria10 and we only chose patients with both HbA1C from 6.5 to 7.5 % and FPG from 7.0 to 13.0 mmol/L. We excluded all patients whose FPG and HbA1C levels felt out of our selection ranges and conditions affecting results of our study, namely type 1 diabetes mellitus, secondary types of diabetes, significant complications of diabetes, and uses of other drugs affecting insulin concentrations. Drug choice was based on the guidelines of the American association of clinical endocrinologists and American College of Endocrinology (AACE/ACE 2009).8 In our study, patients with T2D had low HbA1C concentrations so we could select Sitagliptin as the first choice for treatment therapy in adjunct to lifestyle modification and exercises. The primary endpoint was the change from baseline in GLP-1, HOMA2-B, HOMA2-IR, HOMA2-S after 3 months of treatment with Sitagliptin. Other variables of interest consisted of FPG, lipid profile, safety laboratory measurements (urea, creatinine, ALT and AST) after 3 months of treatment with Sitagliptin. No adverse events occurred during the study duration.
Ethical Statements
All participants were dispensed with written informed consents, and the protocol was approved by the Ethical Review Committee of Military Medical University, Vietnam (No. 57/2014/VMMU-IRB). The study was also conducted using good clinical practice following the Declaration of Helsinki.
Blood Glucose, Beta-Cell Function and Insulin Resistance
Fasting blood glucose levels of all participations were collected on the first day of trial and every month afterwards. For beta-cell function, fasting plasma glucose, fasting insulin and C-peptide were collected on the first day of trial and after 3 months. Serum insulin and C-peptide were measured by electrode chemiluminescence (COBAS E411, USA). HbA1c was measured in percentage by high-performance liquid chromatography method (Adams A1C, Japan). FPG was measured by hexokinase methods (Beckman AU 680, USA). We used the HOMA-2 calculator which was available online at https://www.dtu.ox.ac.uk/homacalculator/ for measurement of beta-cell function, peripheral insulin sensitivity and insulin resistance by fasting plasma glucose and fasting C-peptide.
Serum GLP-1 Concentrations
Fasting blood samples were aspirated on the first day of trial and after 3 months for patients with T2D but only on the first day of trial for the control group. On the day of blood samples collection, patients were asked to be fasting and not to use Sitagliptin. At the time of clinical visiting, patients were fasted at least 8 hours and blood was collection for fasting serum GLP-1 analysis (fGLP1). All collecting tubes were prepared with DPP4-inhibitor before containing blood to prevent GLP-1 from degradation by DPP4 (DPP IV Inhibitor, K579, BioVision, USA). These blood samples were centrifuged at the velocity of 3000rpm for 15 minutes. Sera were then analysed with commercially available kits (ELISA-GLP-1 kits, Sigma, Japan) according to the manufacturer’s protocol by ELISA apparatus (ThermoFisher). Changes in color were checked at a wavelength of 450nm. The intra-assay and inter-assay coefficients of variation were less than 10% in enzyme immunoassays.
Data Analysis and Statistics
The size of the sample was calculated by the formula: 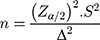 (n is the total of patients with newly diagnosed T2D, Zα/2 = 1.96 (with α = 0.05)) and S was the standard deviation of serum GLP-1 (3.9 pmol/L). Δ was the expected standard deviation for serum GLP-1 before and after treatment, in our study, it was 1.2pmol/L. Normally distributed data were expressed as mean±SD. Differences between groups were examined with Student’s t-test or Mann Whitney’s test. Correlations between serum GLP-1 levels and other variables were evaluated with Pearson’s and Spearman correlation analysis for normal distribution and skewed distribution variables, respectively. Two-tailed P values < 0.05 were defined as statistically significant. To test the difference between before and after treatments with Sitagliptin, we used Cohen’s d test, if d was greater than or equal to 0.2, it was assigned as a significant difference.
(n is the total of patients with newly diagnosed T2D, Zα/2 = 1.96 (with α = 0.05)) and S was the standard deviation of serum GLP-1 (3.9 pmol/L). Δ was the expected standard deviation for serum GLP-1 before and after treatment, in our study, it was 1.2pmol/L. Normally distributed data were expressed as mean±SD. Differences between groups were examined with Student’s t-test or Mann Whitney’s test. Correlations between serum GLP-1 levels and other variables were evaluated with Pearson’s and Spearman correlation analysis for normal distribution and skewed distribution variables, respectively. Two-tailed P values < 0.05 were defined as statistically significant. To test the difference between before and after treatments with Sitagliptin, we used Cohen’s d test, if d was greater than or equal to 0.2, it was assigned as a significant difference.
Results
Study Population Characteristics
44 patients with newly diagnosed T2D were treated with Sitagliptin (dose of 100mg/day) for the duration of 3 months and 52 healthy participants. The two groups have the same characteristics but there were a significant decrease of serum GLP-1 concentrations, and significant increases of FPG, HbA1C, C-peptide, insulin in patients with T2D in comparison to those in healthy participants (Table 1).
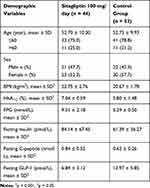 |
Table 1 Baseline Demographic Background Characteristics of Study Population |
Glycemic Controls, Insulin Resistance, Peripheral Insulin Sensitivity, and Beta-Cell Function
When we measured insulin resistance and beta-cell function indices with HOMA2 calculators, T2D group showed significantly worse peripheral insulin sensitivity, insulin resistance and beta-cell function than the control group (60.03 vs 84.91, p < 0.01; 2.36 vs 1.39, p < 0.001; 53.77 vs 105.85, p < 0.01; respectively), especially the decrease of beta-cell function (Table 2).
 |
Table 2 Indices of Beta-Cell Function, Insulin Resistance and Insulin Sensitivity Before Treatment |
Treatment with Sitagliptin alone in patients with T2D reduced HbA1C and FPG statistically in comparison to before treatment and there were no hypoglycemic events. Whilst there were increases in fasting insulin and C-peptide levels, the difference was not significant (p > 0.05) after treatment. BMI indices and weight of patients with T2D decreased significantly in comparison to those before treatment. Regarding lipid profile, whilst triglyceride changed slightly, cholesterol, LDL-C, and HDL-C concentrations witnessed significant differences.
After treatment of Sitagliptin with the stable dose of 100 mg/day for 3 months, changes in HOMA2-B and HOMA2-IR were found significantly in patients with T2D (with the different of 36.28 and - 0.55, respectively). Despite an insignificant change, there was an upward trend of HOMA2-S. Serum GLP-1 concentrations in patients with T2D treated with Sitagliptin increased significantly after 3 months and did not show a significant difference in comparison to those in the control group (12.83 ± 5.78 and 12.97 ± 5.85 pmol/L, respectively, p>0.05). Besides, patients with T2D showed no significant changes in both liver and renal functions via creatinine, ALT and AST after treatment with Sitagliptin (Table 3).
 |
Table 3 Changes in Paraclinical Parameters in Patients with T2D Treated with Sitagliptin 100 mg/Day |
After a 3-month duration of Sitagliptin usage, whereas both FPG and HbA1C decreased significantly with reductions of - 3.14 mmol/L and - 0.43 %, respectively; both serum insulin and C-peptide concentrations increased but differences were not significant. In our study, we chose the threshold for normal beta-cell function was the value of mean – 2SD in the control group, and as the result, we assigned abnormal beta-cell function if beta-cell function in patients with T2D was less than 43.37%. There were 4 patients after treatment with Sitagliptin witnessing a low beta-cell function but it was still improved in comparison to that at onset (Figure 1).
Independent Variables Correlated with Beta-Cell Function and Insulin Indices
Among patients with T2D treated with Sitagliptin 100mg/day, we found that only 40 patients appeared to have increased in beta-cell function. And hence, we made an insight on correlations of independent variables with beta-cell function and insulin indices for only this group to find out some potential factors contributed to this change.
When adjusted with other related factors (namely, weight, age, HbA1C, lipid profile), only HbA1C was found to be negatively correlated with HOMA2-S and positively correlated with HOMA2-IR whilst serum GLP-1 concentrations were found to be negatively and positively correlated with HOMA2-IR and HOMA2-S, respectively (Tables 4 and 5).
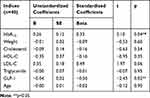 |
Table 4 Multivariate Regression of Health Profiles Associated with Insulin Resistance in Patients with an Increase in Beta-Cell Function 90 Days After Sitagliptin Treatment |
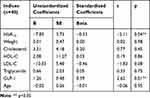 |
Table 5 Multivariate Regression of Health Profiles Associated with Insulin Sensitivity in Patients with an Increase in Beta-Cell Function 90 Days After Sitagliptin Treatment |
Discussion
In our present study, we compared changes in peripheral insulin sensitivity, insulin resistance and beta-cell function of Sitagliptin prescribed patients to healthy participants and measured the efficacy of Sitagliptin. We conducted this study comprising newly diagnosed patients with T2D and healthy subjects and a follow-up of 3 months. To minimize the effects of different demographic characteristics, we choose participants with similar demographic characteristics. Vietnamese patients with T2D had some characteristics, namely the age of diabetes establishing diagnose was at their 50s, normal-to-high BMI, high HbA1C, and high FPG levels, which were in line with previous studies among Asian populations.11–13
Changes in BMI and Lipid Profiles After 3 Months of Treatment with Sitagliptin
After 3 months of treatment, there were no adverse events among patients with T2D. Albeit their BMI and bodyweight changed significantly, Cohen’s d values were not greater than 0.2. Additionally, there were also changes in the lipid profile. Particularly, while triglyceride changed slightly, cholesterol, LDL-C, and HDL-C concentrations witnessed significant differences. Our results were in line with previous studies.14–17 Although the role of Sitagliptin on lipid metabolism has been scrutinized to decrease cholesterol, triglyceride and LDL-C,18,19 these effects could be overlapped by some factors, such as diets, lipid-lowering drug usages, and exercises. In our study, we could not distinguish the role of each mentioned factors contributing to the decrease of each feature in the lipid profile and body weight.
Beta-Cell Function Decreased Sharply and Early in Vietnamese Patients with T2D
Although insulin resistance remained the main pathology factor in type 2 diabetes mellitus, previous studies pointed out that there were some differences in characteristics of T2D among the Asian population. The degeneration of beta-cell function occurred earlier than that in Caucasian populations.2,11 In our study in Vietnamese patients, we also found that there was a significant decrease (of 44.48%) in patients with T2D, while only −20.35 and 0.79 for insulin sensitivity and insulin resistance in the same order. Though all patients were newly diagnosed and their mean age was 52.70 (year), they still suffered from a decrease of nearly 45% of beta-cell function.
Glycemic Controls
After the 3-month duration of treatment with Sitagliptin 100 mg/day, we found the significant amelioration in glycemic controls in patients with T2D. The reduction of HbA1C and FPG were significantly different in comparison to those before the initiation of treatment with −0.43 (95% CI, - 0.69 to - 0.17, p < 0.001) and - 3.14 (95% CI, - 4.13 to - 2.15, p < 0.001), respectively. Our results were consistent with previous studies.12,13,20-22 Despite the slight increase in insulin and C-peptide levels, these changes were not significant. We hypothesized that due to the direct effect of DPP4-inhibitor to increase the half-life of incretin, and as a result, the drug mainly affected serum GLP-1 levels instead of insulin from beta-cells. The increase in insulin was due to the net effect of GLP-1 depends on glucose levels.23 In particular, serum GLP-1 concentrations in patients with T2D treated with Sitagliptin increased significantly after 3 months and did not show a significant difference in comparison to those in the control group.
Treatment with Sitagliptin Increased Beta-Cell Function, Insulin Sensitive and Decreased Insulin Resistance
After 3 months of treatment with 100mg/day Sitagliptin, patients illustrated higher HOMA2-B, HOMA2-S and lower HOMA2-IR to those before interventions. Sitagliptin, one of the anti-DPP4 agents, has been consistently demonstrated to have effects on beta-cell and insulin concentrations via indirectly prolongs active incretins and exhibits L-cells to secrete more GLP-1.24,25 Recently, this group of agents were approved to be a second-line therapy for patients with type 2 diabetes mellitus internationally but as recommended by AACE/ACE (2009), the anti-DPP4 agents may be used to start monotherapy for type 2 diabetes patients.8 One model-based analysis (a placebo-controlled clinical study) found that Sitagliptin improved beta-cell function relative to placebo in both fasting and postprandial states in patients with T2D.26 Albeit Sitagliptin has been shown numerous efficacies in antidiabetic therapy overall, these effects varied from different races. A meta-analysis showed that, among patients with T2D in Asia, Sitagliptin had increased insulin sensitivity and weight much higher in comparison to that in the Caucasian population and the between-group (Asia-Caucasian) different in HOMA2-B was - 4.97 (95% CI, - 9.86 to - 0.09, p < 0.05). One suggestion for these differences could be due to Asian anthropometric indices including low BMI and high blood glucose due to insulin resistance rather than insulin deficiency.11 DPP4 inhibitors might induce beta-cell regeneration, prevention from pancreas islet hypertrophy and insulin synthesis in vitro studies.27,28 Clinically speaking, DPP4-inhibitors also improved beta-cell function both inside and outside the setting of food consumption, but some studies found there was no change in the incretin effect.29 Moreover, DPP-4 inhibitors would allow beta-cells to adapt to the degree of insulin resistance and have a better response to glucose overload and as the result, they decrease the overall insulin exposure and the proinsulin-to-insulin ratio.14,30,31 Some recent placebo-controlled studies denoted that Sitagliptin significantly improved HOMA-B, the proinsulin-to-insulin ratio in different races.9,13,21 Besides effects on beta-cell function, DPP4-inhibitors agents showed controversially contribution to insulin sensitivity. Some studies found that there was no different in HOMA-IR and QUICKI index among patients treated with Sitagliptin alone or in combination with other antihyperglycemic agents.17,32 Interestingly, on a long placebo-and active-controlled study, there was a significant improvement on disposition indices of insulin sensitivity from baseline at week 24 with all active treatments (with Sitagliptin alone or in combination with metformin) relative to placebo.20 Our results were in line with Goldstein et al and these results could suggest that Sitagliptin might make progress in insulin resistance at the early stage of diagnosed T2D.20 Albeit beta-cell function and insulin resistance based on HOMA2-B and HOMA2-IR, respectively, significantly improved, insulin and C-peptide concentrations witnessed upward trends but the difference was not significant (Table 3). Our results were in concordance with those of Mohan.12 There were some reasons for these changes. Several studies illustrated that Sitagliptin elevated serum GLP-1 concentrations, which affected beta-cell function (such as an increase in beta-cell differentiation and proliferation), and as a result, using Sitagliptin increased HOMA2-B index. Moreover, our results showed that there were good controls in both glycemic targets and lipid profile in patients with T2D (Table 3), which contributes to improving insulin sensitivity. To sum up, Sitagliptin helps to improve insulin sensitivity rather than increasing solely insulin concentrations and increases in insulin levels might take time more than only 3 months of treatment duration.
GLP-1 Might Contribute to the Amelioration of Insulin Sensitivity and Insulin Resistance in Patients with T2D Treated with Sitagliptin
GLP-1 is a potent insulin secretagogue that exhibits glucose-dependent insulin secretion. In vitro study, GLP-1 was found to be capable of healing beta-cell function which was reduced with age for some reasons: i) recruit beta-cells into a secretory mode; ii) activate the gene for glucose sensitivity of beta-cells; and, iii) reduction of beta-cell apoptosis.23 Treatment of old Wistar rats with GLP-1 led to the normal insulin secretion via increases of beta-cell mass and pancreas cell proliferation.28,33 And, we hypothesized that besides the hypoglycemic effect of anti-DPP4 agents, it may be the increase of GLP-1 that contributed to the increase of beta-cell functions.34 In our study, serum GLP-1 concentrations increased sharply after treatment and regression analysis confirmed that serum GLP-1 concentrations were independent variable making a great contribution to the amelioration of insulin sensitivity and insulin resistance. We found that there were improvements in beta-cell function but there were also 4 patients still had low beta-cell functions in comparison to those in the control group. These discordances might be due to the extreme low baseline levels of beta-cell function of these 4 patients. After the treatment, changes in beta-cell function were marginal and remained low. To neglect bias, we analyzed serum GLP-1 concentrations correlation with HOMA-IR and HOMA-S among patients with improvements in HOMA-B. Our data denoted that serum GLP-1 concentrations negatively and positively correlated to HOMA-IR and HOMA-S, respectively when adjusted for some related factors (age, weight, HbA1C, and lipid profile), which also contributed to the improvements of beta-cell function besides effects on glucose-independent insulin secretagogue.
There were some limitations to our study. Firstly, the size of our study population was relatively small and this was the cross-sectional study with a 3-month follow-up. Moreover, to measure beta-cell function and insulin indices, we solely employed HOMA2 calculator so we did not have continuous data to measure changes of insulin, glucose before and after patients consumptions. Finally, we just compared between diabetes mellitus group treated with Sitagliptin and control group so it is cannot scrutinize advantages of utilizing Sitagliptin in comparison to other oral antihyperglycemic drugs.
Conclusions
In summary, we showed that Sitagliptin with the dose of 100 mg enhanced beta-cell function and serum GLP-1 concentrations in newly diagnosed patients with T2D. It is the amelioration of serum GLP-1 concentrations and blood glucose that makes Sitagliptin a potential agent to decrease insulin resistance.
Abbreviations
GLP-1, glucagon like peptide-1; T2D, type 2 diabetes mellitus; DPP4, dipeptidyl peptidase-IV; BMI, body mass index; FPG, fasting plasma glucose; HOMA, homeostasis model assessment; QUICKI, quantitative insulin sensitivity index; ADA, The American Diabetes Association; SD, standard deviation; vs, versus.
Ethical Statements
All participants were dispensed with written informed consents, and the protocol was approved by the Ethical Review Committee of Military Medical University, Vietnam (No. 57/2014/VMMU-IRB). The study was also conducted using good clinical practice following the Declaration of Helsinki.
Acknowledgments
We thank all the staffs in Outpatients Department of the Vietnam Endocrinology Hospital and Department of Endocrinology and Rheumatology, Military Hospital 103 for collecting the samples and supporting the study.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Disclosure
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. IDF. IDF diabetes atlas 2015. Int Diabetes Fed. 2015.
2. Gujral UP, Pradeepa R, Weber MB, Narayan KMV, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann. N. Y. Acad. Sci. 2013;1281(1):51–63. doi:10.1111/j.1749-6632.2012.06838.x
3. Phillips LK, Prins JB. Update on incretin hormones.. Ann N Y Acad Sci. 2011;00:1–20.
4. Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520. doi:10.1172/JCI990
5. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33(2):187–215. doi:10.1210/er.2011-1052
6. Campbell IW, Day C. Sitagliptin - Enhancing incretin action. Br. J. Diabetes Vasc. Dis. 2007;7(3):134–139. doi:10.1177/14746514070070030601
7. Cernea S, Itamar RAZ. Therapy in the early stage: incretins. Diabetes Care. 2011;34(SUPPL):2. doi:10.2337/dc11-s223
8. Rodbard HW, Jellinger P, Davidson J, et al. Statement by an American association of clinical endocrinologists/American college of endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr. Pract. 2009;15(6):540–559. doi:10.4158/EP.15.6.540
9. Hanefeld M, Herman GA, Wu M, Mickel C, Sanchez M, Stein PP. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007;23(6):1329–1339. doi:10.1185/030079907X188152
10. American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37:S14.
11. Cai X, Han X, Luo Y, Ji L. Efficacy of dipeptidyl-peptidase-4 inhibitors and impact on β-cell function in Asian and Caucasian type 2 diabetes mellitus patients: A meta-analysis. J Diabetes. 2015;7(3):347–359. doi:10.1111/1753-0407.12196
12. Mohan V, Yang W, Son H-Y, et al. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res. Clin. Pract. 2009;83(1):106–116. doi:10.1016/j.diabres.2008.10.009
13. Nonaka K, Kakikawa T, Sato A, et al. Efficacy and safety of sitagliptin monotherapy in Japanese patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2008;79(2):291–298. doi:10.1016/j.diabres.2007.08.021
14. Sakaoto Y, Oyama J, Ikeda H, et al. Effects of sitagliptin beyond glycemic control: focus on quality of life. Cardiovasc Diabetol. 2013;12(35):1–9. doi:10.1186/1475-2840-12-1
15. Shigematsu E, Yamakawa T, Kadonosono K, et al. Effect of sitagliptin on lipid profile in patients with type 2 Diabetes Mellitus. J Clin Med Res. 2014;6(5):327–335. doi:10.14740/jocmr1889w
16. Husain M, Atif MA, Tunio AG, et al. Effect of Sitagliptin on glycemic control, bodyweight, blood pressure and serum lipid profile in type 2 diabetic hyperlipidemic patients. J Ayub Med Coll Abbottabad. 2016;28(2):369–372.
17. Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: A 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28(10):1556–1568. doi:10.1016/j.clinthera.2006.10.007
18. Steinberg H, Anderson MS, Musliner T, et al. Management of dyslipidemia and hyperglycemia with a fixed-dose combination of sitagliptin and simvastatin. Vasc Health Risk Manag. 2013;9:273–282. doi:10.2147/VHRM.S44330
19. Mells JE, Ananis FA. The role of gastrointestinal hormones in hepatic lipid metabolism. Semin Liver Dis. 2013;33(4):343–357. doi:10.1055/s-0033-1358527
20. Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30(8):1979–1987. doi:10.2337/dc07-0627
21. Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61(1):171–180. doi:10.1111/j.1742-1241.2006.01246.x
22. Scott R, Loeys T, Davies MJ, Engel SS. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes, Obes. Metab. 2008;10(10):959–969. doi:10.1111/j.1463-1326.2007.00839.x
23. Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470–512. doi:10.1124/pr.108.000604
24. Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension. 2010;56(4):728–733. doi:10.1161/HYPERTENSIONAHA.110.156554
25. Sangle GV, Lauffer LM, Grieco A, Trivedi S, Iakoubov R, Brubaker PL. Novel biological action of the dipeptidylpeptidase-IV inhibitor, sitagliptin, as a glucagon-like peptide-1 secretagogue. Endocrinology. 2012;153(2):564–573. doi:10.1210/en.2011-1732
26. Xu L, Man CD, Charbonnel B, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on beta-cell function in patients with type 2 diabetes: A model-based approach. Diabetes Obes Metab. 2008;10(12):1212–1220.
27. Conarello SL, Li Z, Ronan J, et al. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc. Natl. Acad. Sci. U. S. A. 2003;100(11):6825–6830. doi:10.1073/pnas.0631828100
28. Mu J, Woods J, Zhou Y-P, et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic β-cell mass and function in a rodent model of type 2 diabetes. Diabetes. 2006;55(6):1695–1704. doi:10.2337/db05-1602
29. Vardarli I, Arndt E, Deacon CF, Holst JJ, Nauck MA. Effects of sitagliptin and metformin treatment on incretin hormone and insulin secretory responses to oral and “isoglycemic” intravenous glucose. Diabetes. 2014;63(2):663–674. doi:10.2337/db13-0805
30. Brazg R, Xu L, Dalla Man C, Cobelli C, Thomas K, Stein P. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and β-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9(2):186–193. doi:10.1111/j.1463-1326.2006.00691.x
31. He YL, Wang Y, Bullock JM, et al. Pharmacodynamics of vildagliptin in patients with type 2 diabetes during OGTT. J. Clin. Pharmacol. 2007;47(5):633–641. doi:10.1177/0091270006299137
32. Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49(11):2564–2571. doi:10.1007/s00125-006-0416-z
33. Wang Y, Perfetti R, Greig NH, et al. Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J. Clin. Invest. 1997;99(12):2883–2889. doi:10.1172/JCI119482
34. Doyle ME, Egan JM. Glucagon-like peptide-1. Recent Prog Horm Res. 2001;56(1):377–399. doi:10.1210/rp.56.1.377
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

