Back to Journals » OncoTargets and Therapy » Volume 11
SIRT1 overexpression protects non-small cell lung cancer cells against osteopontin-induced epithelial-mesenchymal transition by suppressing NF-κB signaling
Authors Li X, Jiang Z, Li XM, Zhang X
Received 15 March 2017
Accepted for publication 5 May 2017
Published 2 March 2018 Volume 2018:11 Pages 1157—1171
DOI https://doi.org/10.2147/OTT.S137146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Xuejiao Li,1 Zhongxiu Jiang,2 Xiangmin Li,2 Xiaoye Zhang2
1The Second Clinical College, China Medical University, 2Fourth Department of Oncology, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province, People’s Republic of China
Abstract: Osteopontin (OPN) is a promoter for tumor progression. It has been reported to promote non-small cell lung cancer (NSCLC) progression via the activation of nuclear factor-κB (NF-κB) signaling. As the increased acetylation of NF-κB p65 is linked to NF-κB activation, the regulation of NF-κB p65 acetylation could be a potential treatment target for OPN-induced NSCLC progression. Sirtuin 1 (SIRT1) is a deacetylase, and the role of SIRT1 in tumor progression is still controversial. The effect and mechanism of SIRT1 on OPN-induced tumor progression remains unknown. The results presented in this research demonstrated that OPN inhibited SIRT1 expression and promoted NF-κB p65 acetylation in NSCLC cell lines (A549 and NCI-H358). In this article, overexpression of SIRT1 was induced by infection of SIRT1-overexpressing lentiviral vectors. The overexpression of SIRT1 protected NSCLC cells against OPN-induced NF-κB p65 acetylation and epithelial-mesenchymal transition (EMT), as indicated by the reduction of OPN-induced changes in the expression levels of EMT-related markers and cellular morphology. Furthermore, SIRT1 overexpression significantly attenuated OPN-induced cell proliferation, migration and invasion. Moreover, overexpression of SIRT1 inhibited OPN-induced NF-κB activation. As OPN induced NSCLC cell EMT through activation of NF-κB signaling, OPN-induced SIRT1 downregulation may play an important role in NSCLC cell EMT via NF-κB signaling. The results suggest that SIRT1 could be a tumor suppressor to attenuate OPN-induced NSCLC progression through the regulation of NF-κB signaling.
Keywords: OPN, SIRT1, EMT, NF-κB, NSCLC
Introduction
Lung cancer is one of the main reasons for cancer-related deaths worldwide.1 Tumor metastasis is considered as the primary cause of mortality. Non-small cell lung cancer (NSCLC) is the dominant form of lung cancer, accounting for nearly 85% of the cases.2 Study has indicated that more than 65% of patients show regional lymph node or distant site metastases when they were initially diagnosed with NSCLC.3 Therefore, it is necessary to explore the mechanisms regulating NSCLC metastasis for the development of potential new therapeutic targets. Epithelial-mesenchymal transition (EMT) is associated with multiple pathologies including lung cancer metastasis, during which epithelial cells acquire enhanced mobility and invasiveness by the loss of E-cadherin expression and the increase of mesenchymal marker (N-cadherin and Vimentin) expression.4,5 Further studies are needed to explore the molecular mechanism that regulates EMT, in order to find therapeutic target for the treatment of tumor invasion and metastasis.
Osteopontin (OPN) is an extracellular matrix protein that plays a key role in tumor progression through binding with avβ3-integrin and CD44 receptor.6 The overexpression of OPN has been shown to correlate with poor prognosis in NSCLC.7 It has been demonstrated that OPN promotes EMT of several types of cancer cells, including endometrial cancer, prostate cancer, breast cancer and liver cancer.8–11 However, the mechanism underlying OPN-induced EMT remains poorly understood. Nuclear factor-κB (NF-κB) is a nuclear transcription factor that stimulates the expression of transcription factors that drive the EMT process. It has been shown to be involved in OPN-induced tumor progression.12–14 It has been shown that the acetylation of RelA/p65, a subunit of NF-κB, can increase its specific transcriptional activity and the deacetylation will inhibit its transactivation.15,16 Therefore, it can be inferred that deacetylation of NF-κB p65 could be a potential target to suppress OPN-induced NSCLC cell EMT. However, the acetylation level of NF-κB p65 in OPN-induced EMT remains unclear.
Sirtuin 1 (SIRT1) is a nicotinamide adenine dinucleotide-dependent lysine deacetylase.17 The role of SIRT1 in tumor progression is still controversial. Initially SIRT1 was shown to suppress apoptosis by deacetylation of p53, a well-known tumor suppressor.18 However, SIRT1 is regarded as a tumor suppressor that inhibits tumor progression by targeting HIF-1a, TGF-β/Smad4 or NF-κB/cyclin D1 signaling pathway.19–21 In addition, resveratrol, the SIRT1 activator, has been shown to activate caspase-3 and reduce chemoresistance in breast tumor cells through the inhibition of NF-κB-specific transcriptional activation.22 However, little is known regarding to the role of SIRT1 as regulator of NF-κB activation during EMT process. SIRT1 inhibits NF-κB activation by deacetylation of the RelA/p65 subunit at lysine 310.16 Therefore, we examined the effect of OPN on SIRT1 expression and the role of SIRT1 in OPN-induced EMT process.
This study aimed to examine the mechanisms of OPN-induced EMT in NSCLC. It is found that OPN inhibits SIRT1 expression, and SIRT1 protects against OPN-induced EMT by inhibiting NF-κB signaling activation.
Materials and methods
Cell culture and reagents
NSCLC cell lines A549, NCI-H358 (H358), NCI-H1299 (H1299) and NCI-H460 (H460) and normal lung epithelium cell line BEAS-2B were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, People’s Republic of China) and were cultured in RPMI 1640 (HyClone, Logan, UT, USA) supplemented with a mixture of 10% fetal bovine serum (FBS) and antibiotics at 37°C in an incubator filled with 5% CO2. Recombinant human OPN protein (rhOPN) was purchased from R&D Systems (Minneapolis, MN, USA).
Enzyme linked immunosorbent assay
A549, NCI-H358, NCI-H1299, NCI-H460 and BEAS-2B cell lines were seeded in T25 culture bottle at a density of 3×106/bottle; cell culture supernatants were collected after cells were cultured for 24 hours. The secretory levels of OPN in these cells were detected using a human OPN enzyme-linked immune detection kit according to the manufacturer’s instructions (R&D Systems).
Cell infection
Lentiviral vectors overexpressing SIRT1 (LV-SIRT1) and their negative control (LV-NC) were constructed by GeneChem Co., Ltd (Shanghai, People’s Republic of China) and used to infect A549 and NCI-H358 cell lines. For infection, cells were plated in 6-well plates at a density of 1×105 cells/well. After 24 hours, the cells were infected with 5 μL of the lentiviral vector by using polybrene (8 μg/mL) according to the manufacturer’s instructions. At 96 hours after infection, the effect of the infection was evaluated by real-time quantitative polymerase chain reaction (qPCR) and Western blot analysis.
CCK-8 assay
Cell suspension (5×104/mL) was seeded in 96-well plate (100 μL per well). Cells were cultured overnight at 37°C in an incubator filled with 5% CO2. The cells infected with LV-SIRT1 and LV-NC were treated with different concentrations of OPN (0, 100, 200 and 400 ng/mL) for 24 hours or treated with 400 ng/mL OPN for various times (0, 12, 24 and 48 hours). The concentration of FBS in culture medium was reduced to 5%. To detect cell proliferation, cells were incubated with 10 μL Cell Counting Kit-8 (CCK-8) solution (Dojindo, Kumamoto, Japan) for 2 hours in an incubator at 37°C. The absorbance was measured at a wavelength of 450 nm.
Real-time PCR (quantitative PCR)
Total RNA was extracted from NSCLC cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed using the PrimeScript™ RT reagent Kit (Takara, Dalian, People’s Republic of China). Aliquots of the resulting cDNA were used for PCR amplification in Roche light cycler 480 (Roche Diagnostics GmbH, Basel, Switzerland) using the SYBR Green Supermix (Takara). The specific primer sequences used to determine the mRNA expressions were as follows: SIRT1 F: 5′-TAGCCTTGTCAGATAAGGAAGGA-3′, R: 5′-ACAGCTTCACAGTCAACTTTGT-3′; OPN F: 5′-CGAGGAGTTGAATGGTGCATAC-3′, R: 5′-TTTCAGCACTCTGGTCATCCA-3′; Vimentin F: 5′-ACCGCTTTGCCAACTACAT-3′, R: 5′-TTGTCCCGCTCCACCTC-3′; E-cadherin F: 5′-ACCATTAACAGGAACACAGGAG-3′, R: 5′-GGCATCAGCATCAGTCACTT-3′; N-cadherin F: 5′-CATCATCATCCTGCTTATCCTTGT-3′, R: 5′-TTCTCCTCCACCTTCTTCATCATA-3′; GAPDH F: 5′-GAGTCAACGGATTTGGTCGT-3′, R: 5′-GACAAGCTTCCCGTTCTCAG-3′. GAPDH was used as an internal control. The relative expressions of the genes were quantified using the 2−ΔΔCT method.
Western blot
Total protein was extracted from cells on ice using RIPA lysing buffer. The nuclear and cytoplasmic proteins were separated by nuclear and cytoplasmic protein extraction kit (Beyotime, Jiangsu, People’s Republic of China) according to the manufacturer’s instructions. The protein content was quantified by using the BCA protein assay kit (Beyotime). Samples containing 40 μg proteins were separated by SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA), which was blocked and incubated overnight at 4°C with specific primary antibodies against SIRT1 (Abcam, Cambridge, UK), acetyl NF-κB p65 Lys310 (Abcam), E-cadherin (Proteintech, Rosemont, IL, USA), N-cadherin (Proteintech), Vimentin (Proteintech), NF-κB p65 (Abcam), IKK-α (Cell Signaling Technology, Danvers, MA, USA), IκB-α (Cell Signaling Technology), Lamin B (Proteintech) and β-actin (Proteintech). The membrane was washed and incubated with HRP-conjugated secondary antibody (Proteintech) at room temperature for 90 minutes. After washing the membrane, signals were detected using an ECL kit (Thermo; Rockford, IL, USA).
Wound healing migration assay
The cells (infected with either LV-SIRT1 or LV-NC) were plated onto 6-well plates and then cultured to 90% confluence. Cell monolayers were carefully wounded by scratching with the 200 μL pipette tip. The cells were then washed twice with PBS to remove dislodged cells and cultured in serum-free RPMI 1640 culture medium with either control buffer (CB) or 400 ng/mL OPN for 48 hours. Wound closure rate = (wound width at 0 hour – wound width at 48 hours)/wound width at 0 hour ×100%.
Transwell invasion assay
Cells (infected with either LV-SIRT1 or LV-NC) were treated with either CB or 400 ng/mL OPN for 48 hours. The cells in serum-free medium were added to the upper chambers (8×104 cells/well) of a 24-well transwell chamber (8 μm, Corning Life Sciences, Corning, NY, USA) coated with 40 μL Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) diluted 1:8 in serum-free medium. The chambers were incubated for 48 hours with 600 μL complete culture medium, which contained 15% FBS added in the lower chamber. Non-invading cells were removed from the upper chamber with cotton swabs. Cells that had invaded through membrane on the lower surface of the membrane were fixed and stained with crystal violet (Beyotime). The membranes were captured with digital images, and the number of invading cells on the filters were counted in random fields.
Immunofluorescence
Cells (infected with either LV-SIRT1 or LV-NC) were cultured in 12-well plates containing coverslips, treated with CB or OPN (400 ng/mL) for 2 hours, and then washed with PBS. The cells were fixed in 4% paraformaldehyde for 30 minutes and permeabilized in 0.1% Triton X-100 solution. Cells were blocked with goat serum for 1 hour and incubated overnight at 4°C with primary antibody against NF-κB p65 (Abcam). Subsequently, cells were washed three times with PBS, followed by incubation with FITC-conjugated anti-rabbit antibody (Proteintech) at room temperature for 3 hours. The cell nuclei were stained with DAPI and the cells were observed by fluorescence microscopy.
Statistical analysis
All of the experiments were repeated at least three times for statistical analysis. All data would be presented as mean ± SD. Student’s t-test was used to compare data between two groups. P<0.05 was considered statistically significant.
Results
OPN is highly expressed by NSCLC cells and promotes NSCLC cells EMT
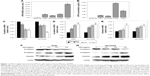
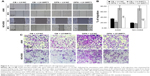
Real-time qPCR revealed that the mRNA expression levels of OPN in human NSCLC cell lines (A549, NCI-H358, NCI-H1299, NCI-H460) were higher than that in normal lung epithelium cell line BEAS-2B (Figure 1A). Consistent with this, enzyme linked immunosorbent assay revealed that the protein secretion levels of OPN in these NSCLC cell lines were higher than that in normal lung epithelium cell line (Figure 1B). In these cells, the protein secretion levels of OPN in A549 and NCI-H358 cells were lower than that in NCI-H1299 and NCI-H460 cells (Figure 1B). Therefore, A549 and NCI-H358 cells were treated with rhOPN for further research. Real-time qPCR and Western blot analyses revealed that incubation with OPN for 24 hours resulted in a decrease of epithelial marker E-cadherin expression and an increase of mesenchymal markers N-cadherin and Vimentin expression. The changes of EMT marker expression in cells treated with 400 ng/mL OPN were the most significant at both mRNA and protein levels, which suggests that OPN significantly promotes EMT of A549 and NCI-H358 cells (Figure 1C–G and Figure S1A–C).
OPN inhibits SIRT1 expression and promotes acetylation of NF-κB p65 subunit
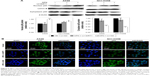
Firstly, the SIRT1 expression levels were examined in response to OPN. Real-time qPCR and Western blot analyses revealed that incubation with OPN (0, 100, 200 and 400 ng/mL) for 24 hours resulted in a decrease of SIRT1 expression in A549 and NCI-H358 cells (Figure 2A–D). As SIRT1 can inhibit the activation of NF-κB by deacetylation of the RelA/p65 subunit at lysine 310,16 the acetylation of RelA/p65 was evaluated after treatment with OPN for 24 hours. Figure 2B, C and E showed that OPN significantly increased the protein levels of acetyl NF-κB p65; this is consistent with the decrease of SIRT1 expression in A549 and NCI-H358 cells. At the same time, the protein expression levels of NF-κB p65 were not significantly changed by OPN treatment.
SIRT1 overexpression inhibits OPN-induced NSCLC cell EMT
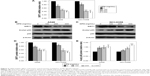
In order to examine the role of SIRT1 in OPN-induced EMT, NSCLC cells (A549 and NCI-H358) were infected with LV-SIRT1 or LV-NC. After 96 hours, infection efficiency was determined by real-time qPCR and Western blot analysis. SIRT1 expression was significantly increased in cells infected with LV-SIRT1 (Figure S2A–C). Cells (infected with either LV-SIRT1 or LV-NC) were stimulated with 400 ng/mL OPN. The cells infected with LV-NC followed by treatment with OPN for 72 hours exhibited mesenchymal fibroblast-like morphology. By contrast, SIRT1-overexpressing cells treated with OPN exhibited epithelial cobblestone-like appearance (Figure S2D). These morphological changes suggested that the overexpression of SIRT1 in NSCLC cells may reverse OPN-induced EMT. Furthermore, the expression changes of E-cadherin, N-cadherin and Vimentin in SIRT1-overexpressing cells followed by treatment with OPN (400 ng/mL) for 24 hours were examined. The results demonstrated that SIRT1 overexpression attenuated OPN-induced downregulation of E-cadherin and upregulation of N-cadherin and Vimentin at both mRNA and protein levels (Figure 3A–H). All these results indicated that OPN promotes NSCLC cell EMT, at least in part, by the inhibition of SIRT1 expression. At the same time, SIRT1 overexpression inhibited OPN-induced NF-κB p65 acetylation (Figure 3D, E and I). However, neither SIRT1 overexpression nor OPN treatment could influence NF-κB p65 protein expression levels (Figure 3D and E).
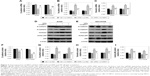
SIRT1 overexpression inhibits OPN-induced NSCLC cell proliferation
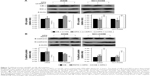
The effect of SIRT1 on OPN-induced NSCLC cell proliferation was assessed. A549 and NCI-H358 cells were infected with LV-SIRT1 or LV-NC followed by treatment with different concentrations of OPN (0, 100, 200, 400 ng/mL) for 24 hours. CCK-8 assays were, then, performed on A549 and NCI-H358 cells. As shown in Figure 4A and B, compared with untreated cells, OPN promoted A549 and NCI-H358 cell proliferation. However, in SIRT1 overexpression cells, OPN could not significantly promote cells proliferation. A549 and NCI-H358 cells infected with LV-SIRT1 or LV-NC were treated with CB or 400 ng/mL OPN for different times (0, 12, 24, 48 hours). CCK-8 assays showed that SIRT1 overexpression inhibited OPN-induced A549 and NCI-H358 cell proliferation at 12, 24 and 48 hours after OPN treatment (Figure 4C and D).
SIRT1 overexpression inhibits OPN-induced NSCLC cell migration and invasion
The roles of SIRT1 in OPN-induced NSCLC cell migration and invasion were explored using wound healing assay and transwell assay, respectively. Wound healing assay showed that OPN significantly increased the migration rate of the NSCLC cell lines A549 and NCI-H358 (Figure 5A and B). The overexpression of SIRT1 in A549 and NCI-H358 cells significantly inhibited OPN-induced cell migration (Figure 5A and B). Similarly, OPN promoted cell invasion, and overexpression of SIRT1 significantly depressed OPN-induced cell invasion (Figure 5C). This phenomenon indicated that SIRT1 is involved in inhibition of OPN-induced NSCLC cell migration and invasion.
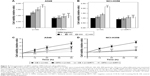
SIRT1 overexpression inhibits OPN-induced NF-κB signaling activation
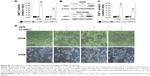
NF-κB is an important signaling pathway that mediates OPN-induced tumor progression. So, acetyl NF-κB p65 protein expression level was explored in cells treated with OPN, revealing that OPN promoted acetyl NF-κB p65 protein expression (Figure 2B, C and E). Furthermore, SIRT1 overexpression inhibited OPN-induced NF-κB p65 acetylation (Figure 3D, E and I). Therefore, the assumption, where OPN promotes NF-κB p65 subunit activation through inhibiting SIRT1 expression was explored. The role of SIRT1 in OPN-induced NF-κB activation was explored using Western blot and immunofluorescence assay in A549 and NCI-H358 cells. The analysis from the Western blot clearly showed that OPN increased the nuclear translocation of the NF-κB p65 subunit and hence OPN-induced NF-κB activation in comparison to untreated cells (Figure 6A). However, OPN-induced NF-κB p65 nuclear translocation was greatly inhibited by the overexpression of SIRT1 (Figure 6A). Similarly, immunofluorescence assay also showed that in both untreated cells and SIRT1-overexpressing cells treated with OPN, NF-κB p65 subunit was mainly located in the cytoplasm. However, in cells treated with OPN alone, NF-κB p65 was translocated to the nucleus (Figure 6B). Furthermore, the effect of OPN on the upstream molecules involved in NF-κB activation was examined. Treatment of A549 and NCI-H358 cells with OPN caused a degradation of IκB-α and an increase in the expression of IKK-α (Figure 7A). SIRT1 overexpression inhibited OPN-induced downregulation of IκB-α and upregulation of IKK-α (Figure 7A). These results indicated that OPN induces NF-κB signaling activation through the inhibition of SIRT1 expression. In order to examine the role of NF-κB signaling in OPN-induced NSCLC cell EMT, A549 and NCI-H358 cells were pretreated with NF-κB inhibitor (PDTC, 10 μM) for 30 minutes followed by treatment with OPN (400 ng/mL) for 24 hours. Western blot showed that PDTC significantly inhibited OPN-induced decrease of E-cadherin expression and increase of N-cadherin expression (Figure 7B). In conclusion, OPN promoted NSCLC cell EMT by activating NF-κB signaling, and SIRT1 overexpression inhibited OPN-induced EMT by inhibiting NF-κB signaling activation.
Discussion
Previous studies indicated that OPN expression varies significantly in non-carcinomatous and carcinomatous tissues and it is a potential biomarker in various types of cancer, including gastric cancer,23 liver cancer,24 bladder urothelial cancer,25 prostate cancer,26 breast cancer27 and NSCLC.28 OPN is involved in multiple tumor-associated biological processes, including the induction of tumor cell immune evasion, survival, invasion, metastasis and angiogenesis.29 The EMT process appears to be important for tumor cells to acquire multiple drug resistance, an increased migration and invasion ability, and cancer stem cells characteristics.5 In our study, NSCLC cell lines were shown to be with a higher OPN expression than normal lung epithelium cell line, which is consistent with previous studies.
In this study, we found that OPN-induced EMT in NSCLC cells may depend, at least partially, on the downregulation of SIRT1. SIRT1 overexpression attenuated OPN-induced NSCLC cell migration and invasion via inhibition of EMT. Previously, it has been shown that SIRT1 regulates various physiological processes by the inhibition of NF-κB signaling pathway as a deacetylase.22,30,31 Here, the results showed that treatment of A549 and NCI-H358 cells with OPN increased the expression of acetyl NF-κB p65, while SIRT1 overexpression attenuated OPN-induced NF-κB p65 acetylation. Furthermore, it is demonstrated that SIRT1 overexpression inhibited OPN-induced NF-κB p65 signaling activation. The results showed that SIRT1 may protect NSCLC cells against OPN-induced EMT by inhibiting NF-κB activity. It is well known that the EMT process plays an important role in the transition of lung cancer from early stage to invasive carcinoma.4 Taken together, these data suggested that SIRT1 might be a new therapeutic modality in OPN-mediated NSCLC progression.
It is worth noting that previous studies have indicated that SIRT1 exerted both stimulative32–34 and inhibitory effects20,35,36 on tumor cell EMT. In prostate cancer, SIRT1 has been reported to induce EMT by cooperating with EMT-related transcription factor ZEB1.32 Moreover, in colorectal cancer, Cheng et al33 found that SIRT1 promotes EMT and metastasis by regulating Fra-1 expression. Recently, non-steroidal anti-inflammatory drugs were found to inhibit TGF-β1-induced EMT via the downregulation of SIRT1.34 However, SIRT1 has been documented to suppress EMT. In lung cancer, hypoxia promoted EMT and cancer metastasis by the repression of SIRT1 expression in a SUMOylation-dependent manner.35 Consistent with this, in ovarian cancer, hypoxic stress induced EMT by the downregulation of SIRT1 transcription level.36 In addition, SIRT1 has been shown to reduce EMT in breast cancer by deacetylating Smad4 in the TGF-β pathway and repressing TGF-β signaling.20 Therefore, it is likely that the effect of SIRT1 on EMT depends on the tumor types, specific downstream targets and tumor microenvironment. However, the underlying mechanisms of these contradictory data in different cell types and culture conditions would require further investigation.
OPN, as a secreted tumor regulatory protein in tumor microenvironment derived from both tumor cells and tumor associated stromal cells, mediates cell–matrix interactions and plays an important role in EMT initiation.37 It is necessary to develop effective approaches to decrease OPN level or block the signaling cascades elicited by OPN. The SIRT1 activator, resveratrol, takes part in anti-nephrolithic activity mediated partially by the inhibition of OPN.38 However, the relationship between OPN and SIRT1 in tumor is still unclear. Recent studies have showed that SIRT1 expression was regulated by various cytokines, such as TGF-β, TNF-α and IL-1β.30,39,40 This is the first study to demonstrate that SIRT1 expression was decreased in NSCLC cells treated with OPN. Since NF-κB is a direct target of SIRT1,16 the effect of SIRT1 overexpression on NF-κB signaling pathway activated by OPN was explored. It was discovered that SIRT1 overexpression inhibits OPN-induced NF-κB activation. Previous studies have demonstrated that SIRT1 suppresses tumor cell proliferation and reduces chemoresistance by inhibiting NF-κB signaling.21,22 In our study, the results exhibited that SIRT1 overexpression could inhibit OPN-induced NSCLC cell EMT by the inhibition of NF-κB activation.
Conclusion
In conclusion, SIRT1 expression was inhibited and NF-κB acetylation was promoted by OPN treatment. Our study suggests that SIRT1 is a potential tumor suppressor that inhibits OPN-induced NF-κB activation by deacetylation of NF-κB p65 to protect NSCLC cells from OPN-induced EMT. Therefore, SIRT1 may be a novel therapeutic target for treating OPN-induced NSCLC progression.
Acknowledgment
This work was supported by the provincial Natural Science Foundation of Liaoning, People’s Republic of China (No 2014021032).
Disclosure
The authors report no conflicts of interest in this work.
References
Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382(9893):709–719. | ||
Langer CJ, Besse B, Gualberto A, Brambilla E, Soria JC. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(36):5311–5320. | ||
Morgensztern D, Ng SH, Gao F, Govindan R. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol. 2010;5(1):29–33. | ||
Soini Y. Tight junctions in lung cancer and lung metastasis: a review. Int J Clin Exp Pathol. 2012;5(2):126–136. | ||
De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. | ||
Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90(10):1877–1881. | ||
Jin Y, Tong DY, Chen JN, et al. Overexpression of osteopontin, αvβ3 and Pim-1 associated with prognostically important clinicopathologic variables in non-small cell lung cancer. PLoS One. 2012;7(10):e48575. | ||
Li Y, Xie Y, Cui D, et al. Osteopontin promotes invasion, migration and epithelial-mesenchymal transition of human endometrial carcinoma cell HEC-1A through AKT and ERK1/2 signaling. Cell Physiol Biochem. 2015;37(4):1503–1512. | ||
Nakamura KD, Tilli TM, Wanderley JL, et al. Osteopontin splice variants expression is involved on docetaxel resistance in PC3 prostate cancer cells. Tumour Biol. 2016;37(2):2655–2663. | ||
Li NY, Weber CE, Mi Z, Wai PY, Cuevas BD, Kuo PC. Osteopontin up-regulates critical epithelial-mesenchymal transition transcription factors to induce an aggressive breast cancer phenotype. J Am Coll Surg. 2013;217(1):17–26. | ||
Dong Q, Zhu X, Dai C, et al. Osteopontin promotes epithelial-mesenchymal transition of hepatocellular carcinoma through regulating vimentin. Oncotarget. 2016;7(11):12997–13012. | ||
Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26(5):711–724. | ||
Liu J, Liu Q, Wan Y, et al. Osteopontin promotes the progression of gastric cancer through the NF-kB pathway regulated by the MAPK and PI3K. Int J Oncol. 2014;45(1):282–290. | ||
Cao L, Fan X, Jing W, et al. Osteopontin promotes a cancer stem cell-like phenotype in hepatocellular carcinoma cells via an integrin-NF-kB-HIF-1α pathway. Oncotarget. 2015;6(9):6627–6640. | ||
Chen LF, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293(5535):1653–1657. | ||
Yeung F, Hoberg JE, Ramsey CS, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. | ||
Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460(7255):587–591. | ||
Chen WY, Wang DH, Yen RC, Luo J, Gu W, Baylin SB. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123(3):437–448. | ||
Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38(6):864–878. | ||
Simic P, Williams EO, Bell EL, Gong JJ, Bonkowski M, Guarente L. SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep. 2013;3(4):1175–1186. | ||
Yang Q, Wang B, Gao W, et al. SIRT1 is downregulated in gastric cancer and leads to G1-phase arrest via NF-kB/Cyclin D1 signaling. Mol Cancer Res. 2013;11(12):1497–1507. | ||
Bourguignon LY, Xia W, Wong G. Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J Biol Chem. 2009;284(5):2657–2671. | ||
Cao DX, Li ZJ, Jiang XO, et al. Osteopontin as potential biomarker and therapeutic target in gastric and liver cancers. World J Gastroenterol. 2012;18(30):3923–3930. | ||
Deng B, Zhang XF, Zhu XC, et al. Correlation and prognostic value of osteopontin and Bcl-2 in hepatocellular carcinoma patients after curative resection. Oncol Rep. 2013;30(6):2795–2803. | ||
Zhao L, Wang Y, Qu N, Huang C, Chen L. Significance of plasma osteopontin levels in patients with bladder urothelial carcinomas. Mol Diagn Ther. 2012;16(5):311–316. | ||
Thoms JW, Dal Pra A, Anborgh PH, et al. Plasma osteopontin as a biomarker of prostate cancer aggression: relationship to risk category and treatment response. Br J Cancer. 2012;107(5):840–846. | ||
Thorat D, Sahu A, Behera R, et al. Association of osteopontin and cyclooxygenase-2 expression with breast cancer subtypes and their use as potential biomarkers. Oncol Lett. 2013;6(6):1559–1564. | ||
Rud AK, Boye K, Oijordsbakken M, et al. Osteopontin is a prognostic biomarker in non-small cell lung cancer. BMC Cancer. 2013;13:540. | ||
Bandopadhyay M, Bulbule A, Butti R, et al. Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets. 2014;18(8):883–895. | ||
Shen J, Fang J, Hao J, et al. SIRT1 inhibits the catabolic effect of IL1-beta through TLR2/SIRT1/NF-kB pathway in human degenerative nucleus pulposus cells. Pain Physician. 2016;19(1):E215–E226. | ||
Pan W, Yu H, Huang S, Zhu P. Resveratrol protects against TNF-α-induced injury in human umbilical endothelial cells through promoting sirtuin-1-induced repression of NF-KB and p38 MAPK. PLoS One. 2016;11(1):e0147034. | ||
Byles V, Zhu L, Lovaas JD, et al. SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene. 2012;31(43):4619–4629. | ||
Cheng F, Su L, Yao C, et al. SIRT1 promotes epithelial-mesenchymal transition and metastasis in colorectal cancer by regulating Fra-1 expression. Cancer Lett. 2016;375(2):274–283. | ||
Cha BK, Kim YS, Hwang KE, et al. Celecoxib and sulindac inhibit TGF-β1-induced epithelial-mesenchymal transition and suppress lung cancer migration and invasion via downregulation of sirtuin 1. Oncotarget. 2016;7(35):57213–57227. | ||
Sun L, Li H, Chen J, et al. A SUMOylation-dependent pathway regulates SIRT1 transcription and lung cancer metastasis. J Natl Cancer Inst. 2013;105(12):887–898. | ||
Sun L, Li H, Chen J, et al. PIASy mediates hypoxia-induced SIRT1 transcriptional repression and epithelial-to-mesenchymal transition in ovarian cancer cells. J Cell Sci. 2013;126(Pt 17):3939–3947. | ||
Shevde LA, Samant RS. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014;37:131–141. | ||
Hong SH, Lee HJ, Sohn EJ, et al. Anti-nephrolithic potential of resveratrol via inhibition of ROS, MCP-1, hyaluronan and osteopontin in vitro and in vivo. Pharmacol Rep. 2013;65(4):970–979. | ||
Palmirotta R, Cives M, Della-Morte D, et al. Sirtuins and cancer: role in the epithelial-mesenchymal transition. Oxid Med Cell Longev. 2016;2016:3031459. | ||
Huang W, Shang WL, Wang HD, Wu WW, Hou SX. Sirt1 overexpression protects murine osteoblasts against TNF-α-induced injury in vitro by suppressing the NF-kB signaling pathway. Acta Pharmacol Sin. 2012;33(5):668–674. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.