Back to Journals » OncoTargets and Therapy » Volume 13
Sinomenine Inhibits Non-Small Cell Lung Cancer via Downregulation of Hexokinases II-Mediated Aerobic Glycolysis
Authors Liu W, Yu X, Zhou L, Li J, Li M, Li W , Gao F
Received 20 December 2019
Accepted for publication 28 March 2020
Published 16 April 2020 Volume 2020:13 Pages 3209—3221
DOI https://doi.org/10.2147/OTT.S243212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Wenbin Liu,1,* Xinfang Yu,2,* Li Zhou,3,* Jigang Li,1 Ming Li,4,5 Wei Li,6 Feng Gao7
1Department of Pathology, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan 410006, People’s Republic of China; 2Department of Cancer Biology, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio 44195, USA; 3Department of Pathology, Xiangya Hospital of Central South University, Changsha, Hunan 410008, People’s Republic of China; 4School of Stomatology, Hunan University of Chinese Medicine, Changsha, Hunan 410208, People’s Republic of China; 5Changsha Stomatological Hospital, Hunan University of Chinese Medicine, Changsha, Hunan, 410004, People’s Republic of China; 6Department of Radiology, The Third Xiangya Hospital of Central South University, Changsha, Hunan 410013, People’s Republic of China; 7Department of Ultrasonography, The Third Xiangya Hospital of Central South University, Changsha, Hunan 410013, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Li; Feng Gao Email [email protected]; [email protected]
Background: Addiction to aerobic glycolysis is a common metabolic phenotype in human non-small cell lung cancer (NSCLC). The natural product Sinomenine (Sin) exhibits significant anti-tumor effects in various human cancers. However, the underlying mechanism remains elusive.
Methods: The inhibitory effect of Sin on NSCLC cells was determined by MTS and soft agar assays. The glycolysis efficacy of NSCLC cells was examined by glucose uptake and lactate production. The activation of Akt signaling and the protein level of hexokinases II (HK2) were examined by immunoblot (IB), qRT-PCR, and immunohistochemical staining (IHC). The in vivo anti-tumor effect of Sin was validated by the xenograft mouse model.
Results: We showed that HK2 is highly expressed in NSCLC tissues and cell lines. Depletion of HK2 suppressed cell viability, anchorage-independent colony formation, and xenograft tumor growth. Sinomenine exhibited a profound inhibitory effect on NSCLC cells by reducing HK2-mediated glycolysis both in vitro and in vivo. Ectopic overexpression of HK2 compromised these anti-tumor efficacies in sinomenine-treated NSCLC cells. Moreover, we revealed that sinomenine decreased Akt activity, which caused the down-regulation of HK2-mediated glycolysis. Knockdown of Akt reduced HK2 protein level and impaired glycolysis. In contrast, overexpression of constitutively activated Akt1 reversed this phenotype.
Conclusion: This study suggests that targeting HK2-mediated aerobic glycolysis is required for sinomenine-mediated anti-tumor activity.
Keywords: sinomenine, non-small cell lung cancer, glycolysis, hexokinase 2
Introduction
Non-small cell lung cancer (NSCLC) is a malignancy with high mortality worldwide.1 In China, NSCLC is the most common type of cancer and a leading cause of cancer-related death. Genetic susceptibility, individual behavioral (tobacco and smoking), and environmental exposures, including air pollution, infection, and occupational exposures, have been demonstrated to be associated with the tumorigenesis of NSCLC.2 The overall age standardized incidence among men and women has only declined slightly over the past decades, and approximately half of NSCLC patients diagnosed at an advanced stage.3 The systemic therapies of NSCLC have revolutionized over recent years. Recently, the great success of the clinical application of epidermal growth factor receptor (EGFR)4 and immune checkpoint5,6 targeted therapies has significantly prolonged the overall survival rate of advanced NSCLC patients. However, owing to the low response rate and the intrinsic and acquired resistance, new treatment strategies are still an urgent demand. Thus, further elucidation of the underlying mechanisms and identification of novel anti-tumor targets would greatly aid clinicians in the development of new therapeutic strategies.
The “Warburg effect” is often observed in most human cancer cells, in which the cancer cells favor glucose metabolism via glycolysis even in the presence of oxygen.7 Hexokinases (HKs) catalyzes the first rate-limiting step of glycolysis and irreversibly phosphorylate glucose to glucose-6-phosphate. So far, mammalian HK isozymes, including HK1, 2, 3, and 4, have been identified.8 Accumulating evidence indicates that HK2 is frequently overexpressed and positively correlates with poor prognosis in multiple human cancers.9–11 HK2 enhances the aerobic glycolysis in tumor cells and suppresses apoptosis by localizing in mitochondria and interacting with the voltage dependent anion channel (VDAC) to prevent the release of cytochrome c.12 Moreover, overexpression of HK2 is associated with chemo or radioresistance in cancer cells.13,14 Thus, HK2 could be a promising target for anti-cancer treatment.
In the present study, we found that sinomenine inhibited NSCLC cell growth both in vitro and in vivo through the reduction of HK2-mediated glycolysis. Our results suggest that sinomenine is a novel anti-tumor agent which could provide a new option for NSCLC prevention and treatment.
Materials and Methods
Cell Lines and Antibodies
Human non-small cell lung cancer, including HCC827, H1650, H1299, H23, H460, and H1975, as well as normal lung epithelial cells HBE and NL20, were obtained from American Type Culture Collection (ATCC, Manassas, VA). All cells were maintained according to ATCC protocols, and subjected to routinely checking for mycoplasma contamination. The Fetal Bovine Serum (FBS), RPMI-1640, and DMEM cell culture medium were obtained from Thermo Fisher Scientific (Waltham, MA). The natural product, sinomenine (7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methylmorphinan-6-one), was obtained from Selleck Chemicals (Houston, TX). Lipofectamine 2000, which used for transient transfection, was a product of Invitrogen (#11668019, Carlsbad, CA). Antibodies against HK1 (#2024; 1:1000), p-H3 Ser10 (#53348; 1:1000), VDAC1 (#4866; 1:1000), cleaved-caspase 3 (#9664; 1:1000), cleaved-PARP (#5625; 1:1000), β-actin (#3700; 1:5000), Cytochrome c (#4280; 1:1000), Bax (#14796; 1:1000), α-Tubulin (#2144; 1:5000), p-Akt (#4060; 1:1000), p-S6 (#4858; 1:2000), HK2 (#2867; 1:1000), and Akt (#2920; 1:1000), were purchased from Cell Signaling Technology, Inc. (Beverly, MA). The Ki67 (ab16667, IHC: 1: 250) antibody and second antibodies used for immunofluorescence were obtained from Abcam (Cambridge, UK).
Knock Out and Knock Down
The HK2 sgRNA (TCAGATCTATGCCATCCCTG) was purchased from Addgene (#76309). For HK2 knockout stable cell generation, the NSCLC cells were transfected with HK2 sgRNA and selected by 1μg/mL puromycin for three weeks. The Akt1/2 siRNA pool (sc-43609) and siCtrl (sc-37007) were purchased from Santa Cruz Biotechnology (Dallas, TX).
MTS Assays
The CellTiter 96® Aqueous One Solution Cell Proliferation Assay kit (MTS) was used for cell viability analysis and performed according to the standard protocol. Briefly, cells (3000/well) in 96-well plates were treated with sinomenine for 0, 24, 48, and 72 h. The MTS reagent (20 μL/well) was added to the cell culture medium and incubated at a 37°C incubator for 1 h. Cell viability was examined using the Microplate reader (Bio-tek).
Anchorage-Independent Cell Growth
Soft agar assay was performed as described previously.15 Briefly, the cells were counted and seeded into 6-well plates at a density of 8×103/per well with 0.3% Basal Medium Eagle agar containing 10% FBS and various doses of sinomenine or DMSO control. The colonies were counted using a microscope (BX60 BF/DF transmitted and reflected light microscope, Olympus) two weeks later.
Immunofluorescence
The NSCLC cells in chamber slides were treated with sinomenine or DMSO control for 24 h, and fixed/permeabilized with ice-cold methanol in a cold room for 20 min. Blocking was performed using 5% BSA in PBS for 1 h at room temperature, followed by hybridization with primary antibodies (p-Histone H3 S10, c-caspase 3) at 4°C in a humidified chamber overnight. The slides were incubated with second antibodies for 45 min at room temperature. DAPI (P36935, Thermo Fisher Scientific) was used for nuclei counterstaining. The images were viewed and captured using the confocal fluorescence microscope system (NIKON C1si; NIKON Instruments Co.).
Subcellular Fractions
The Mitochondria Isolation Kit for Cultured Cells (#89874, Thermo Fisher Scientific) was used for the isolation of subcellular fractions according to the manufacturer’s protocol.
Western Blotting
The immunoblotting was performed as described previously.16 Briefly, NSCLC cells were treated with sinomenine or DMSO control. Whole-cell extract (WCE) was prepared using RIPA buffer and concentrated with the Protein Assay Kit (Thermo Fisher Scientific). WCE (20 μg) was subjected to SDS-PAGE analysis, followed by transfer onto PVDF membranes, and blocking with 5% non-fat milk. The membrane was incubated with the primary antibody overnight at 4°C. After a wash with PBST, the membrane was hybridized with a secondary antibody for 30 min at room temperature. The horseradish peroxidase (HRP) chemiluminescent substrate was used for visualization.
Glucose Uptake and Lactate Production
The glucose uptake and lactate production analysis were performed as described previously.17 NSCLC cells with sinomenine or DMSO control treatment were seeded in 6-well plates at a density of 1×106/well. The cells were cultured at the incubator for 10 h, and the cell culture medium was subject to glucose and lactate measurement at the Clinical Laboratory of Hunan Cancer Hospital (Changsha, China). Relative glucose consumption and lactate production were normalized by protein concentration.
Xenograft Mouse Model
The in vivo animal assay was approved by the Animal Ethics Committee of Hunan Cancer Hospital. Human H460 cells (1× 106) and HCC827 cells (4× 106) were inoculated s.c. into the right flank of 6-week-old female athymic nude mice. Sinomenine treatment at a dose of 40 mg/kg was initiated daily by i.p. injection when tumor volume reached 100 mm3. Tumor volume was recorded by vernier caliper and calculated as A ×B2 × 0.5. The A is the longest diameter of the tumor, B is the shortest diameter, and B2 is B squared.
Immunohistochemical Staining (IHC)
The NSCLC tissue array was purchased from Shanghai Outdo Biotech Co., Ltd. (Shanghai, China) (Cat. Hlug-NSCLC150PT-01) and used for HK2 protein level analysis. This array contains 75 cases of NSCLC tumor tissues and paired adjacent tissues. The staining was performed as described previously.18 Briefly, the slide was deparaffinized for 1 h at 60°C and rehydrated in ddH2O. The antigen retrieval was performed by immersing into boiling sodium citrate buffer (10 mM, pH 6.0) for 10 min. After treated with 3% H2O2 for 10 min, the slide was blocked with 50% goat serum albumin for 1 h at room temperature, followed by incubated with primary and second antibodies. The DAB Substrate Kit (Thermo Fisher Scientific) was used for visualization. Immunohistochemical staining evaluation was performed as previously described.18 The percentage of positive cells was scored as follows: 0, no positive cells; 1, ≤ 10% positive cells; 2, 10–50% positive cells; 3, > 50% positive cells. Staining intensity was scored as follows: 0, no staining; 1, faint staining; 2, moderate staining; 3, dark staining. The comprehensive score = staining percentage × intensity.
Statistical Analysis
The Statistics Package for Social Science (SPSS) software (version 13.0; SPSS, Chicago, IL, USA) was used for Standard statistical analysis. The qualified data were presented as mean values ± S.D. and analyzed using the Student’s t-test or Brown-Forsythe and Welch ANOVA test. A p value < 0.05 was considered statistically significant.
Results
HK2 Is Highly Expressed in Human NSCLC Cancer Cells
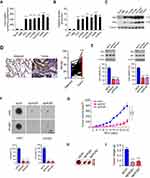
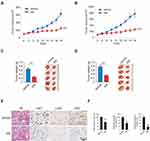
We first examined the 2-DG uptake and lactate production in NSCLC cells and two immortalized lung epithelial cells under normoxic conditions. Our data demonstrated that the aerobic glycolysis in NSCLC cells was significantly upregulated. The efficacy of 2-DG uptake (Figure 1A) and lactate production (Figure 1B) were increased robustly in NSCLC cancer cells. Moreover, the immunoblotting (IB) data showed that HK2 was highly expressed in NSCLC cells, but not the HBE and NL20 cells (Figure 1C). We further determined HK2 expression using a human NSCLC tissue array by immunohistochemistry (IHC) staining. As data shown in Figure 1D, HK2 is highly expressed in tumor tissues when compared to that of the matched adjacent tissues. To validate the effect of HK2 on NSCLC cell viability, we constructed HK2 knockout stable cells in H460 and HCC827 (Figure 1E) cells. The sgRNA stable expressing cells blocked HK2 expression, whereas the HK1 was unaffected. The MTS result showed that the depletion of HK2 decreased cell viability (Figure 1E) and inhibited the colony formation in soft agar (Figure 1F). Also, the tumor formation efficacy of HK2 deficient H460 cells was significantly impaired in nude mice, as the tumor volume form H460-sgHK2 cells was smaller than that of the H460-sgCtrl (Figure 1G and 1H). Consistently, the xenograft tumor weight form the sgHK2 cell was much lighter when compared with that of the sgCtrl cell (Figure 1I). These results suggest that the depletion of HK2 in NSCLC cells reduces tumorigenic properties both in vitro and in vivo.
Sinomenine Inhibits Glycolysis and Cell Growth in NSCLC Cells
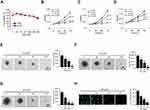
Sinomenine (Figure 2A) exhibits a profound anti-tumor efficacy against several human cancers.19,20 However, the effect of sinomenine on glycolysis is not unclear. We found that the culture medium of sinomenine-treated HCC827cells turned yellow much slower than that of untreated cells. This phenotype indicates that sinomenine might decrease the glycolysis in NSCLC cells. Our data showed that the control (DMSO-treated HCC827) cells showed a much stronger capacity to reduce the pH values of cell culture medium than the sinomenine-treated HCC827 (Figure 2B), we thus hypothesized that this phenotype might be due to lactate acidosis. We further examined the effect of sinomenine on the expression of a panel of glycolytic enzymes by qRT-PCR and Western blotting in HCC827 cells. The result showed that the mRNA and protein level of HK2, but not HK1 or other glycolytic enzymes, was reduced significantly in sinomenine-treated HCC827 cells (Figure 2C, Supplementary Figure 1).
Furthermore, the immunoblotting (IB) result indicated that sinomenine decreased the protein level of HK2 dose-dependently, whereas the HK1 was unaffected (Figure 2D). The efficacy of glucose consumption and lactate production in sinomenine -treated HCC827 cells were impaired significantly (Figure 2D). The similar inhibitory effect of sinomenine on HK2 expression, as well as glucose consumption and lactate production, were observed in H1975 (Figure 2E) and H460 cells (Figure 2F). We next determined whether the decrease of glycolysis in NSCLC cells suppressed cell growth. The results showed that sinomenine did not affect the cell growth of immortalized lung epithelial cells HBE and NL20 (Figure 3A). However, sinomenine decreased the cell viability of NSCLC cells, including HCC827, H1975, and H460 cells, in a time- and dose-dependent manner. (Figure 3B-D). Also, the soft agar assay showed that sinomenine inhibited colony formation dose-dependently in HCC827, H1975, and H460 cells (Figure 3E-G). We next examined the phosphorylation of histone H3 S10, a marker for cancer cell proliferation. As shown in Figure 3H, treatment with sinomenine significantly inhibited the expression of p-H3 Ser10. This data further confirmed that sinomenine decreased the growth of NSCLC cells.
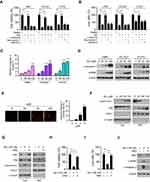
Overexpression of HK2 Reduces Sinomenine-Induced Apoptosis in NSCLC Cells
We next determined whether treatment with sinomenine could cause cell death. The result showed that pre-treated with apoptosis inhibitor z-VAD-fmk, but not the necroptosis inhibitor necrostatin-1 or GSK’873, rescued sinomenine-induced cell death (Figure 4A). The trypan blue exclusion assay also showed that z-VAD-fmk increased the population of live cells in the presence of sinomenine treatment (Figure 4B). These results indicated that sinomenine promoted apoptosis in NSCLC cells. By analyzing the activity of caspase 3, we showed that sinomenine increased the activity of caspase 3 dose-dependently (Figure 4C). Furthermore, the IB data demonstrated that the cleaved-caspase 3 and -PARP were up-regulated robustly (Figure 4D). The immunofluorescence results indicated that sinomenine increased the population of cleaved-caspase 3 positive cells (Figure 4E). To determine whether intrinsic apoptosis was involved, the subcellular fractions, including mitochondrial and cytosolic fractions, were isolated. As shown in Figure 4F, sinomenine decreased the expression of Bax in cytosolic fraction but enhanced its protein levels in the mitochondrial fraction. Consistently, the release of cytochrome c from mitochondria to the cytoplasm was increased with sinomenine treatment (Figure 4G). Overexpression of HK2 compromised sinomenine-induced intrinsic apoptosis (Figure 4G). The results showed that overexpression of HK2 increased the population of live cells (Figure 4H-J), and reduced the expression of cleaved-caspase 3 and -PARP (Figure 4J). These data suggest that sinomenine induced mitochondrial apoptosis is partly dependent on the downregulation of HK2.
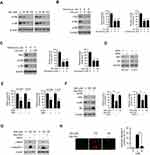
Inhibition of Akt Signaling Is Required for Sinomenine-Mediated Glycolysis Suppression
We next examined which signaling pathway was required for glycolysis in NSCLC cells. Interestingly, the results revealed that sinomenine inhibited the activity of Akt and downstream kinase S6 dose-dependently (Figure 5A). Furthermore, treated with wortmannin, an Akt signaling inhibitor, decreased Akt activity, HK2 expression, and glycolysis in HCC827 (Figure 5B) and H1975 (Figure 5C) cells. To further validate the function of Akt in sinomenine-induced glycolysis suppression, Akt knockdown cells were generated using the Akt siRNA in HCC827 and H1975 cells. The IB data showed that transfection with Akt siRNA caused a robust decrease of Akt and HK2 protein levels simultaneously (Figure 5D). Meanwhile, the glycolysis was compromised in Akt silencing NSCLC cells (Figure 5E). Ectopically overexpression of constitutively activated Akt1, Myr-Akt1, rescued sinomenine-induced HK2 reduction, glycolysis suppression (Figure 5F), and apoptosis induction. (Figure 5G). Consistently, the IF data revealed that Myr-Akt1 transfected HCC827 cells exhibited a significant reduction of cleaved-caspase 3 positive cells (Figure 5H). These results indicated that suppression of Akt signaling is required for sinomenine-mediated glycolysis reduction in NSCLC cells.
Sinomenine Inhibits Tumor Development in a Xenograft Mouse Model
We next determined the anti-tumor effect of sinomenine in xenograft mouse models. Results indicated that treatment with sinomenine significantly delayed in vivo tumor development. The average volume of the vehicle-treated HCC827 xenograft tumor was 632 ± 112 mm3, whereas the size of the sinomenine-treated tumor was 253 ± 43 mm3 (Figure 6A). Furthermore, we observed similar anti-tumor efficacy in H1975 xenograft tumors. The average tumor volume of the vehicle-treated control group and the sinomenine-treated group were 779± 128 mm3 and 266 ± 68 mm3, respectively (Figure 6B). In addition, the tumor mass and tumor weight were reduced significantly in sinomenine-treated group in both HCC827 and H1975 cells derived tumors (Figure 6C and D). The population of Ki-67 positive cells was decreased dramatically in sinomenine-treated tumors. Moreover, the p-Akt and HK2 staining were greater in tumors with vehicle treatment (Figure 6E and F). These results further confirmed that sinomenine exhibited a profound anti-tumor effect in the animal models.
Discussion
Recently studies have shown that the natural product, sinomenine, an active alkaloid which isolated from the Chinese medicinal plant Sinomenium acutum, exhibits a remarkable anti-tumor activity against a wide range of human malignancies. The anti-tumor activity of sinomenine was well studied in gastric,21,22 breast,23 prostate,24 cervical25 cancer, renal cell carcinoma,26 and glioma.27 Inhibition of cell cycle progression,27 angiogenesis,26 metastasis,28 and induction of apoptosis or autophagy are involved in sinomenine-mediated anti-tumor activity.19,29 However, the function of sinomenine on cancer cell metabolism, especially glycolysis, is not clear. In this study, we found that sinomenine attenuated HK2-mediated glycolysis and induced mitochondrial apoptosis. Importantly, we revealed that suppression of the Akt signaling was required for sinomenine-induced HK2 and glycolysis reduction.
Hyperactivation of glycolysis is a hallmark of human cancers.30 Overexpression of glycolytic enzymes, including GLUT1, HK-2, and PKM2, was related to the dysfunction of aerobic glycolysis.31 Notably, aerobic glycolysis provides a large number of intermediates for macromolecular biosynthesis in cancer cells. Moreover, the acidic microenvironment, which was caused by the accumulation of lactate in tumor tissues contributes to metastasis and anti-cancer therapy resistance.32,33 Recent studies revealed that HK2 is highly expressed in NSCLC cells and related to lung tumorigenesis and positively correlated with poor prognosis.34 Glycolysis was activated under hypoxic conditions in various human cancer cells. Here, we demonstrated that a panel of human NSCLC cells displayed aerobic glycolysis phenotype even under normoxic conditions, and the glucose consumption and lactate production were increased significantly. Depletion of HK2 by sgRNA suppressed cell growth, colony formation, and tumor development in the xenograft mouse model, further confirmed that HK2 is required for maintaining of malignancy properties of NSCLC cells. Furthermore, we demonstrated that sinomenine exhibited a profound anti-tumor effect by inhibition of HK2-mediated glycolysis. This evidence consistent with early findings that targeting HK2 and glycolysis inhibited tumor growth and induced apoptosis both in vitro and in vivo in human cancer cells.35–37
Akt signaling plays a critical role in controlling cell growth, survival, and metabolic reprogramming.38 Accumulating evidence revealed that mutation of the PIK3CA and/or loss of PTEN, which caused the hyperactivation of Akt signaling, enhanced tumor cell growth, and conferred chemo/radiotherapy resistance.38 Moreover, Akt signaling is required for maintaining of HK2 expression and activated Akt phosphorylated HK2 and promoted its localization on mitochondria to regulate apoptosis and cisplatin sensitivity.39 HK2 is dominantly regulated by various oncogenic pathways, including c-myc, PI3K/Akt, NF-κB, and HIF-1α.40–42 Our data showed that suppression of Akt signaling was required for sinomenine inhibited HK2 and glycolysis. Ectopically overexpression of constitutively activated Akt1, Myr-Akt1, partly rescued sinomenine-induced glycolysis inhibition and apoptosis induction. The effect of sinomenine on c-myc and HIF-1α as well as HK2 expression in human cells is not clear. Even sinomenine suppressed the activation of NF-κB signaling in cancer cell,43 the function regarding sinomenine on NF-κB regulated HK2 expression in NSCLC cells need further elucidate.
Overall, this study indicates that HK2 plays a critical role in ma intaining aerobic glycolysis in human NSCLC cells. Sinomenine inhibits NSCLC cells is partly dependent on the inhibitory effect on HK2-mediated glycolysis. This study expended the anti-tumor mechanisms of sinomenine, and targeting HK2 might be an alternative approach for NSCLC treatment.
Abbreviations
NSCLC, non-small cell lung cancer; HK2, hexokinases II; SIN, sinomenine; IB, immunoblot; VDAC, voltage dependent anion channel; EGFR, epidermal growth factor receptor; WCE, whole-cell extract; IHC, immunohistochemical; SD, standard deviation; HRP, horseradish peroxidase; IHC, immunohistochemistry staining.
Ethics Approval
The use and care of experimental animals were approved by the Institutional Animal Care and Use Committee, Central South University (KYJJ-2019-154), according to the Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC).
Funding
This work was supported by the National Natural Science Foundation of China (No. 81904262, 81972,837), the Natural Science Foundation of Hunan Province (2018JJ3787, 2018JJ2604, 2019JJ50682), and the Natural Science Foundation of Changsha Science and Technology Bureau (kq1701018).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi:10.3322/caac.21551
2. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi:10.1038/nature25183
3. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. doi:10.1001/jama.2019.11058
4. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725–737. doi:10.1038/s41416-019-0573-8
5. Tun AM, Thein KZ, Thein WL, Guevara E. Checkpoint inhibitors plus chemotherapy for first-line treatment of advanced non-small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Future Sci OA. 2019;5(9):FSO421. doi:10.2144/fsoa-2019-0081
6. Bianco A, Perrotta F, Barra G, Malapelle U, Rocco D, De Palma R. Prognostic factors and biomarkers of responses to immune checkpoint inhibitors in lung cancer. Int J Mol Sci. 2019;20:19. doi:10.3390/ijms20194931
7. Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi:10.1146/annurev-cellbio-092910-154237
8. Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–2057. doi:10.1242/jeb.00241
9. Zhou L, Li M, Yu X, Gao F, Li W. Repression of hexokinases II-mediated glycolysis contributes to piperlongumine-induced tumor suppression in non-small cell lung cancer cells. Int J Biol Sci. 2019;15(4):826–837. doi:10.7150/ijbs.31749
10. Wolf A, Agnihotri S, Micallef J, et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208(2):313–326. doi:10.1084/jem.20101470
11. Katagiri M, Karasawa H, Takagi K, et al. Hexokinase 2 in colorectal cancer: a potent prognostic factor associated with glycolysis, proliferation and migration. Histol Histopathol. 2017;32(4):351–360.
12. Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277(9):7610–7618. doi:10.1074/jbc.M109950200
13. Shi T, Ma Y, Cao L, et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10(4):308. doi:10.1038/s41419-019-1549-6
14. Zhong JT, Zhou SH. Warburg effect, hexokinase-II, and radioresistance of laryngeal carcinoma. Oncotarget. 2017;8(8):14133–14146. doi:10.18632/oncotarget.13044
15. Li W, Yu X, Xia Z, et al. Repression of Noxa by Bmi1 contributes to deguelin-induced apoptosis in non-small cell lung cancer cells. J Cell Mol Med. 2018;22(12):6213–6227. doi:10.1111/jcmm.13908
16. Li W, Yu X, Tan S, Liu W, Zhou L, Liu H. Oxymatrine inhibits non-small cell lung cancer via suppression of EGFR signaling pathway. Cancer Med. 2018;7(1):208–218. doi:10.1002/cam4.1269
17. Li W, Ma X, Li N, et al. Resveratrol inhibits Hexokinases II mediated glycolysis in non-small cell lung cancer via targeting Akt signaling pathway. Exp Cell Res. 2016;349(2):320–327. doi:10.1016/j.yexcr.2016.11.002
18. Liu H, Li W, Yu X, et al. EZH2-mediated Puma gene repression regulates non-small cell lung cancer cell proliferation and cisplatin-induced apoptosis. Oncotarget. 2016;7(35):56338–56354. doi:10.18632/oncotarget.10841
19. Sun Z, Zheng L, Liu X, Xing W, Liu X. Sinomenine inhibits the growth of melanoma by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Drug Des Devel Ther. 2018;12:2413–2421. doi:10.2147/DDDT.S155798
20. Fu S, Jin L, Gong T, et al. Effect of sinomenine hydrochloride on radiosensitivity of esophageal squamous cell carcinoma cells. Oncol Rep. 2018;39(4):1601–1608. doi:10.3892/or.2018.6228
21. Liu Y, Liu C, Tan T, Li S, Tang S, Chen X. Sinomenine sensitizes human gastric cancer cells to cisplatin through negative regulation of PI3K/AKT/Wnt signaling pathway. Anticancer Drugs. 2019;30(10):983–990. doi:10.1097/CAD.0000000000000834
22. Yuan H, Zhang J, Li F, Li W, Wang H. Sinomenine exerts antitumour effect in gastric cancer cells via enhancement of miR-204 expression. Basic Clin Pharmacol Toxicol. 2019;125(5):450–459. doi:10.1111/bcpt.13285
23. Gao G, Liang X, Ma W. Sinomenine restrains breast cancer cells proliferation, migration and invasion via modulation of miR-29/PDCD-4 axis. Artif Cells Nanomed Biotechnol. 2019;47(1):3839–3846. doi:10.1080/21691401.2019.1666861
24. Xu F, Li Q, Wang Z, Cao X. Sinomenine inhibits proliferation, migration, invasion and promotes apoptosis of prostate cancer cells by regulation of miR-23a. Biomed Pharmacother. 2019;112:108592. doi:10.1016/j.biopha.2019.01.053
25. Zhang D, Dong Y, Zhao Y, et al. Sinomenine hydrochloride sensitizes cervical cancer cells to ionizing radiation by impairing DNA damage response. Oncol Rep. 2018;40(5):2886–2895. doi:10.3892/or.2018.6693
26. Zhao B, Liu L, Mao J, et al. Sinomenine hydrochloride attenuates the proliferation, migration, invasiveness, angiogenesis and epithelial-mesenchymal transition of clear-cell renal cell carcinoma cells via targeting Smad in vitro. Biomed Pharmacother. 2017;96:1036–1044. doi:10.1016/j.biopha.2017.11.123
27. He X, Maimaiti M, Jiao Y, Meng X, Li H. Sinomenine induces G1-phase cell cycle arrest and apoptosis in malignant glioma cells via downregulation of sirtuin 1 and induction of p53 acetylation. Technol Cancer Res Treat. 2018;17:1533034618770305. doi:10.1177/1533034618770305
28. Jiang Y, Jiao Y, Liu Y, et al. Sinomenine hydrochloride inhibits the metastasis of human glioblastoma cells by suppressing the expression of matrix metalloproteinase-2/-9 and reversing the endogenous and exogenous epithelial-mesenchymal transition. Int J Mol Sci. 2018;19:3.
29. Deng F, Ma YX, Liang L, Zhang P, Feng J. The pro-apoptosis effect of sinomenine in renal carcinoma via inducing autophagy through inactivating PI3K/AKT/mTOR pathway. Biomed Pharmacother. 2018;97:1269–1274. doi:10.1016/j.biopha.2017.11.064
30. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
31. Abbaszadeh Z, Cesmeli S, Biray Avci C. Crucial players in glycolysis: cancer progress. Gene. 2019;144158.
32. Payen VL, Porporato PE, Baselet B, Sonveaux P. Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci. 2016;73(7):1333–1348. doi:10.1007/s00018-015-2098-5
33. Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152. doi:10.1186/1476-4598-12-152
34. Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–228. doi:10.1016/j.ccr.2013.06.014
35. Liu W, Li W, Liu H, Yu X. Xanthohumol inhibits colorectal cancer cells via downregulation of Hexokinases II-mediated glycolysis. Int J Biol Sci. 2019;15(11):2497–2508. doi:10.7150/ijbs.37481
36. Gao F, Li M, Liu WB, et al. Epigallocatechin gallate inhibits human tongue carcinoma cells via HK2mediated glycolysis. Oncol Rep. 2015;33(3):1533–1539. doi:10.3892/or.2015.3727
37. Li W, Gao F, Ma X, Wang R, Dong X, Wang W. Deguelin inhibits non-small cell lung cancer via down-regulating Hexokinases II-mediated glycolysis. Oncotarget. 2017;8(20):32586–32599. doi:10.18632/oncotarget.15937
38. Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381–405. doi:10.1016/j.cell.2017.04.001
39. Yu X, Wang R, Zhang Y, et al. Skp2-mediated ubiquitination and mitochondrial localization of Akt drive tumor growth and chemoresistance to cisplatin. Oncogene. 2019;38(50):7457–7472. doi:10.1038/s41388-019-0955-7
40. Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27(21):7381–7393. doi:10.1128/MCB.00440-07
41. Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–389. doi:10.1016/j.apsb.2015.05.007
42. Londhe P, Yu PY, Ijiri Y, et al. Classical NF-kappaB metabolically reprograms sarcoma cells through regulation of Hexokinase 2. Front Oncol. 2018;8:104. doi:10.3389/fonc.2018.00104
43. Song L, Liu D, Zhao Y, et al. Sinomenine inhibits breast cancer cell invasion and migration by suppressing NF-kappaB activation mediated by IL-4/miR-324-5p/CUEDC2 axis. Biochem Biophys Res Commun. 2015;464(3):705–710. doi:10.1016/j.bbrc.2015.07.004
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.