Back to Journals » International Journal of Nanomedicine » Volume 15
Single- and Multi-Arm Gadolinium MRI Contrast Agents for Targeted Imaging of Glioblastoma
Authors Patil R , Galstyan A , Grodzinski ZB, Shatalova ES , Wagner S , Israel LL , Ding H, Black KL, Ljubimova JY, Holler E
Received 21 November 2019
Accepted for publication 25 February 2020
Published 1 May 2020 Volume 2020:15 Pages 3057—3070
DOI https://doi.org/10.2147/IJN.S238265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Rameshwar Patil,1 Anna Galstyan,1 Zachary B Grodzinski,1 Ekaterina S Shatalova,1 Shawn Wagner,2 Liron L Israel,1 Hui Ding,1 Keith L Black,1 Julia Y Ljubimova,1,3 Eggehard Holler1
1Nanomedicine Research Center, Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, CA, USA; 2Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA; 3Oncology Translational Program, Samuel Oschin Comprehensive Cancer Center, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Correspondence: Eggehard Holler
Nanomedicine Research Center, Department of Neurosurgery, Cedars Sinai Medical Center, 127 S. San Vicente Boulevard, Suite A8304, Los Angeles, CA 90048 Tel +1 310 423-6630
Email [email protected]
Background: Position of gadolinium atom(s) plays a key role in contrast enhancement of gadolinium-based contrast agents. To gain a better understanding of effects of distance of gadolinium in relation to the nanoconjugate platform, we designed and synthesized single- and multi-arm (“star”) gadolinium conjugates equipped with antibody and peptides for targeting. The contrast agents were studied for their tumor imaging performance in a glioma mouse model.
Materials and Methods: Antibody- and peptide-targeted nano contrast agents (NCAs) were synthesized using polymalic acid platforms of different sizes. Gadolinium-DOTA and intermediates were attached as amides and targeting agents such as antibodies and peptides as thioethers. For in vivo experiments, we used human U87MG xenografts as glioma models. Magnetic resonance imaging (MRI) was performed on a Bruker BioSpec 94/20USR 9.4 T small-animal scanner. Delivery of contrast agents across the blood–brain barrier was studied by fluorescent microscopy.
Results: All contrast agents accumulated into tumor and showed composition-dependent imaging performance. Peptide-targeted mini-NCAs had hydrodynamic diameters in the range 5.2– 9.4 nm and antibody-targeted NCAs had diameters in the range 15.8– 20.5 nm. Zeta potentials were in the range of – 5.4–− 8.2 mV and − 4.6–− 8.8 mV, respectively. NCAs showed superior relaxivities compared to MultiHance at 9.4 T. The signal enhancement indicated maximum accumulation in tumor 30– 60 minutes after intravenous injection of the mouse tail vein. Only targeted NCAs were retained in tumor for up to 3 hours and displayed contrast enhancement.
Conclusion: The novel targeted NCAs with star-PEG features displayed improved relaxivity and greater contrast compared with commercial MultiHance contrast agent. The enhancement by mini-NCAs showed clearance of tumor contrast after 3 hours providing a suitable time window for tumor diagnosis in clinics. The technology provides a great tool with the promise of differential MRI diagnosis of brain tumors.
Keywords: magnetic resonance imaging, Gd-DOTA, structure variation, blood–brain barrier, tumor targeting, accelerated diagnosis
Introduction
Magnetic Resonance Imaging (MRI) is a powerful and safe imaging technique and has become an integral part of modern diagnostics, monitoring disease progression, and treatment achievements. Because of their high magnetic moment and effect on water relaxation, gadolinium(III) (Gd) contrast agents (GBCAs) are highly suitable for disease monitoring and are widely used in medical MRI.1,2 As free Gd is toxic,3 it is used in the form of stable chelates. Its value as a contrast agent depends on the strength to orientate protons of water molecules at the site of imaging. For best imaging, contrast agents should display high relaxation rate at the site of interest. To achieve greater selectivity, contrast agents are often conjugated to a targeting molecule. While this strategy shows promise, its success for contrast enhancement is highly dependent on expression of affinity receptors on target tumor cells. If the affinity for binding is low, high concentrations of contrast agent are needed to achieve desirable enhancement.4
Another requirement for success is the presence of an optimal number of Gd3+ ions as part of the agent. Thus, for short-time MRI, an affine, receptor targetable, multi-Gd3+ contrast enhancer is needed. In addition, other structural requirements apply such as motion restricted fixation on a common platform of the contrast enhancer is optimal which increases the number of uniformly oriented Gd3+ ions.5,6 Besides, another prerequisite is the availability of water molecules which are mobile to be oriented under the influence of Gd3+. Packaging of Gd3+ at high amounts into nano capsules could be unfavorable by reducing the accessibility of water molecules.7,8 Similarly, the attachment close to a carrier scaffold could be disadvantageous because of a structure forming nature of the scaffold constituents, eventually reducing the interaction of Gd3+ to surrounding water molecules in the tissue.
PMLA-based macromolecular nanoconjugates for fluorescence imaging and treatment of primary and metastatic brain tumors have been highlighted.9–15 Starting from these conjugates, Gd3+ DOTA derivatives have been developed to cross blood–brain barrier (BBB) and increase the contrast in MR imaging. In the nanodrugs and nano contrast agents, targeting was achieved by conjugation of specific antibodies. Here, we describe systematically optimized MRI contrast agents, the effect of Gd3+ distance from the polymeric scaffold, the influence of PEG-linkers and the length of the polymer. Antibodies are replaced by Angiopep-2 (Ap2), a ligand specific for the LRP-1 transcytosis pathway across the BBB.10,16-18
The purpose of substituting previously involved BBB-penetrating antibodies by small peptides was to develop (“mini”) contrast agents for fast and deep tissue penetration. Moreover, agents with low hydrodynamic diameters are likely to wash out through kidneys and minimize the implication to liver. At short serum tissue half-life, a series of clinical MRI tests can be carried out on a daily basis.13 Moreover, a faster extravasation into tumor and a short residing time in the blood/serum would be favorable for translation into clinicals.
Materials and Methods
Reagents
Highly purified poly(β-L-malic acid) (PMLA) of molecular weight 20,000–100,000 g/mol was prepared from the culture broth of Physarum polycephalum as described.19 Rat anti-mouse TfR mAb clone R17217 were obtained from BioLegend (San Diego, CA, USA). Cetuximab (Erbitux) was obtained from Bristol-Myers Squibb Inc (New York City, NY, USA). Maleimide-PEG3400-maleimide (mal-PEG3400-mal) was obtained from Laysan Bio Inc (Arab, AL, USA). Gd-DO3A-Butylamine (Gd-DOTA-amine) was purchased from Macrocyclics (Plano, TX, USA). t-boc-N-amido-dPEG®₁₂-acid, mal-dPEG®₂₄-NHS ester, mal-dPEG®₁₂-Tris(-TFP ester)₃, t-boc-NH-dPEG®₁₂-Tris(-TFP ester)₃ and t-boc-NH-dPEG®₁₂-Tris(-TFP ester)₃ were obtained from Quanta BioDesign Ltd. (Plain city, OH, USA). Alexa Fluor 680 C2-mal (Alexa-680) was purchased from Life Technologies (Carlsbad, CA, USA) and Rhodamine Red® C2-mal (Rh) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Anti-mouse specific transferrin receptor antibody (a-MsTfR) was obtained from Protein Expression Center, California Institute of Technology (Pasadena, CA, USA). 3-(2-Pyridyldithio)-propionate (PDP) was synthesized as described.20 Unless otherwise indicated, all chemicals and solvents of highest purity were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Analytical Methods Used in Synthesis of Intermediates and NCAs
The conjugation reaction of Gd-DOTA-amine and MEA with PMLA was followed by thin layer chromatography (TLC) on precoated silica gel 60 F254 aluminum sheets and visualization of spots under UV light and/or by ninhydrin staining.13 Size exclusion chromatography (SEC-HPLC) was performed on an Elite LaChrom analytical system with an L2455 diode array detector (Hitachi), and MW was measured using PolySep-GFC-P 4000 (300 x 7.80 mm) (Phenomenex) with phosphate buffered saline (PBS) pH 7.4 as a mobile phase and polystyrene sulfonates as molecular weight standards. Thiol residues attached to PMLA were assayed by the method of Ellman. Enzyme-linked immunosorbent assay (ELISA) was used to determine the functional activity of conjugated antibody using an ELISA protein detector kit (KPL, Inc.). The amount of Gd in nanoconjugates was determined by ICP-MS at Element Materials Technology (Huntington Beach, CA, USA). Amounts of mPEG, were quantified by the colorimetric method using ammonium ferrothiocyanate.21 The content of monoclonal IgG antibody (mAb) and Ap2 was determined by a Pierce BCA protein assay kit (Thermo Scientific, Waltham, MA, USA). Unlabeled antibodies and peptides were used as standards in quantitative measurements. Quantification of malic acid in nanoconjugates was performed by the malate dehydrogenase assay.19 Percentage (%) of ligand loading on PMLA conjugates was calculated by using the formula % = 100 x (μmol ligand)/(μmol malic acid).
Synthesis of Gd-DOTA-PEG600-amine
Step-1. Attachment of PEG linker: A solution of N-hydroxysuccinimide (NHS; 0.07 mmol) and N,N’-dicyclohexylcarbodiimide (DCC; 0.07 mmol) dissolved in 0.3 mL of dimethylformamide (DMF) was added consecutively to the solution of 50 mg of t-Boc-PEG600-acid (0.07 mmol), dissolved in DMF (0.3 mL). The reaction mixture (RM) was stirred at RT for 2 hours. Then 45.96 mg of Gd-DOTA-amine (0.7 mmol) was dissolved in 0.2 mL of DMSO and added to the RM followed by 8.1 µL of 2.6-Lutidine (0.7 mmol). RM was stirred at ambient temperature for an additional 2 hours. After TLC and indication of a ninhydrin reaction, 1.5 mL of methanol was added, and the product was purified on LH20 columns with methanol as a mobile phase. Product containing fractions were collected and mixed. Methanol was removed by rotary evaporator. A sticky substance, which was obtained and used in the following step without further purification. Step 2. Removal of t-boc group: After addition of 3M Methanolic HCl (2 mL) the removal of the t-boc group accomplished under stirring at ambient temperature for 16 hours. After removal of excess methanol by rotary evaporation, Gd-DOTA-PEG600-amine was washed with 5 mL of diethyl ether to obtain a white solid. Reaction yield was 97% over two steps.
Synthesis of mal-PEG600(Gd-DOTA)3
To a solution of 50 mg of mal-PEG600(TFP)3 ester (0.032 mmol) dissolved in 0.3 mL of DMF was added a solution of Gd-DOTA-NH2 (65.11 mg 0.096 mmol) dissolved in 0.3 mL of DMSO followed by 11.42 μL (0.096 mmol) of 2.6-Lutidine. RM was stirred at ambient temperature for 3 hours and purified by LH20 column in methanol. Fractions containing pure product were collected and methanol was removed by rotary evaporation. Finally, the sticky substance was dissolved in water and lyophilized to obtain mal-PEG600(Gd-DOTA)3 as a white solid. Reaction yield 95%.
Synthesis of mal-PEG10000(Gd-DOTA)7
To a solution of PEG10000-COOH (137 mg, 13.7 µmol) in 1 mL DMF was added a mixture of NHS/DCC (110 µmols each) and stirred at ambient temperature for 2 hours. Gd-DOTA-NH2 (68.5 µmols) dissolved in 0.4 mL DMSO was added to the RM followed by 2.6-Lutidine (68.5 µmol) and stirred for 1 hour. mal-PEG3400-NH2 (46.6 mg, 13.7 µmols) dissolved in 0.3 mL of DMF was added to RM followed by an equivalent amount of 2.6-Lutidine and stirred for 1 hour. Reaction completion was confirmed by TLC and ninhydrin test. Again, a slight excess amount of Gd-DOTA-NH2 (41.1 µmol) dissolved in 0.2 mL DMSO was added to the RM followed by 2.6-Lutidine (41.1 µmol) and stirred for 2 hours. A portion of 3 mL water was added, and the solution was passed over PD-10 column. After lyophilizing, mal-PEG10000(Gd-DOTA)7 was obtained as a white solid. Reaction yield was 68%.
Synthesis of Ap2-PEG3400-mal
Ap2-PEG3400-mal was synthesized using procedures published earlier.10
Synthesis of Preconjugates 1–4
Synthesis of preconjugates was performed after activating pendent carboxylates following similar procedures as published earlier.13
Attachment of Antibodies to Preconjugates
To the solution of mAb (5 mg, 33 nmol) dissolved in 1 mL of 100 mM sodium phosphate buffer containing 150 mM NaCl pH 5.5 was added tris(2-carboxyethyl)phosphine hydrochloride (TCEP, to a final concentration of 5 mM, prepared as a 50 mM solution in water). The mixture was incubated for 30 minutes at RT. TCEP was removed using Sephadex PD10, and the reduced antibody was immediately added dropwise to mal-PEG3400-mal (10 mmol) dissolved in 1 mL of freshly prepared, sterile 100 mM sodium phosphate buffer with 150 mM NaCl (pH 5.5). The reaction mixture was stirred at RT for 1 hour and then was concentrated using a centrifuge membrane filter (Vivascience, cutoff 30 kDa, 20 mL, 100 mM sodium phosphate buffer containing 150 mM NaCl, pH 5.5) and purified over Sephadex G75 pre-equilibrated with 100mM sodium phosphate buffer and 150 mM NaCl, pH 6.3. Pure fractions containing antibody were collected, adjusted to 4 mg/mL Mal-functionalized antibodies and intermediates were conjugated to preconjugates by formation of thioether.13
Synthesis of Amino-PEG600(Gd-DOTA)3
Step-1. Attachment of Gd-DOTA: To a solution of Boc-PEG600(TFP)3 ester (116 mg, 0.078 mmol) dissolved in 0.5 mL of dimethylformamide (DMF) was added Gd-DOTA-amine (157 mg, 0.23 mmol) dissolved in 1 mL of DMSO followed by 55 μL of 2,6-Lutinine (0.47 mmol) and RM was stirred at ambient temperature for 16 hours. After TLC and ninhydrin test, 1.5 mL of methanol was added, and the product was purified by LH20 columns. Pure fractions from the column were mixed and methanol was removed by rotary evaporator to obtain a sticky substance and used for next step without any further purification. Step 2. Removal of t-boc group: 2 mL of 3M Methanolic HCl was added to the intermediate and stirred at ambient temperature for 16 hours. Excess methanol was removed by rotary evaporator and material was washed with 5 mL of diethyl ether to obtain a white solid. Reaction yield 97% over two steps.
Hydrodynamic Diameter and Zeta Potential
Synthesized NCAs and intermediates were characterized by their size and ζ potential using Zetasizer Nano ZS90 (Malvern Instruments). For the particle size measurements at 25°C, the solutions were prepared in PBS at a concentration of 2 mg/mL. For the measurement of the ζ potential, the concentration of the sample dissolved in a PBS (pH 7.4) at 2 mg/mL, and the voltage applied was 150 mV. Data represent the mean of three or more independent measurements (standard deviation).
MRI Measurements
For MRI, a 200 µL solution of contrast reagents was administered by intravenous (IV) injection via tail vein. All contrast agents were injected at a dose of 0.1 mmol Gd/kg. T1-weighted images were acquired as spin-echo images with a short repetition time of 450 ms, and an echo time of 8.77 ms. A field of view (2 cm) was used with an in-plane resolution of 78 x 102 μm and thickness of 1 mm, total time of 10 minutes 7 seconds for seven averages. The T1 maps were generated at a resolution of 78 x 102 μm using a RARE imaging sequence (Rare factor 4,) using 9 repetition times with a maximum time of 10 seconds. Animals underwent MRI scanning on a Bruker BioSpec 94/20USR 9.4 T small-animal scanner (Bruker Biospin MRI GmbH). Each animal was placed inside a transmission whole body coil (T10325 V3, Bruker Biospin MRI GmbH) with a four-channel surface array coil (T11071 V3, Bruker Biospin MRI GmbH) positioned over the brain. The transmission body coil was used for all radio frequency transmission; the surface coil was used for detection. Contrast enhancement quantification was performed using our earlier methods.13
MRI Contrast Measurement and Quantification
To quantify contrast enhancement over time, we used contrast intensity generated by T1-weighted images (three contiguous slices through the center of each tumor) and determined relative increase in consort at various times after administration of contrast agents. An MRI scan was measured before the injection of contrast agent and contrast from that scan was used as a reference/background. For each time point, MRI scans of the whole brain included several slices of 1.0 mm thickness. We chose a minimum of three slices for each time point and traced the regions of interest for the brain tumor and chose reference regions for normal brain on the same plane as shown in our earlier work.13 On each slice, multiple ROI were selected. At least three ROI were used for each analysis. MRI images were stored in TIFF format and analyzed by Leica MM AF 1.6 software. An average intensity/pixel was calculated for each ROI individually. The presented data refer to intensity ratios with reference to normal brain tissue and are given as mean values (standard error of mean (SEM)). They are shown as a function of the time elapsed after the injection of the contrast agents.
Cell Lines, Culture Conditions and Tumor Xenografts
Primary glioblastoma multiforme (GBM) U87MG cell line was obtained from ATCC (Manassas, VA, USA) and was cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS), 1% MEM nonessential amino acids, 1 mM sodium pyruvate, and 2 mM L- glutamine at 37°C with CO2. All animal procedures were performed in accordance with the ethical principles and animal welfare considerations detailed in the Guide for the Care and Use of Laboratory Animals, 8th Edition (The National Academies Press, 2011), and experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Cedars-Sinai Medical Center. Animal facility at Cedars-Sinai Medical Center has full accreditation from AAALAC and AAALAC guidelines were followed for the welfare of the laboratory animals. Athymic NCr-nu/nu female mice were obtained from NCI-Frederick. Tumors were stereotactically implanted using EGFR+ U87MG GBM cells at 2.5 x 104 into the right basal ganglia of mouse brain. All animals were followed for neurological symptoms during the entire study.
Immunohistochemistry and BBB Crossing Efficiency
Along with hematoxylin and eosin (H&E) staining, fluorescence immunohistochemistry was used with various antibodies. After the last MRI measurement (3 hours) animals were euthanized, and harvested brains were embedded in OCT tissue freezing medium (Tissue Tek) and frozen in liquid nitrogen. Frozen tissue blocks were sectioned at 8 μm thickness using a Leica CM 3050S cryostat. Before staining, tissue sections were air-dried at room temperature, fixed with ice-cold xylene for 10 minutes, and then rinsed three times with PBS. Sections were incubated in a humidified chamber with blocking buffer (4% normal goat serum, 4% normal donkey serum, 1% BSA, and 0.1% Triton X-100 in PBS) for 1 hour at RT to block nonspecific sites. The blocked sections were incubated overnight at 4°C with primary antibodies diluted in staining buffer and later washed with PBS and sections mounted with Prolonged Gold Antifade (Thermo Fisher Scientific) mounting medium containing DAPI. Anti-von Willebrand factor (vWF) antibody labelled with Alexa fluor® 488 or Lectin from Lycopersicon esculentum (Millipore Sigma, Burlington, MA, USA) was used to identify blood vessels. Images were captured using a Leica DM6000B microscope (Germany). Efficiency of BBB crossing of NCA was evaluated by a fluorescence-based quantification method developed in our lab.10
Measurement of Gd in Tumor Samples
Frozen tissue blocks of brains were sectioned for H&E and a tumor sample was carefully scoped under a microscope using H&E staining as a guide. The amount of gadolinium was determined using Inductively coupled plasma mass spectrometry (ICPMS) at Element Materials Technology, Huntington Beach, CA, USA.
Results
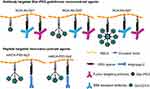
To develop novel NIA to enhance the contrast in MRI by structure variation, we synthesized intermediates with structurally varied linear and star-like PEG linkers and varied chain length of PMLA. Schematics of NCA is shown in Figure 1. Syntheses of intermediates carrying several gadolinium moieties and glioma targeting groups per agent are described below.
Synthesis of NCA with Unbranched PEG Spacer
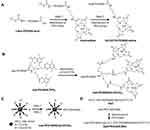
Starting with bifunctional PEG (tBoc-PEG600-acid), the carboxylic end of PEG was attached to commercially available t-boc protected Gd-DOTA-amine forming a stable amide linkage after NHS/DCC activation of terminal carboxylate. In the next step, the t-boc group was removed by using methanolic HCl (Figure 2A). For details see the syntheses section under Materials and Methods. Preconjugate-1 was synthesized by attaching Gd-DOTA-PEG600-amine and 2-mercapto-1-ethylamine (MEA) after activating PMLA pendent carboxylic acid groups using the NHS/DCC chemistry. Preconjugate-1 was purified by size exclusion chromatography and obtained after lyophilization as a white floppy solid, stable at −20°C for months. The sulfhydryl groups (-SH) provided by the attached amidoethyl-SH were conjugated with mal-functionalized mAbs, or peptides for tumor targeting, BBB crossing, as well as with fluorescent dyes such as rhodamine for optical imaging (Figure 3).
Synthesis of NCA with Star-PEG Linker
The synthetic strategy for these NCAs was divided into two parts. In the first part of the synthesis Gd-DOTA intermediates with varied chain length and branches (4- and 8-arm PEG) were prepared (Figure 2B and C). For the synthesis of mal-PEG600(Gd-DOTA)3, we used 4-arm bifunctional “Star-PEG” providing mal-group on one end and three activated TFP-esters on the other end. In a one-step reaction, TFP esters were conjugated with Gd-DOTA-amine to form mal-PEG600(Gd-DOTA)3 (Figure 2B). By reaction of the mal-group with amidoethyl-SH functionalized PLMA, the 3-armed PEG-(Gd-DOTA)3 PMLA-antibody MRI enhancer construct (NCA-Ab-Gd3) in Figure 1. The synthesis with 8-arm PEG yielding mal-PEG3400-PEG10000(Gd-DOTA)7 was carried out in two steps. The 8-arm Star-PEG (MW 10,000 g/mol) provides eight terminal carboxylates. At first, carboxylate groups were activated using NHS/DCC chemistry and one of the carboxylates was substituted with mal-PEG3400-amine. The remaining seven carboxylates were then conjugated with Gd-DOTA-amine to form mal-functionalized star-peg (Figure 2C). The 7-armed Gd-DOTA PEG-mal was attached to PMLA-amidoethyl-SH together with mal-functionalized antibodies to yield the 7-armed-PEG-(Gd-DOTA)7 PMLA-antibody MRI enhancer construct (NCA-Ab-Gd7) in Figure 1.
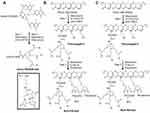
Synthesis of Mini-NCA with Ap2 as Targeting Agent
Synthetic strategy for this mini-NCA was divided in two parts. First, intermediates Ap2-PEG3400-mal, amino-PEG600-Gd3, preconjugate-3 and preconjugate-4 (Figure 4AC) were separately prepared. Ap2 peptide (S-C-YEETKFBNRKGRSGGYFFT-H) was selectively attached to one of the ends of bifunctional PEG to form Ap2-PEG3400-Mal (Figure 2D). Amino-PEG600-Gd3 was obtained in two steps from commercially available PEG (t-boc-amine-PEG600-TFP3) by conjugating Gd-DOTA to the TFP triple-ester followed by deprotection of t-boc-amine-PEG600-GD-DOTA3 to generate the amine (Figure 4A). The amine was conjugated to PMLA by the NHS/DCC method. Finally, Ap2-PEG3400-mal was attached to PMLA backbone along with mal-PEG600(Gd-DOTA)3 and mal-Rh to form the mini-NCAs (Figure 4B).
Physicochemical Characterization
Detailed physicochemical characterization of all NCAs and intermediates was performed. Composition of the contrast reagents in terms of malic acid, mAbs, and gadolinium were quantified post-synthetically. Reaction progress was monitored by thin layer chromatography and SEC-HPLC. Hydrodynamic diameter of antibody containing NCA increased from 15.8 (±1.5) nm to 17.1 (±1.4) nm and 20.5 (±1.2) nm from single arm to 8-arm contrast agent (NCA-Ab-Gd1, NCA-Ab-Gd3, NCA-Ab-Gd7), while zeta potential became less negative from −8.8 (±0.9) to −5.5 (±0.5) mV and −4.6 (±0.6) mV. Peptide (Ap2) containing contrast agents were prepared with PMLA of molecular mass either 20,000 (NCA-P20-Ap2) or 60,000 (NCA-P60-Ap2) g/mol PMLA and referred as mini-NCAs. Mini-NCA-P20-Ap2 showed smaller hydrodynamic diameter of 5.2 (±1.1) nm and the zeta potential −5.4 (±0.4) mV, while mini-NCA-P60-Ap2 had the hydrodynamic diameter of 9.4 (±1.6) nm and zeta potential of −8.2 (±1.1) mV. These structure variations towards higher molecular weights and branching of PEG revealed a decrease of the (negative) zeta potential, while the increase in the molecular weight of the single-armed mini-NCA revealed an increase in the (negative) zeta potential. In our studies, MRI equipment of 9.4 T was used. We measured relaxivities for the contrast agents ranging from 5.42, 5.98 to 5.69 (mM Gd)−1s−1 and 397, 438 and 416 (mM NCA)−1s−1 in measurements of single, 4-arm and 8-arm contrast agents, respectively. While the increase in the size of these NCAs related to less than 10% change in the Gd-relaxivity and the NCA-relaxivity, corresponding changes were 2-fold and 5-fold for the mini-NCAs, namely 3.2 (mM Gd)−1s−1 for NCA-P20-Ap2 to 5.63 (mM Gd)−1s−1 for (NCA-P60-Ap2) and from 182 (mM NCA)−1s−1 (NCA-P20-Ap2) to 961 (mM NCA)−1s−1 (NCA-P60-Ap2). This 2- and 5-fold changes in relaxivity for mini-NCA in comparison to the almost invariant relaxivity for the other contrast agents indicate that the chain length of polymalic acid had a pronounced impact, while variations in size and branching of PEG were negligible.
Enhanced Contrast Imaging with Targeted NCAs
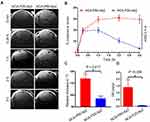
Mice bearing intracranial GBM (U87MG) were IV injected with the contrast agents at a dose of 0.1 mmol Gd/kg and MRI scans were recorded in the time interval of 0 min (no contrast), 0.2, 1, 2 and 3 hours after NCA administration (Figure 5A). For all NCA, peak contrast enhancement was reached at 1 hour and maintained for 3 hours at which point animals were euthanized. NCAs were similar except for the linker of Gd-DOTA. In NCA-Ab-Gd1, Gd-DOTA was attached via a linear PEG linker of molecular weight 600 g/mol. In NCA-Ab-Gd3, three molecules of Gd-DOTA were attached via a linear PEG of the same chain length (600 g/mol). In case of NCA-Ab-Gd7, we used a relatively large Star-PEG linker with molecular weight of 10,000 g/mol. The NCAs maintained the contrast intensity over time at different per cent contrast enhancement (Figure 5B). NCA-Ab-Gd3 showed the highest contrast intensity. All animals were scanned using clinical contrast agent MultiHance 2–3 days before NCAs administration to conform the presence of tumors.
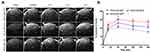
Contrast Imaging with Targeted Mini-NCAs
The dynamic contrast enhancement in tumor, relative to intensity in healthy brain was measured (Figure 6A). The measurement of contrast enhancement followed the method described above for contrast agents NCA-Ab-Gd1.13 High contrast in the tumor was observed with NCA-P60-Ap2 and retained for 3 hours. Contrast from low molecular weight NCA-P20-Ap2 showed a comparably low maximum between 0.3 and 1 hour and declined rapidly to only 3.43% contrast at 3 hours compared to 29.6% for NCA-P60-Ap2 (Figure 6B). A similar difference was also reflected in the relative increase in 1/T1 values at 3 hours for the mini-NCAs measured in slices covering the middle of the tumor. In accordance with the contrast enhancement NCA-P60-Ap2 generated significantly higher 1/T1-value (p =0.017) than NCA-P20-Ap2 (Figure 6C). This referred to a higher Gd content in the tumor for NCA-P60-Ap2 than for NCA-P20-Ap2 (p= 0.036, Figure 6D).
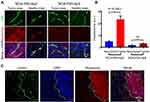
Penetration of Contrast Agents Through BBB
In our prior work13 we have shown that certain antibodies were required to target polymalic acid conjugates across BBB and deliver antisense and contrast agents to GBM cells. Recently, we studied the efficiency of certain peptides which allowed mini-NCA to cross BBB.10 When NCAs were fluorescence-labelled with rhodamine for microscopic analysis, high fluorescence intensity outside the blood vessels (green) was observed for NCA-P60-Ap2 (Figures 6 and 7). In healthy brain most of the intensity was visually noticed for mini-NCA NCA-P60-Ap2 only in vessels, but upon microscopic optical quantification, fluorescence in tumor out of vessels was also detected, however fluorescence intensity was significantly higher for NCA-P60-Ap2 than for NCA-P20-Ap2 (P<0.0001, Figure 7). This suggested that Ap2 targeting provided high BBB penetration activity and usefulness of glioma targeting MRI contrast agents.
Discussion
In the past few decades, various attempts have been made to improve contrast agents for superior image quality and accurate disease detection and progression.22 Gd complexes with small molecular weight peptides showed promising results, nevertheless since the emergence of nanotechnology Gd complexes bound to macromolecules gained interest due to advantages such as high loading of Gd per molecule, high relaxivity due to slow tumbling rates, and passive and active targeting. Though macromolecules offer these advantages, they also circulate much longer in the blood and cause increased background and higher accumulation/deposition of excess metal ions in some healthy organs causing potential long-term side effects.23–26 The concept of a mini-nanoconjugates approach shares common advantages such as higher loading, better relaxivity and targetability and yet offers relatively faster clearance for eliminating background signal for high quality contrast image providing grater molecular details for superior imaging.
Our approach in the nanodrug design takes into account not just the size but also the potential interaction of Gd with water molecules from the tissue of interest, which is the key for producing higher contrast for superior imaging.27 Chemical modification and physicochemical properties of contrast agents highly affect the ability of a contrast agent.28 We chose an optimum length of PEG spacer to orient Gd away from the polymer backbone to facilitate the interaction with water molecules from the tissue of interest. PEG is an excellent molecule for linker, but too much PEG would increase the molecular weight and steric hindrance. To avoid this problem, we attached three Gd-DOTA molecules at the end of PEG molecule of defined monomers and conjugated this to PMLA backbone.
The degree of Gd relaxivity is thought to depend on the distance between Gd-DOTA and the polyanion as a function of the length and degree of branching on linear and branched PEG. In NCA-Ab-Gd1 we placed one Gd complex at the end of PEG spacer and in NCA-Gd-Gd3 we introduced three Gd complexes with the same length of PEG spacer. To introduce more Gd on the same length of PEG would have created crowding, hence we used “star-PEG” for NCA-Ab-Gd7. In order to have the same number of Gd ions per NCA we varied the ratio of Gd-PEG spacer on each NCA. An inspection of the Gd relaxivity values and comparison with those obtained for the previously investigated multi-arm PEGs indicates, however, only incremental variations including relatively small change in molecular size and zeta potential (Table 1). The highest values of relaxivities were measured for the 3-arm PEG containing antibody targeting agents and for the Ap2 targeting agents. This indicated a small effect of PEG “crowding” in the case of the 8-armPEG10,000 linker as compared with the 3-armPEG600 linker as well as a protein “crowding” effect by the antibodies compared with peptides. Based on the Gd relaxivity values, we consider contrast agents containing 3-arm PEG linkers and Ap2 targeted linkers as lead compounds.
 |
Table 1 Physicochemical Characterization of Contrast Agents |
The longitudinal relaxation rate in tumor relative to non-tumor brain was measured for NCA-P20-Ap2, and NCA-P60-Ap2 (Figure 6A) using the T1 maps generated before injection and 3 hours after injections. The higher contrast and persistence with time in the T1-weighted images (Figure 6A) correlates with the higher increase in relaxation rate relative to the effects by smaller NCA-P20-Ap2. This can be attributed to the higher affinity of NCA-P60-Ap2, which contains 3 times as much targeting Ap2 peptide molecules than the smaller NCA-P20-Ap2. Because of firm binding and thus longer life-time of the NCA-P60-Ap2–receptor complex, the Gd-ions were held in the tumor area for a relatively longer time (Figure 6C), generating better contrast at the 3 hour time point than the less firmly bound NCA-P20-Ap2 and shorter life-time. Based on the results in Figure 6, we consider NCA-P60-Ap2 as the primary lead mini-NCA for contrast imaging, because of the better clearance than antibody targeted NCA while still providing a measurable contrast enhancement, affording more frequent periodical repetition of MRI measurements under preclinical and clinical conditions. Values obtained by quantification of Gd amount in tumor (Figure 6D) correlate with the relaxation data obtained by T1 (Figure 6 B and C).
Conclusions
We optimized the dynamic contrast-enhanced MRI agents for brain tumor targeted imaging using polymalic acid-based NCAs. In our earlier synthesized antibody targeted contrast agents, we noticed that the contrast agents displayed prolonged target residing times, which would be unfavorable for repeated testing over short intervals and causing toxicity risks when translated into clinics. We also reasoned that the use of specific antibodies afforded circumstantial chemical synthesis, high production costs, instabilities, problematic storage and large volume preparation for clinical imaging. We resolved the issue by replacing antibodies using affine peptides, which recognized tumor markers and were successfully tested in novel polymer conjugates for imaging and drug delivery. Ap2 is a well-known BBB shuttle peptide and was chosen for new nano imaging agent development. We developed a lead conjugate containing Ap2 that recognizes lipid protein delivery pathway specifically overexpressed on tumor BBB and glioblastoma tumor cell membranes. We established synthesis and characterization of the agents for fluorescence and MR enhanced imaging of the tumor. We have developed a mini-NCA which crosses BBB highly efficiently. The mini-NCA offers all the benefits of a nanoparticle yet, has relatively faster clearance from the blood stream minimizing background for enhanced image quality. Mini-NCA also possess high molecular relaxivity and can potentially be used for lower grade tumor which are otherwise very hard to detect by MRI. Additionally, Ap2 targeted mini-NCA can be useful to deliver anticancer agent for better survival benefit of brain tumors.
Acknowledgments
This work was supported by the following grants: NIH/NCI R01 CA188743 (JYL), NIH/NCI R01 CA206220 (JYL), NIH/NCI R01 CA230858-01 (JYL), NIH/NCI R01 CA209921 (EH).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
KLB, JYL and EH are shareholders of Arrogene, Inc. Keith L Black reports grants from NIH, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99(9):2293–2352. doi:10.1021/cr980440x
2. Lee SH, Kim BH, Na HB, Hyeon T. Paramagnetic inorganic nanoparticles as T1 MRI contrast agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(2):196–209. doi:10.1002/wnan.1243
3. Ranganathan RS, Raju N, Fan H, et al. Polymethylated DOTA ligands. 2. Synthesis of rigidified lanthanide chelates and studies on the effect of alkyl substitution on conformational mobility and relaxivity. Inorg Chem. 2002;41(25):6856–6866. doi:10.1021/ic025695e
4. Park JA, Lee JJ, Jung JC, et al. Gd-DOTA conjugate of RGD as a potential tumor-targeting MRI contrast agent. Chembiochem. 2008;9(17):2811–2813. doi:10.1002/cbic.200800529
5. Ananta JS, Godin B, Sethi R, et al. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrast. Nat Nanotechnol. 2010;5(11):815–821. doi:10.1038/nnano.2010.203
6. Karfeld-Sulzer LS, Waters EA, Davis NE, Meade TJ, Barron AE. Multivalent protein polymer MRI contrast agents: controlling relaxivity via modulation of amino acid sequence. Biomacromolecules. 2010;11(6):1429–1436. doi:10.1021/bm901378a
7. Nam T, Park S, Lee SY, et al. Tumor targeting chitosan nanoparticles for dual-modality optical/MR cancer imaging. Bioconjug Chem. 2010;21(4):578–582. doi:10.1021/bc900408z
8. Ayyagari AL, Zhang XD, Ghaghada KB, Annapragada A, Hu XP, Bellamkonda RV. Long-circulating liposomal contrast agents for magnetic resonance imaging. Magn Reson Med. 2006;55(5):1023–1029. doi:10.1002/(ISSN)1522-2594
9. Patil R, Galstyan A, Sun T, et al. Polymalic acid chlorotoxin nanoconjugate for near-infrared fluorescence guided resection of glioblastoma multiforme. Biomaterials. 2019;206:146–159. doi:10.1016/j.biomaterials.2019.03.029
10. Israel LL, Braubach O, Galstyan A, et al. A combination of tri-leucine and angiopep-2 drives a polyanionic polymalic acid nanodrug platform across the blood-brain barrier. ACS Nano. 2019;13(2):1253–1271. doi:10.1021/acsnano.8b06437
11. Ljubimova JY, Sun T, Mashouf L, et al. Covalent nano delivery systems for selective imaging and treatment of brain tumors. Adv Drug Deliv Rev. 2017;113:177–200. doi:10.1016/j.addr.2017.06.002
12. Chou ST, Patil R, Galstyan A, et al. Simultaneous blockade of interacting CK2 and EGFR pathways by tumor-targeting nanobioconjugates increases therapeutic efficacy against glioblastoma multiforme. J Control Release. 2016;244(Pt A):14–23.
13. Patil R, Ljubimov AV, Gangalum PR, et al. MRI virtual biopsy and treatment of brain metastatic tumors with targeted nanobioconjugates: nanoclinic in the brain. ACS Nano. 2015;9(5):5594–5608. doi:10.1021/acsnano.5b01872
14. Ding H, Inoue S, Ljubimov AV, et al. Inhibition of brain tumor growth by intravenous poly (beta-L-malic acid) nanobioconjugate with pH-dependent drug release [corrected]. Proc Natl Acad Sci U S A. 2010;107(42):18143–18148. doi:10.1073/pnas.1003919107
15. Lee BS, Fujita M, Khazenzon NM, et al. Polycefin, a new prototype of a multifunctional nanoconjugate based on poly(beta-L-malic acid) for drug delivery. Bioconjug Chem. 2006;17(2):317–326. doi:10.1021/bc0502457
16. Wang L, Hao Y, Li H, et al. Co-delivery of doxorubicin and siRNA for glioma therapy by a brain targeting system: angiopep-2-modified poly(lactic-co-glycolic acid) nanoparticles. J Drug Target. 2015;23(9):832–846. doi:10.3109/1061186X.2015.1025077
17. Ke W, Shao K, Huang R, et al. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials. 2009;30(36):6976–6985. doi:10.1016/j.biomaterials.2009.08.049
18. Sun X, Li Y, Liu T, Li Z, Zhang X, Chen X. Peptide-based imaging agents for cancer detection. Adv Drug Deliv Rev. 2017;110–111:38–51. doi:10.1016/j.addr.2016.06.007
19. Ljubimova JY, Ding H, Portilla-Arias J, et al. Polymalic acid-based nano biopolymers for targeting of multiple tumor markers: an opportunity for personalized medicine? J Visualized Exp. 2014;(88). doi:10.3791/50668.
20. Carlsson J, Drevin H, Axen R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978;173(3):723–737. doi:10.1042/bj1730723
21. Nag A, Mitra G, Ghosh PC. A colorimetric assay for estimation of polyethylene glycol and polyethylene glycolated protein using ammonium ferrothiocyanate. Anal Biochem. 1996;237(2):224–231. doi:10.1006/abio.1996.0233
22. Ibrahim MA, Dublin AB. Magnetic Resonance Imaging (MRI), Gadolinium. Treasure Island (FL): StatPearls; 2019.
23. Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals. 2016;29(3):365–376. doi:10.1007/s10534-016-9931-7
24. Garcia J, Liu SZ, Louie AY. Biological effects of MRI contrast agents: gadolinium retention, potential mechanisms and a role for phosphorus. Philos Trans a Math Phys Eng Sci. 2017;375(2107):20170180. doi:10.1098/rsta.2017.0180
25. Robert P, Fingerhut S, Factor C, et al. One-year retention of gadolinium in the brain: comparison of gadodiamide and gadoterate meglumine in a rodent model. Radiology. 2018;288(2):424–433. doi:10.1148/radiol.2018172746
26. Fingerhut S, Sperling M, Holling M, et al. Gadolinium-based contrast agents induce gadolinium deposits in cerebral vessel walls, while the neuropil is not affected: an autopsy study. Acta Neuropathol. 2018;136(1):127–138. doi:10.1007/s00401-018-1857-4
27. Sherry AD, Wu Y. The importance of water exchange rates in the design of responsive agents for MRI. Curr Opin Chem Biol. 2013;17(2):167–174. doi:10.1016/j.cbpa.2012.12.012
28. Zhou Z, Lu ZR. Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(1):1–18. doi:10.1002/wnan.1198
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.