Back to Journals » Infection and Drug Resistance » Volume 12
Simvastatin improves the eradication rate of Helicobacter pylori: upper Egypt experience
Authors Hassan AM, Shawky MAEG , Mohammed AQ, Haridy MA , Eid KAEA
Received 21 January 2019
Accepted for publication 28 March 2019
Published 5 June 2019 Volume 2019:12 Pages 1529—1534
DOI https://doi.org/10.2147/IDR.S202346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Amro Metwaly Hassan, Muhammad Abd El-Gawad Shawky, Ahmed Qasem Mohammed, Mustafa Ahmed Haridy, Khaled Abd-El-Azeem Eid
Hepatology, Gastroenterology and Infectious Diseases Department, Faculty of Medicine, Al-Azhar University, Assiut, Egypt
Background: Helicobacter pylori infection is one of the most prevalent chronic bacterial human infections worldwide. Helicobacter pylori colonizes the gastric mucosa and causes persistent gastritis that may progress to gastric cancer. Increased resistance of H. pylori presents a major problem in most countries. Statins, including simvastatin, which are currently used to treat hypercholesterolemia, appear to have potential synergistic role to antibiotics. This study aimed to assess the value of adding simvastatin as adjuvant to standard triple therapy in patients infected with H. pylori.
Methods: This study was conducted on 100 patients diagnosed with H. pylori by the presence of antigen in stools. All patients were randomly subjected either to the standard triple regimen (clarithromycin 500 mg bid + amoxicillin 1 g bid + omeprazole 20 mg bid) (group 1, N=50) or to the standard triple regimen plus simvastatin (clarithromycin 500 mg bid + amoxicillin 1 g bid + omeprazole 20 mg bid + simvastatin 20 mg bid) (group 2, N=50). Both groups were treated for 14 days and eradication of H. pylori was assessed by a stool antigen test 4 weeks after therapy.
Results: Eradication of H. pylori infection was significantly higher in patients treated with the standard triple therapy plus simvastatin (n=41, 82%) than in patients treated with the standard triple therapy (n=31, 62%) (P<0.022).
Conclusion: Simvastatin significantly improves the H. pylori eradication rate.
Keywords: eradication of H. Pylori, simvastatin, standard triple therapy, clarithromycin resistance
Introduction
Helicobacter pylori is a Gram-negative microaerophilic spiral bacillus and one of the most prevalent chronic bacterial human infections worldwide. About 4.4 billion individuals worldwide are estimated to have H. pylori infection.1
Helicobacter pylori colonizes the gastric mucosa, leading to gastritis, peptic ulcer disease,2,3 gastric adenocarcinoma,4,5 and type B low-grade mucosal-associated lymphoma.6 Also, many extra-digestive diseases are associated with H. pylori infection, such as demyelinating neuropathies, ischemic heart disease, and chronic urticaria.7–9 Treating H. Pylori markedly decreases the risk of non-cardia gastric adenocarcinoma so there is a will all over the world to eradicate H. Pylori to decrease the risk of gastric adenocarcinoma and other several gastrointestinal complications.4,5
The standard triple therapy which is used for eradication of H. pylori combines a proton pump inhibitor (PPI), clarithromycin, and either amoxicillin or metronidazole.10
Antimicrobial resistance has increased in many countries as a consequence; the standard triple therapy eradication rate is less than 80%.11,12
In Egypt, the standard triple therapy eradication rates show regional variation, ranging between 56.25% and 88.2%; therefore, because of the decreasing eradication rates of this regimen, an alternative regimen, or adding other drugs to this regimen, has been used to augment its efficacy.13,14
Statins are antihyperlipidemic agents that inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, thus diminishing cholesterol biosynthesis.15 Statins also have potential direct antibacterial activity, synergistic activity with antibiotics, and ability to stimulate human immune system.16
Helicobacter pylori has lipid rafts which are composed of cholesterol, phospholipids, and sphingolipids for internalization of cells. Treating cells with cholesterol-lowering agents can dissociate the raft-associated proteins and lipids and render the structure non-functional.17–19
In an in vitro study, Liao et al20 showed that statins reduce the risk of H. pylori infection by decreasing the H. pylori burden in macrophages. Also, Lai et al21 showed that depletion of cholesterol has been demonstrated to attenuate CagA-induced pathogenesis.
Aim
The aim of this study was to assess value of adding simvastatin as an adjuvant to standard triple therapy in patients infected with H. pylori.
Patients and methods
Study design and participants
This study was a randomized controlled trial conducted on 100 patients infected with H. pylori attending the outpatient clinics of Hepatology, Gastroenterology and Infectious diseases Department, Al-Azhar-Assiut University Hospital, Egypt, from December 2017 to June 2018.
Approval was obtained from the Al-Azhar Assiut Faculty of Medicine ethical committee before the start of the study, and informed written consent was signed by every patient before enrollment in the study, in accordance with the World Medical Association Declaration of Helsinki, as revised in 2000, Edinburgh, UK.
Inclusion criteria
Patients above the age of 18 years with upper gastrointestinal symptoms related to H. pylori infection were included in the study. Patients infected with H. pylori were diagnosed by a stool antigen test (SAT) (One Step® H. pylori Antigen Test Device; Abon Biopharm, Hangzhou, China).
Exclusion criteria
Patients aged less than 18 years, who had undergone prior gastric surgery or H. pylori eradication therapy, who had a recent intake of antibiotics, PPI, histamine (H2) receptor blockers, non-steroidal anti-inflammatory drugs within the past month, known allergy to any of the antibiotics used in the study, gastrointestinal malignancy, or active upper gastrointestinal bleeding, and pregnant and lactating women were excluded from study.
Investigatory work-up
Eligible patients were randomized and divided into two groups. Group 1 comprised 50 patients who were treated with the standard triple therapy clarithromycin (Klacid®; Abbott Laboratories, Cairo, Egypt) 500 mg bid, amoxicillin (Amoxil®; GlaxoSmithKline, Cairo, Egypt) 1,000 mg bid, and omeprazole (Pepzol®; Hikma Pharmaceuticals, Giza, Egypt) 20 mg for 14 days. Group 2 comprised 50 patients treated with the standard triple therapy plus simvastatin (Zocor®; Global Napi, Giza, Egypt) 20 mg bid, prescribed for 14 days.
All patients were assessed by taking a full history and conducting a clinical examination, with special stress on upper gastrointestinal symptoms, including epigastric pain, heartburn, and vomiting. Body mass index (BMI) was calculated for each patient. All patients were assessed by the following laboratory and imaging tests after fasting since midnight: complete blood count, renal function, liver function (aspartate transferase [AST], alanine transferase [ALT], serum bilirubin, international normalized ratio [INR], total protein, and albumin), fasting blood sugar (FBS), lipid profile including cholesterol and triglycerides, erythrocyte sedimentation rate (ESR), and pelvi-abdominal ultrasonography.
The eradication of H. pylori was assessed 4 weeks after end of treatment by the SAT (One Step H. pylori Antigen Test Device). During this period, patients were instructed not to use PPIs, H2 blockers, or antibiotics.
Statistical analysis
Statistical analysis was carried out using SPSS version 22 for Windows 10 (IBM Corp., Armonk, NY, USA). Data are expressed as mean ± SD, frequency and percentage. The Student’s t-test was used to compare the results of continuous variables between groups and the chi-squared test for categorical variables. P-values were considered significant if <0.05.
Results
The mean age of patients enrolled in the study was 35.5±12.9 years, 29% of patients were male and 71% were female, and no significant differences were found between the two studied groups.
No significant differences were found between the two groups for mean BMI, gender or symptoms (P>0.05 for each). Patients infected with H. pylori presented with epigastric pain in 53%, bloating in 34%, heartburn in 22%, vomiting in 22%, early satiety in 20%, and urticaria in 5%.
Table 1 shows that there were no significant differences between the two groups concerning laboratory parameters, including complete blood count, renal function, liver function tests (AST, ALT, serum bilirubin, INR, total protein, and albumin), fasting blood sugar, or lipid profile (P>0.05 for each).
 | Table 1 Baseline laboratory data for patients in both groups |
With regard to ultrasonography, about 52% of patients had normal ultrasonographic findings and 48% of patients had fatty liver. No significant differences were found between the two groups (P>0.05 for each) (Table 2).
 | Table 2 Ultrasonographic findings of the studied patients |
After 4 weeks of treatment, patients' response was assessed by SAT. The results showed that the eradication rate of H. pylori infection was significantly higher in patients treated with the standard triple therapy plus simvastatin (n=41, 82%) than in patients treated with the standard triple therapy (n=31, 62%) (p<0.022) (Table 3).
 | Table 3 Helicobacter pylori stool antigen test 4 weeks after treatment in the study groups |
In this study, all patients were compliant with treatment (100%) and adherent to treatment, and the number needed to treat/benefit ratio was 5 (95% CI 2.7–35.2) (P<0.05).
Minor adverse effects were reported in the form of headache, epigastric pain, nausea, and diarrhea, with no significant difference between the two groups (Table 4).
 | Table 4 Side effects of treatment in both groups |
Discussion
As infection with H. pylori may be associated with severe gastric or duodenal ulcers, gastric lymphoma or adenocarcinoma, and many other extra-gastrointestinal diseases, eradication of this microorganism from the human body is a goal with worldwide agreement.4,5
Although many regimens have been used to treat H. pylori, triple therapy is still being used as a first line therapy in many parts of the world,22 but unfortunately the standard triple therapy eradication rate is less than 80%10 owing to antimicrobial resistance.11 In areas with high clarithromycin resistance, the triple therapy is no longer preferred as the standard first line therapy,23 as any regimen with an eradication rate lower than 80% is not acceptable as a first line eradication therapy.24
In Egypt, the standard triple therapy eradication rates show regional variation, ranging between 56.25% and 88.2%.13,14 Thus, because of the decreasing efficacy of this standard triple therapy regimen, an alternative regimen or adding another drug to this regimen may be used to augment its efficacy.
Simvastatin has potential direct antibacterial activity, synergistic activity with antibiotics, and the ability to stimulate the human immune system.14 Simvastatin also promotes lysosomal fusion, resulting in degradation of sequestered bacteria, and in turn attenuates interleukin-1β production.20 In addition, Lai et al21 showed that depletion of cholesterol can attenuate CagA-induced pathogenesis. On this basis, the present study was conducted to investigate the value of adding simvastatin as an adjuvant to standard triple therapy in patients infected with H. pylori.
Eradication of H. pylori infection was significantly higher in patients treated with the standard triple therapy plus simvastatin (n=41, 82%) than in patients treated with the standard triple therapy (n=31, 62%) (P<0.022), proving that simvastatin has an additive adjuvant effect on eradication of H. pylori (Figure 1). This result agrees with the study by Nseir et al,25 in which the addition of statin to the standard triple therapy increased the eradication rate of H. pylori infection from 72% to 91%.
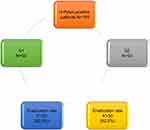 | Figure 1 Flow chart of the studied patients. |
Minor adverse effects were reported in the simvastatin-based regimen, in the form of headache in 3%, epigastric pain in 14%, nausea in 8%, and diarrhea in 10%, with no significant differences between the two study groups. This makes simvastatin a good adjuvant drug when added to the standard triple therapy.
Our study is a single-center study and has some limitations, such as the small number of patients. Further multicenter randomized clinical trials on larger numbers of patients in different locations are needed to show the efficacy and safety of simvastatin as an adjuvant treatment in the eradication of H. pylori.
Conclusion
Adding simvastatin 20 mg twice daily to the standard triple therapy improved the eradication rate of H. pylori from 62% to 82%.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hooi KJY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori Infection: systematicReview and meta-analysis. Gastroenterology. 2017;153:420–429. doi:10.1053/j.gastro.2017.04.022
2. Ghotaslou R, Ebrahimzadeh Leylabadlo H, Mohammadzadeh Asl Y. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J Methodol. 2015;5(3):164–174. doi:10.5662/wjm.v5.i3.164
3. Smolka AJ, Backert S. How Helicobacter pylori infection controls gastric acid secretion. J Gastroenterol. 2012;47:609–618. doi:10.1007/s00535-012-0592-1
4. Doorakkers E, Lagergren J, Engstrand L, Brusselaers N. Helicobacter pylori eradication treatment and the risk of gastric adenocarcinoma in a western population. Gut. 2018;67(12):2092–2096. doi:10.1136/gutjnl-2017-315363
5. Talebi Bezmin Abadi A, Yamaoka Y. Helicobacter pylori therapy and clinical perspective. J Glob Antimicrob Resist. 2018;14:111–117. doi:10.1016/j.jgar.2018.03.005
6. Asaka M, Dragosics BA. Helicobacter pylori and gastric malignancies. Helicobacter. 2004;9(Suppl 1):35–41. doi:10.1111/j.1083-4389.2004.00252.x
7. Kountouras J, Zavos C, Deretzi G, et al. Helicobacter pylori may play an important role in both axonal type Guillain – barre syndrome and acute inflammatory demyelinating polyradiculoneuropathy. Clin Neurol Neurosurg. 2011;113:520. doi:10.1016/j.clineuro.2011.01.004
8. Jafarzadeh A, Nemati M, Tahmasbi M, et al. The association between infection burden in Iranian patients with acute myocardial infarction and unstable angina. Acta Med Indones. 2011;43:105–111.
9. Ben Mahmoud L, Ghozzi H, Hakim A, Sahnoun Z, Zeghal K. Helicobacter pylori associated with chronic urticaria. J Infect Dev Ctries. 2011;5:596–598. doi:10.3855/jidc.1363
10. Malfertheiner P, Megraud F, O‘Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66(1):6–30. doi:10.1136/gutjnl-2016-312288
11. Huang AH, Sheu BS, Yang HB, et al. Antimicrobial resistance of H. pylori to the outcome of one-week lansoprazole based triple therapy. J Formosan Med Assoc. 2000;90:704–709.
12. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–331. doi:10.1038/ncpgasthep1138
13. Shehata MAH, Talaat R, Soliman S, et al. Randomized controlled study of a novel triple nitazoxanide (NTZ)-containing therapeutic regimen versus the traditional regimen for eradication of Helicobacter pylori infection. Helicobacter. 2017;22:1–5. doi:10.1111/hel.12395
14. Salamah AM, Gad M, Deghady A, et al. Effectiveness of 14-days course of clarithromycin-based triple therapy as first line therapy for h.pylori infection in egyptian elderly patients. EJGG. 2015;2(1):19–26. doi:10.12816/0030878
15. Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi:10.1016/S0140-6736(07)60716-8
16. Hennessy E, Adams C, Reen FJ, et al. Is there potential for repurposing statins as novel antimicrobials? Antimicrob Agents Chemother. 2016;60:5111–5121. doi:10.1128/AAC.00192-16
17. Wunder C, Churin Y, Winau F, et al. Cholesterol glucosylation promotes immune evasion by Helicobacter pylori. Nat Med. 2006;12:1030–1038. doi:10.1038/nm1480
18. Murata-Kamiya N, Kikuchi K, Hayashi T, Higashi H, Hatakeyama M. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe. 2010;7:399–411. doi:10.1016/j.chom.2010.04.005
19. Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi:10.1038/42408
20. Liao W-C, Huang M-Z, Wang ML, et al. Statin decreases Helicobacter pylori burden in macrophages by promoting autophagy. Front Cell Infect Microbiol. 2017;6:203. doi:10.3389/fcimb.2016.00203
21. Lai CH, Chang YC, Du SY, et al. Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA-induced responses in AGS cells. Infect Immun. 2008;76:3293–3303. doi:10.1128/IAI.00365-08
22. Bang CS, Baik GH. Attempts to enhance the eradication rate of Helicobacter pylori infection. World J Gastroenterol. 2014;20(18):5252–5262. doi:10.3748/wjg.v20.i18.5252
23. Pellicano R, Ribaldone DG, Fagoonee S, et al. A 2016 panorama of Helicobacter pylori infection: key messages for clinicians. Panminerva Med. 2016;58(4):304–317.
24. Kuo C-H, Kuo F-C, Hu H-M, et al. The optimal first-line therapy of Helicobacter pylori infection in year 2012. Gastroenterol Res Pract. 2012;2012:1–8. doi:10.1155/2012/168361
25. Nseir W, Diab H, Mahamid M, et al. simvastatin as adjuvant therapy improves significantly the Helicobacter pylori eradication rate -a placebo-controlled study. Aliment Pharmacol Ther. 2012;36:231–238. doi:10.1111/j.1365-2036.2012.05161.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
