Back to Journals » Cancer Management and Research » Volume 11
Silibinin restores the sensitivity of cisplatin and taxol in A2780-resistant cell and reduces drug-induced hepatotoxicity
Authors Yang Z, Pan Q, Zhang D, Chen JQ, Qiu Y, Chen X, Zheng F, Lin F
Received 12 January 2019
Accepted for publication 6 June 2019
Published 26 July 2019 Volume 2019:11 Pages 7111—7122
DOI https://doi.org/10.2147/CMAR.S201341
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Zhichun Yang,*,1 Qionghui Pan,*,2 Dingfang Zhang,3 Jianqiang Chen,3 Yinda Qiu,3 Xiaojing Chen,3 Feiyun Zheng,1 Feng Lin1
1Department of Obstetrics and Gynecology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325000, People’s Republic of China; 2Department of Gynecology, Wenzhou People’s Hospital, Wenzhou 325027, Zhejiang, People’s Republic of China; 3School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou 325035, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Purpose: Ovarian cancer is the most lethal cancer among all gynaecological malignancies. The combination theraputics of cisplatin and taxol is widely used in clinicals for ovarian cancer treatment. However, long-term use of cisplatin and taxol induces strong tolerance and hepatotoxicity. Since silibinin is a commonly used anti-hepatotoxic drug in Europe and Asia, the aim of this study was to determine whether silibinin could restore the sensitivity of combination use of cisplatin and taxol in drug-resistant human ovarian cancer cells and reduce drug-induced hepatotoxicity.
Patients and methods: Normal hepatocyte LO2 cells and A2780/DDP cells were treated with silibinin, cisplatin, taxol, cisplatin and taxol plus silibinin for 48 h. Cell viability was determined by MTT and long-term proliferation assay, while apoptosis and cell cycle progression were assessed by flow cytometric analysis. DNA damage was evluated by immunofluorescence assays. The metastatic activity of A2780/DDP was determined by cell adhesion assay.
Results: The addition of silibinin on cisplatin and/or toxal could sensitize the antitumor activity of cisplatin and toxal on A2780/DDP cells, supress cell-matrix adhesion of A2780/DDP, inhibit the cell proliferation, result in A2780/DDP cells apoptosis. In addition, silibinin could effectively reduce cisplatin and/or toxal-induced hepatotoxicity by protecting DNA from damage and restoring the potential of cell proliferation in cisplatin and/or toxal-treated LO2 cells.
Conclusion: Our results suggest that silibinin could restore the sensitivity of cisplatin and taxol in drug-resistant human ovarian cancer cells and reduce durg-induced hepatotoxicity in cell level.
Keywords: silibinin, cisplatin and/or taxol, drug resistance, human ovarian cancer, hepatotoxicity
Introduction
Ovarian cancer is one of the most lethal malignancies in women and is responsible for 5% of all the cancer deaths in women.1 There has been a steady decline in the incidence of ovarian cancer since the mid-1970s.1 However, ovarian cancer is difficult to detect and many patients are still diagnosed in advanced stages (III-IV) of the disease (60%) which significantly decrease their survival rates (≈46%).1,2 Unlike other epithelial cancer cells, ovarian cancer cells can disseminate directly to the peritoneum cavity due to the absence of anatomical barriers.3 In addition, recent data indicates that the majority of patients will relapse despite a satisfactory response to the initial treatment.4,5
Cisplatin is widely used in clinical ovarian cancer treatment. However, long-term use of cisplatin could induce strong tolerance with high metastasis. Drug resistance and metastasis are the main causes of treatment failure in ovarian cancer patients in clinic.6,7 The ovarian cancer cells have drug resistance and metastasis are due either to selection of more aggressive cells or to an increase in metastatic potential following chemotherapeutic insults.8 Thus, there is an urgent need for novel treatment strategies to overcome drug resistance and tumor metastasis.
Combination of cisplatin-taxol is the first-line treatment in ovarian cancer.9 Despite encouraging clinical effects, the side effects of the combination therapy, such as the fact that the combination of cisplatin-taxol therapy cannot overcome or reduce the resistance in ovarian cancer cells,10 cannot be ignored. Moreover, taxol may have a cross tolerance with cisplatin, as well as strong hepatotoxicity. Consequently, the efficacy of taxol plus cisplatin chemotherapy schedule used for ovarian cancer is limited after the treatment for a period of time. Hence, the combination of other drugs has been suggested to deal with drug tolerance and induced hepatotoxicity.
Silibinin, a plant extraction, has been extensively used for its hepato-protective effects in Europe and Asia.11 In the last decade, numerous studies have reported that silibinin has anticancer efficacy in vitro and in vivo.12–15 Silibinin has been demonstrated to be an efficacious chemopreventive and chemotherapeutic agent,16,17 without any toxicity or adverse effects.18 Therefore, it could have potential clinical application in combination with chemotherapy.
However, whether silibinin could sensitize ciaplatin and/or taxol antitumor activity on A2780/cisplatin-resistant (A2780/DDP) human ovarian carcinoma cells and reduce their induced hepatotoxicity has never been reported. In this study, we treated A2780/DDP cells and normal hepatocyte cells (LO2) with silibinin, cisplatin, and taxol plus silibinin for 48 h, and then assessed the effect of silibinin restores the sensitivity of cisplatin and taxol in drug-resistant (A2780/DDP) human ovarian cancer cells together with reducing their induced-hepatotoxicity.
Materials and methods
Cell culture
LO2 and A2780/DDP cells were obtained from the Cell Resource Center of Peking Union Medical College. Cells were cultured at 37°C under 5% CO2 in DMEM (Sigma, St. Louis, MO), supplemented with 10% fetal calf serum (PPA-GE, Marlborough, MA) and 100 U/mL penicillin and streptomycin (HyClone-GE, Marlborough, MA).
Drugs administrations
Silibinin, cisplatin and taxol were purchased from Sigma and were dissolved in dimethyl sulfoxide (DMSO) for storage and further diluted to final concentrations. The control groups were administrated with the same amount of DMSO. For all experiments, the silibinin, cisplatin, and cisplatin plus silibinin, taxol, and taxol plus silibinin were added to the cells simultaneously for 48 h with the full culture medium.
Cell viability assay
The cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells (4,000 cells/well in 96-well plates) were incubated at 37°C for 48 h and then MTT was added with a final concentration of 0.5 mg/mL per well for another 4 h. The reaction product formazan was dissolved in DMSO after discarding the culture medium. The cell viability was determined by reading the absorbance at 570 nm by a spectrophotometer (DTX880, Beckman Coulter, CA, USA). Results are presented as the mean of three measurements ± standard deviation (n=3).
Long-term proliferation studies
Cells (5.0×105) were seeded in a 10 cm2 dish. After 6 h, the drugs were co-cultured. The medium was changed every three days until the 6th days was passed. During the proliferation process, the cell numbers of each dish was counted using a cytometer (Countstar, China), then the cells (5.0×105) were passed into a 10 cm2 dish until the cells were less than 5.0×105. 2PDs=M/N (PDs: population doublings, M: number of every count cells, N: number of cells implanted).
Cell cycle distribution analysis
Cells were cultured in the absence or presence of 0, 25, 50 and 100 μM of silibinin for 48 h, trypsinized, washed and stained with propidium iodide before cell cycle distribution was assessed on a flow cytometer (BD FACSCalibur, BD Biosciences).
Annexin V/PI apoptosis assay
Cells were seeded in a 6 cm2 dish at a density of 3.0×105 cells per dish and incubated at 37 °C for 6 h until cells were attached to the dish. Silibinin with a final concentration of 0, 25, 50 and 100 μM were then added into medium. After 48 h, cells were harvested for Anexin V/PI apoptosis assay. The assay was performed following the protocol provided by the Annexin V/PI apoptosis Kit (Sigma) and was assessed on a flow cytometer (BD FACSCalibur, BD Biosciences).
Immunofluorescence (IF) assay of DNA damage
Immunofluorescence (IF) assay was performed as previously described.19 Briefly, cells on a coverslip were fixed with 4% paraformaldehyde, permeabilized in 0.5% Triton X-100 (in 1× PBS). Cells were incubated overnight at 4 °C with primary antibodies against 53BP1 (CST), washed three times, and incubated with secondary antibodies (DyLight 488-conjugated anti-mouse). The coverslip was washed with PBS three times. The cells were washed and mounted with DAPI. Fluorescence was detected and imaged using a Nikon Ti microscope. To quantify the damage degree of DNA, over 700 cells from each group were randomly chosen from three independent experiments.
Cell adhesion assay
The 96-well plate was coated with 2.5 μg/ml human fibronectin in PBS (Millipore, CA) at room temperature. The cells were then seeded in serum-free medium at the density of 4×104 cells/well, and cultured at 37 °C under 5% CO2 for 1 h. The medium was then removed gently and cells were rinsed three times with 10% formalin. The attached cells were stained with crystal violet for 5 min at room temperature and color intensity was determined by absorbance at 560 nm. A relative number of cells attaching to extracellular matrix was calculated using the following equation: mean OD of treated cells/mean OD of control cells. Cells treated with vehicle (0.1% DMSO) were used as a control.
Statistical analysis
The Student’s two-tailed unpaired t-test was used to determine statistical significance and the resulting p-values are indicated in figures (*p<0.05; **p<0.01; ***p<0.001).
Results
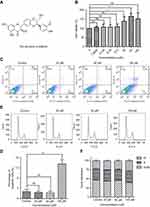
Silibinin has no side effects for normal hepatocyte LO2 cells
The physiological effect of silibinin (Figure 1A) to normal hepatocyte LO2 cells was firstly evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The results show that silibinin has no toxic effect on hepatic cells but promotes cell proliferation in a concentration-dependent manner in the range of 0–50 µM (Figure 1B). The data are well correlated with the data obtained from flow cytometry experiments. Flow cytometry results shown that silibinin treatment neither causes LO2 cells apoptosis (Figure 1C and D) nor cell arrest (Figure 1E and F). Based on the analysis of obtained data, the conclusion that silibinin has no side effects for hepatic cells is proposed. More importantly, the results suggest that the concentration of 50 μM of silibinin is the optimum concentration for the following experiments in this study.
Silibinin restores the sensitivity of cisplatin and taxol on A2780/cisplatin-resistant (A2780/DDP) human ovarian carcinoma cells
A2780/DDP cells were treated with different concentrations of cisplatin and taxol for 48 h and cell viability was measured by the MTT assay (the IC50 values are 113.78 μM ±4.98 μM and 48.51 μM ±0.45 μM, respectively) (Figure 2A and S1A). MTT assay was carried out to further evaluate the impact of the silibinin addition to the treatment in the whole concentration-response of cisplatin and taxol with/without silibinin (Figure S1B and C). The results showed that silibinin could restore the sensitivity of cisplatin and/or taxol to A2780/DDP cells in the whole concentration, with lower IC50 values (35.32 μM ±1.24 μM and 12.51 μM ±0.89 μM, respectively) compared to cisplatin and/or taxol alone (Figure 2A). Moreover, in order to manifest significant sensibilization of silibinin on cisplatin and/or taxol, A2780/DDP cells were treated with silibinin (50 μM), cisplatin (115 μM) and/or taxol (50 μM), silibinin (50 μM) plus cisplatin (115 μM) and/or silibinin (50 μM) plus taxol (50 μM) for 48 h, respectively, the cell viabilities were determined. As shown in Figure 2B, silibinin had little anti-tumor effects on A2780/DDP cells, but silibinin could markedly restore the sensitivity of cisplatin and/or taxol to A2780/DDP cells. This conclusion was further supported by flow cytometry assay, which shown that cells treated with silibinin (50 μM) plus cisplatin (115 μM) and/or taxol (50 μM) would induce more apoptotic cells than cells treated with taxol (50 μM) alone (Figure 3A and B). And the sensitizing effect of silibinin on cisplatin and/or taxol did not change over time (Figure S2A and B).
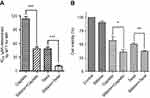
The combination of silibinin and cisplatin or taxol decreases cell-matrix adhesion and inhibits A2780/DDP cells proliferation
Cell resistance is always accompanied by metastasis. To explore whether silibinin could decrease cell-matrix adhesion of A2780/DDP, cell adhesion assay was performed. To test this, cell adhesion assay that determines the adhesion between cell and attached matrix was also performed in A2780/DDP cells. As shown in Figure 4A, the result displayed a significant decrease in cell adhesion to the matrix after treatment with silibinin (50 μM) plus cisplatin (115 μM) and/or taxol (50 μM) than cells treated with cisplatin (115 μM) and/or taxol (50 μM) alone. All results are well consistent with the results of the long-term cell proliferation assay (Figure 4B).
Silibinin reduce cisplatin and/or taxol-induced hepatotoxicity and restore the potential of hepatocyte cell proliferation
Firstly, the cytotoxic effect of cisplatin and taxol on normal hepatocyte LO2 cells were assessed by MTT assay with the half inhibitory concentration (IC50) values of taxol on LO2 are 23.81 μM ±0.68 μM, 3.65 μM ±0.45 μM, respectively (Figure S3A). And then, the detoxication effects of silibinin to cisplatin and/or taxol-induced hepatotoxicity was determined in the whole concentrations (Figure S3B and C). The results suggested that silibinin could effectively reduce cisplatin and/or toxal-induced hepatotoxicity in the whole concentration with higher IC50 values (43.50 μM ±1.68 μM and 8.34 μM ±0.46 μM, respectively) compared to cisplatin and/or taxol alone. In addition, LO2 cells were treated with silibinin (50 μM), cisplatin (24 μM), taxol (4 μM) and silibinin (50 μM) plus cisplatin (24 μM) and/or taxol (4 μM) for 48 h, respectively. The results shown that LO2 cell vitality was strongly enhanced by silibinin (131.53%) and sharply inhibited by cisplatin (47.83%) and taxol (51.33%), in particular, silibinin treatment significantly reversed the cisplatin and/or taxol-induced cell death, and could rescue the cell viability to ~75% (Figure 5A). In addition, we found silibinin could restore the potential of cisplatin and/or taxol-treated hepatocyte cell proliferation (Figure 5B). To further confirm the hepatocyte cell protective effects of silibinin, LO2 cells were further detected under Annexin V/PI apoptosis assay after treatment (Figure 5C). The results shown that cisplatin and/or taxol treatment for 48 h led to massive cell apoptosis (59.8% and 65.52%) compared with the control group (7.58%). However, cisplatin and/or taxol combined with silibinin could rescue cell apoptosis compared to only cisplatin and/or taxol treatment (31.4% and 41.09%) (Figure 5D). This result is well consistent with the results of MTT assay and long term cell proliferation assay, which strongly supporting the conclusion that silibinin could reduce cisplatin and/or taxol-induced hepatotoxicity.
Silibinin protects the DNA of normal hepatocyte cell against damage
It is known that apoptosis is induced when the degree of DNA lesions in a cell exceeds the capacity of the cell to repair the lesions.20 To explore the mechanism that silibinin could reduce cisplatin and/or taxol-induced hepatotoxicity, we carried out immunofluorescence assay (IF), using an antibody to 53BP1. In spite of another possibly minor role for 53BP1 other than in DDR,21,22 53BP1 has been widely used as a marker for double-strands breaks (DSBs). We treated LO2 cells with silibinin (50 μM), cisplatin (24 μM), taxol (4 μM) and silibinin (50 μM) plus cisplatin (24 μM) and/or taxol (4 μM) for 24 h, respectively. As expected, silibinin treatment would not increase the number of 53BP1 foci. And the number of 53BP1 foci increased to an average of ~28 and ~38 foci per nucleus after cisplatin and taxol treatment. However, the values (~7 and ~15 foci per nucleus) dramatically decreased in silibinin plus cisplatin and/or taxol treated cells (Figure 6A and B). Taken the above results together, our data support the conclusion that silibinin could reduce cisplatin and/or taxol-induced hepatotoxicity by protecting hepatocyte cell DNA from damage.
Discussion
Ovarian cancer is one of the leading causes of mortality in women over the world.23 Cisplatin, sometime combine with taxol, is widely used in the treatment of advanced ovarian cancer.24 However, long-term use of cisplatin with or without taxol would result in drug resistance and strong hepatotoxicity, of which extremely limit the efficiency and application of the drugs in the clinic. Therefore, the rationale of our study based on the notion that silibinin not only has the protective effect on the liver,25 but also enhance the efficacy of cisplatin and/or taxol on human ovarian cancer cells.26–29 We speculated that silibinin might restore the sensitivity of cisplatin and/or taxol on cisplatin-resistant human ovarian cancer (A2780/DDP) cells and reduce the drug-induced hepatotoxicity in the drug combination. Firstly, our findings suggest that silibinin at the concentration of 50 µM has the strongest protective effects for liver in the range from 0 µM to 100 µM (Figure 1). Therefore, the concentration (50 µM) was used in downstream assays. We observed that silibinin could restore the sensitivity of the antitumor activity of cisplatin and/or taxol on A2780/DDP cells (Figures 2B and 3).
In addition to drug resistance, metastasis is another biggest impediments and problems in the treatment of human cancer, including ovarian cancer, which might result in the failure of treatment.30,31 In general, metastasis concomitant with drug resistance has been found in patient samples.6,7 Hence, one strategy that would reduce or reverse drug resistance might inhibit the metastasis in chemotherapy. Indeed, silibinin could decrease the cell-matrix adhesion (Figure 4A) and inhibit proliferation of A2780/DDP cells (Figure 4B).
Hepatotoxicity is a very common side effect of chemotherapy drugs (eg, cisplatin and taxol). It might seriously affect the quality of living life of patients or even leads to death.32 Increased production of reactive oxygen species (ROS) is one of the most recognized mechanisms that underlies the hepatotoxicity.33 In this regard, the strategies of protecting the liver from cisplatin and/or taxol-induced hepatotoxicity would include scavenge ROS and/or enhance DNA repairing ability. Silibinin could take effect on both. Firstly, silibinin is a scavenger of intracellular ROS.34 It’s significantly reduced drug-induced hepatocyte line LO2 cell death and even rescued the LO2 cell proliferative ability (Figure 5). The further study of mechanism indicated that silibinin protects DNA from cisplatin and/or taxol-induced toxic damage might through enhancing the DNA repairing ability (Figure 6). These results indicated that silibinin could reduce cisplatin and/or taxol-induced hepatotoxicity by protecting DNA from cisplatin and/or taxol-induced toxic damage.
Conclusion
Our study demonstrated that silibinin could restore the sensitivity the antitumor activity of cisplatin and/or taxol on cisplatin-resistant human ovarian carcinoma (A2780/DDP) cells and reduce cisplatin and/or taxol-induced hepatotoxicity in cell level. If these effects are confirmed in vivo, silibinin in combination with cisplatin and/or taxol may be a beneficial chemotherapeutic strategy, especially in patients with tumors refractory to cisplatin.
Acknowledgments
This work was supported by the Natural Science Foundation of China Project 81803781 and Zhejiang Provincial Natural Science Foundation Project LQ18H280007.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Matulonis UA. Highlights in ovarian cancer from the 2017 American Society of clinical oncology annual meeting: commentary. Clin Adv Hematol Oncol. 2017;7(8):13–17.
2. Goodman MT, Howe HL, Tung KH, et al. Incidence of ovarian cancer by race and ethnicity in the United States, 1992–1997. Cancer. 2003;97(10 Suppl):2676–2685. doi:10.1002/cncr.11349
3. Sehouli J, Senyuva F, Fotopoulou C, et al. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J Surg Oncol. 2009;99(7):424–427. doi:10.1002/jso.21288
4. Luvero D, Milani A, Ledermann JA. Treatment options in recurrent ovarian cancer: latest evidence and clinical potential. Ther Adv Med Oncol. 2014;6(5):229–239. doi:10.1177/1758834014544121
5. Bagnato A, Rosano L. Understanding and overcoming chemoresistance in ovarian cancer: emerging role of the endothelin axis. Curr Oncol. 2012;19(1):36–38. doi:10.3747/co.19.895
6. Weinstein RS, Jakate SM, Dominguez JM, et al. Relationship of the expression of the multidrug resistance gene product (P-glycoprotein) in human colon carcinoma to local tumor aggressiveness and lymph node metastasis. Cancer Res. 1991;51(10):2720–2726.
7. Osmak M, Niksic D, Brozovic A, Ristov AA, Vrhovec I, Skrk J. Drug resstant tumor cells have increased levels of tumor markers for invasion and metastasis. Anticancer Res. 1999;19(4B):3193–3197.
8. De Larco JE, Wuertz BR, Manivel JC, et al. Progression and enhancement of metastatic potential after exposure of tumor cells to chemotherapeutic agents. Cancer Res. 2001;61(7):2857–2861.
9. Al-Farsi A, Ellis PM. Treatment paradigms for patients with metastatic non-small cell lung cancer, squamous lung cancer: first, second, and third-line. Front Oncol. 2014;4:157. doi:10.3389/fonc.2014.00157
10. Park BK, Kitteringham NR, Maggs JL, et al. The role of metabolic activation in drug-induced hepatotoxicity. Annu Rev Pharmacol Toxicol. 2005;45:177–202. doi:10.1146/annurev.pharmtox.45.120403.100058
11. Ferenci P, Dragosics B, Dittrich H, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9(1):105–113.
12. Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010;29(3):447–463. doi:10.1007/s10555-010-9237-0
13. Cho HJ, Suh DS, Moon SH, et al. Silibinin inhibits tumor growth through downregulation of extracellular signal-regulated kinase and Akt in vitro and in vivo in human ovarian cancer cells. J Agric Food Chem. 2013;61(17):4089–4096. doi:10.1021/jf400192v
14. Momeny M, Ghasemi R, Valenti G, et al. Effects of silibinin on growth and invasive properties of human ovarian carcinoma cells through suppression of heregulin/HER3 pathway. Tumour Biol. 2016;37(3):3913–3923. doi:10.1007/s13277-015-4220-6
15. Giacomelli S, Gallo D, Apollonio P, et al. Silybin and its bioavailable phospholipid complex (IdB 1016) potentiate in vitro and in vivo the activity of cisplatin. Life Sci. 2002;70(12):1447–1459.
16. Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41(13):1969–1979. doi:10.1016/j.ejca.2005.03.033
17. Sharma G, Singh RP, Chan DC, et al. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23(3B):2649–2655.
18. Flaig TW, Gustafson DL, Su LJ, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25(2):139–146. doi:10.1007/s10637-006-9019-2
19. Chen Y, Deng Z, Jiang S, et al. Human cells lacking coilin and Cajal bodies are proficient in telomerase assembly, trafficking and telomere maintenance. Nucleic Acids Res. 2015;43(1):385–395. doi:10.1093/nar/gku1277
20. Li D, Huang Q, Lu M, et al. The organophosphate insecticide chlorpyrifos confers its genotoxic effects by inducing DNA damage and cell apoptosis. Chemosphere. 2015;135:387–393. doi:10.1016/j.chemosphere.2015.05.024
21. Harrigan JA, Belotserkovskaya R, Coates J, et al. Replication stress induces 53BP1-containing OPT domains in G1 cells. J Cell Biol. 2011;193(1):97–108. doi:10.1083/jcb.201011083
22. Lukas C, Savic V, Bekker-Jensen S, et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol. 2011;13(3):243–253. doi:10.1038/ncb2201
23. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
24. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
25. Bijak M, Synowiec E, Sitarek P, et al. Evaluation of the cytotoxicity and genotoxicity of flavonolignans in different cellular models. Nutrients. 2017;9(12):1356. doi:10.3390/nu9121356
26. Tyagi AK, Agarwal C, Chan DC, et al. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncol Rep. 2004;11(2):493–499.
27. Scambia G, De Vincenzo R, Ranelletti FO, et al. Antiproliferative effect of silybin on gynaecological malignancies: synergism with cisplatin and doxorubicin. Eur J Cancer. 1996;32A:877–882.
28. Fatima PA, Roghiyeh PA, Khodadad K, et al. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif Cell Nanomed B. 2018;46(7):1483–1487. doi:10.1080/21691401.2017.1374281
29. Zhou L, Liu P, Chen B, et al. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Res. 2008;28:1119–1128.
30. Nikolaou M, Pavlopoulou A, Georgakilas AG, et al. The challenge of drug resistance in cancer treatment: a current overview. Clin Exp Metastasis. 2018;35(4):309–318. doi:10.1007/s10585-018-9903-0
31. Robert J. Biology of cancer metastasis. Bull Cancer. 2013;168(4):670–691.
32. Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013;2013:815105. doi:10.1155/2013/815105
33. Lim SC, Choi JE, Kang HS, et al. Ursodeoxycholic acid switches oxaliplatin-induced necrosis to apoptosis by inhibiting reactive oxygen species production and activating p53-caspase 8 pathway in HepG2 hepatocellular carcinoma. Int J Cancer. 2010;126(7):1582–1595. doi:10.1002/ijc.24853
34. Lee B, Lee DG. Reactive oxygen species depletion by silibinin stimulates apoptosis-like death in escherichia coli. J Microbiol Biotechnol. 2017;27(12):2129–2140. doi:10.4014/jmb.1710.10029
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.