Back to Journals » Drug Design, Development and Therapy » Volume 13
Silibinin attenuates high-fat diet-induced renal fibrosis of diabetic nephropathy
Authors Liu K, Zhou S, Liu J, Wang Y, Zhu F, Liu M
Received 25 March 2019
Accepted for publication 8 July 2019
Published 29 August 2019 Volume 2019:13 Pages 3117—3126
DOI https://doi.org/10.2147/DDDT.S209981
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Kun Liu,1,* Shiju Zhou,1,* Jinyan Liu,1,2 Yingying Wang,1 Fengxian Zhu,1 Man Liu1
1Department of Nephrology, Jining No. 1 People’s Hospital, Jining 272000, Shandong, People’s Republic of China; 2Department of Nephrology, Jining Medical University, Jining 272000, Shandong, People’s Republic of China
Correspondence: Jinyan Liu
Department of Nephrology, Jining No. 1 People’s Hospital, No. 6 Jiankang Road, Jining 272000, Shandong, People’s Republic of China
Tel +86 0 537 225 3104
Email [email protected]
*These authors contributed equally to this work
Aim: Diabetic nephropathy (DN) is the leading cause of end-stage renal disease. Silibinin is a flavonoid compound which has medicinal value. Previous studies revealed that silibinin exhibited an anti-fibrotic effect. However, whether silibinin could attenuate high-fat diet (HFD)-induced renal fibrosis remains unclear. Therefore, this study aimed to explore the molecular mechanism by which silibinin regulated renal fibrosis induced by HFD.
Methods: In the present study, human renal glomerular endothelial cells (HRGECs) were treated with various concentrations of silibinin. Then, cell viability and apoptosis were measured by MTT assay and flow cytometry, respectively. In addition, HRGECs were exposed to 100 nM TGF-β1 for mimicking in vitro renal fibrosis. The expressions of collagen I, fibronectin, and α-SMA were detected by reverse transcription-quantitative polymerasechain reaction and Western blot. Protein levels of p-IκB and p-p65 were examined by Western blot; meanwhile, level of NF-κB was measured by immunofluorescence staining. Furthermore, HFD-induced mouse model of renal fibrosis was established. The mouse body weight, fasting glucose, kidney weight/body weight, microalbuminuria, kidney histopathology, and fibrotic area were measured to assess the severity of renal fibrosis.
Results: Low concentration of silibinin (≤50 μM) had no cytotoxicity, while high concentration of silibinin (≥75 μM) exhibited significant cytotoxicity. Additionally, TGF-β1 increased the expressions of collagen I, fibronectin, α-SMA, p-IκB, and p-p65 and decreased the level NF-κB, while these effects were notably reversed by 50 μM silibinin. Moreover, both 50 and 100 mg/kg silibinin greatly decreased HFD-induced the upregulation of kidney weight/body weight, microalbuminuria, and fibrotic area. 100 mg/kg silibinin markedly reduced collagen I, fibronectin, and p-p65 expressions in mice renal tissues.
Conclusion: Silibinin was able to attenuate renal fibrosis in vitro and in vivo via inhibition of NF-κB. These data suggested that silibinin may serve as a potential agent to alleviate the renal fibrosis of DN.
Keywords: silibinin, renal fibrosis, diabetic nephropathy, high-fat diet, TGF-β1, NF-κB pathway
Introduction
Diabetes mellitus (DM) is a chronic disease characterized by high blood glucose, which consists of two main forms insulin-dependent DM (type 1) and non-insulin-dependent DM (type 2).1 Over the past decades, the incidence of type 2 diabetes mellitus (T2DM) has significantly increased in the world, especially in developing country.2 The risk factors of T2DM include family history, lack of exercise, unhealthy diet, overweight, and obesity.3 In addition, high-fat diet (HFD) is associated with the disruption of intestinal barrier, immune response, and/or insulin resistance state, resulting in the increasing risk of T2DM.4 Therefore, it is necessary to focus on HFD-induced hyperglycemia.
Patients with T2DM are generally accompanied by various complications, such as cardiovascular diseases, diabetic neuropathy, nephropathy, and retinopathy.5 Diabetic nephropathy (DN) is a microvascular complication of DM, which is the leading cause of end-stage renal disease. Inadequate glucose control is the most common reason for the occurrence and development of DN.6 DN is charactered by podocytes and glomerular enlargement, interstitial fibrosis or tubular atrophy, microalbuminuria increase, and decline in glomerular filtration rate.7,8 Importantly, renal fibrosis is one of the prominent features of DN, which is the main reason for high morbidity and mortality of DN.9,10 In renal fibrosis, transforming growth factor-Beta (TGF-β) is a major regulator to promote the fibrosis.9 Chronic kidney diseases are characterized by the accumulation of extracellular matrix (ECM) components in the glomeruli.11 Fibronectin is expressed in the ECM, the accumulation of which in the glomeruli is associated with degradation of renal function in patients with DN.12 In addition, type II collagen is one of the main components of the ECM.13 Fibronectin accretion could lead to an upregulation of collagen in patients with diabetes.14 Current treatments of DN are targeted on controlling hyperglycemia and hypertension, as well as inhibiting the renin-angiotensin system. However, these traditional therapies are not optimal.15,16 Therefore, it is necessary to find more effective methods and drugs for the treatment of DN.
A medicinal plant Silybum marianum was commonly used as a hepatoprotective medication to treat jaundice and enlarged liver and spleen.17 Silibinin (Silybin) (Figure 1A), a non-toxic polyphenolic flavonoid, is the main biologically active component extracted from S. marianum.18 It was reported that silibinin has anti-inflammation, antifungal, antioxidant, and anti-cancer effect.19–22 In addition, previous study had revealed that silibinin could be beneficial for T2DM because of its inhibitory effect on glucose-6-phosphatase and gluconeogenesis.23 Moreover, silibinin ameliorates DN via preventing kidney injury, oxidant stress, and activating AKT pathway.24 However, the effect of silibinin on renal fibrosis of DN remains unclear. In the present study, we explored the effect of silibinin on renal fibrosis in vitro and in vivo, and investigated the underlying molecular mechanism.
Materials and methods
Cell culture
Human renal glomerular endothelial cells (HRGECs) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (All from Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and maintained in an incubator at 37°C with 5% CO2. To establish in vitro renal fibrosis model, HRGECs were seeded into six-well plates. When cell confluence reached nearly 80%, the cells were treated with 100 nM recombinant human TGF-β1 (Pepro Tech, Rocky Hill, NJ, USA) for 48 hrs.
Cell viability
Cell viability was analyzed by using MTT assay (Beyotime, Shanghai, China). The cells were seeded in 96-well plates at the density of 2×103 cells/well. After treatment of silibinin (0, 25, 50, 75, and 100 μM) for 72 hrs, 10 μL MTT solution was added into each well and incubated for another 4 hrs. Then, the cells were added with 100 μL Formazan dissolving solution. The absorbance of cell was determined at 570 nm using a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell apoptosis
Cell apoptosis was determined by flow cytometry using FITC Annexin V Apoptosis Detection Kit I (BD Biosciences, Franklin Lakes, NJ, USA). Briefly, after treatment with various concentrations of silibinin (0, 50, 75, and 100 μM) for 72 hrs, the cells were harvested (1×106 cells/mL), washed twice with PBS and resuspended in Binding Buffer. 5 μL FITC Annexin V and 5 μL PI were incubated with 100 μL cell solution at room temperature for 15 mins in the dark. The staining cells were analyzed by flow cytometry using Gallios instrument (Beckman Coulter, Miami, FL, USA). The percentage of apoptotic cells was quantified.
Reverse transcription-quantitative polymerase chain reaction
Total RNA was extracted by using TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.). RNA integrity was measured by agarose gel electrophoresis. Then, the reverse transcription was conducted by using PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). PCR reactions were carried out by SYBR Premix Ex Taq II (Takara, Dalian, China) with Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The specific primers used were Collagen I F: 5ʹ-CGACGGGAGCAGCATTAGCA-3ʹ, R: 5ʹ-GCGCAGGGGCAAAATTCGAG-3ʹ; Fibronectin F: 5ʹ-CGGGAGCCTCGAAGAGCAAG-3ʹ, R: 5ʹ-GTAAAGCGCGCACACACTCG-3ʹ; α-SMA, F: 5ʹ-CTGTGGAACCAGCCTTGCCA-3ʹ, R: 5ʹ-TTTGGTGCAGCCCAGTGGAG-3ʹ; GAPDH, F: 5ʹ-TCATCCCTGCATCCACTGGT-3ʹ, R: 5ʹ-CTGGGATGACCTTGCCCA-3ʹ. The PCR conditions were as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s. The relative expression of gene was calculated using 2−ΔΔCt method by normalizing to GAPDH expression.
Western blot
The cells were lysed using RIPA lysis buffer (Beyotime, Shanghai, China) on the ice. The concentration of the protein was quantified using the BCA Protein Assay Kit (Beyotime, Shanghai, China). A total of 30 μg of protein was separated by SDS-PAGE and transferred onto PVDF membranes (Beyotime, Shanghai, China). After blocking with 5% skim milk, the membranes were incubated with primary antibodies (anti-Collagen I: ab34710, 1:1,000; anti-Fibronectin: ab2413, 1:1,000; anti-α-SMA, ab5694, 1:1,000; anti-p-IkB, ab133462, 1:10,000; anti-p-p65, ab86299, 1:2,000; anti-GAPDH, ab181602, 1:10,000, Abcam, Cambridge, MA, USA) at 4°C overnight, and then incubated with secondary antibodies (Goat Anti-Rabbit IgG: ab205718, 1:2,000, Abcam, Cambridge, MA, USA) at room temperature for 1 hr. The bands of proteins were visualized using BeyoECL Plus (Beyotime, Shanghai, China) and examined using IPP Image-Pro Plus software. GAPDH was used as the internal control.
Immunofluorescence staining
After exposure to TGF-β1 and silibinin, the cells were fixed with 4% paraformaldehyde for 20 mins, permeabilized with 1% Triton X for 20 mins. After blocking with 5% skim milk, the cells were incubated with antibody against NF-κB (ab131546, 1:200, Abcam, Cambridge, MA, USA) at 4°C overnight. On the second day, the cells were incubated with goat-anti-rabbit IgG H&L secondary antibody, ab150077, 1:1,000, Abcam, Cambridge, MA, USA for 50 mins at 37°C, then incubated with DAPI for 10 mins at 37°C in the dark. Immunofluorescence was visualized under fluorescence microscope (Olympus, Tokyo, Japan).
Immunohistochemistry staining
The paraffin tissue sections were cut to 4 μm thick. After deparaffinizing with xylene and eluting with 100%, 95% 75%, and 50% alcohol, the sections were boiled with 0.01 M sodium citrate buffer for 8 mins. After blocking with 10% normal goat serum for 1 hr, the sections were incubated with anti-collagen I (ab34710, 1:200, Abcam, Cambridge, MA, USA) overnight at 4°C, and incubated with secondary antibody (Goat Anti-Rabbit IgG: ab205718, 1:2,000, Abcam, Cambridge, MA, USA) for 30 mins at room temperature. Finally, the sections were stained with DAB, counterstained with hematoxylin. Regarding as Masson staining, paraffin sections were stained with Masson composite staining solution, washed with 0.2% acetic acid solution and 5% phosphotungstic acid solution. After stained with bright green staining solution, the tissues were washed with 0.2% acetic acid solution, dehydrated in absolute alcohol. Finally, the tissues were sealed with neutral gum. The results were imaged with a microscope (Olympus, Tokyo, Japan).
Mouse model and silibinin treatment
Adult male C57BL/6J mice (6–8 weeks) were selected for this study. Every four mice housed in a cage under regular conditions (12 hrs light/dark cycles) with free access to water and food. Mice were fed two different diets. Randomly selected mice (n=36) fed HFD diet (60% fat, 20% protein, and 20% carbohydrate) for 30 weeks were used to establish hyperglycemia model, and other 12 mice were fed standard diet for 30 weeks. In the next 6 weeks, these mice were divided into four groups: control group (standard diet, n=12); HFD group (HFD diet, n=12); HFD+50 mg/kg silibinin (n=12); and HFD+100 mg/kg silibinin (n=12). Mice in HFD+50 mg/kg silibinin and HFD+100 mg/kg silibinin groups were treated with 50 or 100 mg/kg/day silibinin dissolved in distilled water, while mice in control and HFD groups were treated with equal volume of distilled water. After treatment, bodyweight of each mouse was detected. Fasting glucose was measured by using a glucometer (Roche, Basel, Switzerland). Microalbuminuria was determined by mouse microalbuminuria ELISA Kit (Boyao, Shanghai, China). The kidney of each mouse was dissected, and kidney weight/body weight and area of fibrosis was examined. Renal fibrosis was measured by staining renal sections with Masson staining.25 The area of fibrosis was quantified by measuring the ratio of blue staining area to total glomerular area. The animal experimental protocol was approved by the Ethics Committee of Jining No.1 People’s Hospital. National Institutes of Health guide for the care and use of laboratory animals was strictly followed by us.
Statistical analysis
Statistical analysis was performed by using GraphPad Prism software (version 7, La Jolla, CA, USA). The data were presented as mean ± standard deviation (SD) of at least three independent experiments. One-way ANOVA followed by Tukey’s test was Student’s t-test was performed to analyze difference among groups. P-value less than 0.05 was considered as a significant difference.
Results
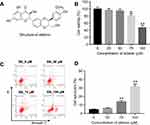
The cytotoxic effect of silibinin on HRGECs
To investigate the cytotoxic effect of silibinin on HRGECs, MTT assay was performed. As shown in Figure 1B, As compared to 0 μM group, 25 and 50 μM silibinin did not affect cell viability, while 75 and 100 μM silibinin significantly inhibited cell viability (75 μM: P<0.05; 100 μM: P<0.01). To assess the effect of silibinin on cell apoptosis, flow cytometry was used. The data demonstrated that 75 or 100 μM silibinin evidently increased apoptosis of HRGECs, compared with 0 μM group (P<0.01, Figure 1C and D). These data suggested that high concentration of silibinin (≥75 μM) had significant cytotoxicity via inducing apoptosis. Therefore, the nontoxic concentration of 50 μM was selected for further analysis.
Silibinin reversed TGF-β1-induced fibrosis of endothelial cells in vitro
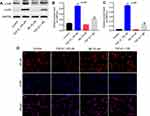
Next, in order to establish cell fibrosis in vitro model, HRGECs were treated with 100 nM TGF-β1, and the expression of fibrotic markers was measured by qPCR and Western blot. As indicated in Figure 2A–E TGF-β1 treatment greatly upregulated the mRNA and protein levels of collagen I, fibronectin and α-SMA compared with the control group (P<0.01). In contrast, 50 μM silibinin markedly attenuated the upregulation of collagen I, fibronectin and α-SMA induced by TGF-β1 (P<0.01). These results indicated that silibinin could reverse TGF-β1-induced fibrosis of endothelial cells in vitro.
Silibinin reversed TGF-β1-induced fibrosis of HRGECs via inhibition of the NF-κB pathway in vitro
The present study further detected the effect of silibinin on p-IκB, p-p65 levels in order to explore the role of NF-κB pathway during TGF-β1-induced renal fibrosis. As illustrated in Figure 3A–C, TGF-β1 induced an increased phosphorylation of IκB and p65 (P<0.01), while silibinin attenuated these elevations (P<0.01). In addition, immunofluorescence data demonstrated that TGF-β1 induced NF-κB nuclear translocation was notably inhibited by silibinin (Figure 3D). These data suggested silibinin reversed TGF-β1-induced fibrosis of HRGECs via inhibition of the NF-κB pathway in vitro.
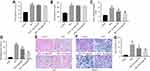
Silibinin inhibited HFD-induced renal fibrosis of mice in vivo
To investigate the anti-fibrosis role of silibinin in vivo, HFD-induced renal fibrosis of mice model was established. The result indicated HFD significantly increased body weight of mice, compared with control group (P<0.01), while silibinin had no effect on body weight of mice (Figure 4A). Similarly, HFD resulted in upregulation of fasting glucose (P<0.01); however, neither 50 mg/kg nor 100 mg/kg silibinin affected fasting glucose in HFD mice (Figure 4B). Additionally, kidney weight/body weight and microalbuminuria of HFD mice were greatly increased, which were markedly reduced in HFD +50 mg/kg silibinin (kidney weight/body weight: P<0.05; microalbuminuria: P<0.01) and HFD+100 mg/kg silibinin groups (kidney weight/body weight: P<0.01; microalbuminuria: P<0.01, Figure 4C and D). Furthermore, the data of HE staining demonstrated that the status and area of fibrosis in kidneys were markedly increased in HFD mice, while fibrosis area induced by HFD was notably reversed by silibinin (50 mg/kg: P<0.05; 100 mg/kg: P<0.01) (Figure 4E–G). All these results suggested that silibinin inhibited HFD-induced renal fibrosis of mice in vivo.
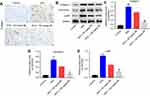
Silibinin reversed HFD-induced upregulation of collagen I, fibronectin, and p-p65 in vivo
Finally, the expression of collagen I, fibronectin, and p-p65 was measured in mice kidneys. Immunohistochemistry results demonstrated that collagen I expression was increased under HFD condition, while 50 or 100 mg/kg silibinin decreased its expression significantly (Figure 5A). The data of Western blot consistent with immunohistochemistry (100 mg/kg: P<0.01; Figure 5B and C). Additionally, the protein expression of fibronectin and p-p65 was upregulated in the HFD group, which was downregulated by 50 or 100 mg/kg silibinin (P<0.01; Figure 5B, D, and E). These results further demonstrated that silibinin reversed HFD-induced upregulation of collagen I, fibronectin, and p-p65 in vivo via inhibition of the NF-κB pathway.
Discussion
In the present study, we explored the effects of silibinin on DN. We found that silibinin reduced the expression of main markers of fibrosis, represented by collagen I, fibronectin, and α-SMA in TGF-β1 treated HRGECs. Additionally, silibinin also decreased kidney hypertrophy and renal fibrosis status in HFD-induced renal fibrosis of mice. Moreover, silibinin downregulated the protein levels of p-IκB, p-p65, and NF-κB. These results suggested that silibinin plays an important role in renal fibrosis of DN through inactivation of the NF-κB pathway.
With the development of the society, HFD is common. However, HFD seriously threaten human health, such as causing gastrointestinal diseases,26 obesity27, and hyperinsulinemia.28 Obesity is a key factor in the development of DN. There is no effective treatment for DN. Previous studies have reported that some compounds as able to prevent DN. For example, thymol afforded protection against HFD-induced DN.29 Myrciaria cauliflora extracts are benefit to DN through inhibiting Ras/PI3K/AKT pathway and suppressing kidney fibrosis-related protein.30 In addition, Ramulus mori polysaccharides exerts antioxidant effect to protect kidney injury and improve the renal function of diabetic rats.31 Silibinin is a kind of flavonoid compound, which has biological activities and medicinal value. Researches have revealed that the effectiveness of silibinin in diabetic complications including neuropathy, hepatopathy, cardiomyopathy, nephropathy, and so on.32 Silibinin could protect high glucose-induced podocyte injury through preventing oxidant stress.33 Moreover, silibinin prevents kidney injury, attenuates oxidant stress, decreases serum insulin level to ameliorate DN.24 Besides, some researches have revealed that silibinin delays the process of fibrosis, including but not limited to intestines,34 lung35, and liver.36 In the present study, silibinin was shown to attenuate renal fibrosis in vitro. 50 μM silibinin downregulated TGF-β1-induced upregulation of the expression of collagen I, fibronectin, and α-SMA. Moreover, silibinin decreased HFD-induced the ratio of kidney weight to body weight, microalbuminuria, and the area of renal fibrosis. These results all demonstrated that silibinin is able to attenuate renal fibrosis in vitro and in vivo.
NF-κB transcription factor regulates various components such as pro-inflammatory cytokines, chemokines, adhesion molecules, and inducible enzymes to adjust the immune response.37 Activation of NF-κB signaling pathway involved in the development of inflammation, atherosclerosis, Alzheimer’s disease, and human cancers.38 Silibinin inhibits the progression of several diseases through this pathway. Silibinin inhibits lipopolysaccharide-induced lung injury, exerts anti-inflammatory effect through suppressing NF-κB signaling pathway.39 In addition, silibinin suppresses colorectal cancer growth and progression through the NF-κB pathway against chronic inflammation.40 Previous studies reported that NF-κB signaling pathway is involved in chronic kidney disease, which mediated inflammation and renal fibrosis.41 Such as protein kinase CK2α and sphingosine kinase 1 ameliorate diabetic renal inflammatory fibrosis through the NF-κB pathway.42,43 In the present study, silibinin decreased the upregulation of phosphorylation of IκB and P65 induced by TGF-β1. In addition, Silibinin prevented an increase in NF-κB activity. Meanwhile, silibinin suppressed the protein level of p-p65 in vivo. These findings suggested that silibinin inhibits renal fibrosis via inhibition of NF-κB. Three major glomerular cell types are involved in the fibrotic process including podocytes or visceral epithelial cells, mesangial cells, and endothelial cells.11 However, this study focused only on the role of silibinin on endothelial cells. Therefore, further studies are needed to clarify the role of silibinin on podocytes or visceral epithelial cells and mesangial cells.
Conclusion
This study for the first time to demonstrate that silibinin attenuated renal fibrosis of DN in vitro and in vivo via inhibition of NF-κB. These findings indicated that silibinin might serve as an effective agent for delaying the treatment of DN.
Abbreviations
DM, diabetes mellitus; DN, diabetic nephropathy; HFD, high-fat diet; HRGECs, human renal glomerular endothelial cells; NF-κB, nuclear factor kappa beta; TGF-β1, transforming growth factor-Beta 1.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tripathi BK, Srivastava AK. Diabetes mellitus: complications and therapeutics. Med Sci Monit. 2006;12:RA130–RA147.
2. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi:10.1016/S0140-6736(16)00618-8
3. Mokashi P, Bhatt LK, Khanna A, Pandita N. Swertisin rich fraction from Enicostema littorale ameliorates hyperglycemia and hyperlipidemia inhigh-fat fed diet and low dose streptozotacin induced type 2 diabetes mellitus in rats. Biomed Pharmacother. 2017;96:1427–1437. doi:10.1016/j.biopha.2017.09.153
4. Matheus VA, Monteiro L, Oliveira RB, Maschio DA, Collares-Buzato CB. Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Exp Biol Med (Maywood). 2017;242:1214–1226. doi:10.1177/1535370217708188
5. Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11:1185–1200. doi:10.7150/ijms.10001
6. Tziomalos K, Athyros VG. Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabet Stud. 2015;12:110–118. doi:10.1900/RDS.2015.12.110
7. Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333–2340. doi:10.1172/JCI72271
8. Furuichi K, Shimizu M, Okada H, Narita I, Wada T. Clinico-pathological features of kidney disease in diabetic cases. Clin Exp Nephrol. 2018;22:1046–1051. doi:10.1007/s10157-018-1556-4
9. Tang F, Hao Y, Zhang X, Qin J. Effect of echinacoside on kidney fibrosis by inhibition of TGF-β1/Smads signaling pathway in the db/db mice model of diabetic nephropathy. Drug Des Devel Ther. 2017;11:2813–2826. doi:10.2147/DDDT.S143805
10. Zanchi C, Macconi D, Trionfini P, et al. MicroRNA-184 is a downstream effector of albuminuria driving renal fibrosis in rats with diabetic nephropathy. Diabetologia. 2017;60:1114–1125. doi:10.1007/s00125-017-4248-9
11. Lopez-Hernandez FJ, Lopez-Novoa JM. Role of TGF-beta in chronic kidney disease: an integration of tubular, glomerular and vascular effects. Cell Tissue Res. 2012;347:141–154. doi:10.1007/s00441-011-1275-6
12. Klemis V, Ghura H, Federico G, et al. Circulating fibronectin contributes to mesangial expansion in a murine model of type 1 diabetes. Kidney Int. 2017;91:1374–1385. doi:10.1016/j.kint.2016.12.006
13. Wang D, Lin N, Tang Y, Lu H. Inhibition of P2Y11R ameliorated TNF-alpha-induced degradation of extracellular matrix in human chondrocytic SW1353 cells. Am J Transl Res. 2019;11:2108–2116.
14. Zent R, Yan X, Su Y, et al. Glomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int. 2006;70:460–470. doi:10.1038/sj.ki.5000359
15. Toth-Manikowski S, Atta MG. Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res. 2015;2015:697010. doi:10.1155/2015/815839
16. Kim Y, Park CW. New therapeutic agents in diabetic nephropathy. Korean J Intern Med. 2017;32:11–25. doi:10.3904/kjim.2016.174
17. Bahmani M, Shirzad H, Rafieian S, Rafieian-Kopaei M. Silybum marianum: beyond hepatoprotection. J Evid Based Complementary Altern Med. 2015;20:292–301. doi:10.1177/2156587215571116
18. Zou H, Zhu XX, Zhang GB, et al. Silibinin: an old drug for hematological disorders. Oncotarget. 2017;8:89307–89314. doi:10.18632/oncotarget.19153
19. Zheng W, Feng Z, Lou Y, et al. Silibinin protects against osteoarthritis through inhibiting the inflammatory response and cartilage matrix degradation in vitro and in vivo. Oncotarget. 2017;8:99649–99665. doi:10.18632/oncotarget.20587
20. Yun DG, Lee DG. Assessment of silibinin as a potential antifungal agent and investigation of its mechanism of action. IUBMB Life. 2017;69:631–637. doi:10.1002/iub.1647
21. Haddad Y, Vallerand D, Brault A, et al. Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid Based Complement Alternat Med. 2011;2011:nep164. doi:10.1155/2011/196190
22. Davatgaran-Taghipour Y, Masoomzadeh S, Farzaei MH, et al. Polyphenol nanoformulations for cancer therapy: experimental evidence and clinical perspective. Int J Nanomedicine. 2017;12:2689–2702. doi:10.2147/IJN.S131973
23. Guigas B, Naboulsi R, Villanueva GR, et al. The flavonoid silibinin decreases glucose-6-phosphate hydrolysis in perfused rat hepatocytes by an inhibitory effect on glucose-6-phosphatase. Cell Physiol Biochem. 2007;20:925–934. doi:10.1159/000110453
24. Liu Y, Ye J, Cao Y, et al. Silibinin ameliorates diabetic nephropathy via improving diabetic condition in the mice. Eur J Pharmacol. 2019;845:24–31. doi:10.1016/j.ejphar.2018.12.031
25. Lu Q, Ma Z, Ding Y, et al. Circulating miR-103a-3p contributes to angiotensin II-induced renal inflammation and fibrosis via a SNRK/NF-kappaB/p65 regulatory axis. Nat Commun. 2019;10:2145. doi:10.1038/s41467-019-10116-0
26. Zhang M, Yang XJ. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J Gastroenterol. 2016;22:8905–8909. doi:10.3748/wjg.v22.i40.8905
27. Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi:10.1017/S0954422410000168
28. Kanno A, Asahara SI, Masuda K, et al. Compensatory hyperinsulinemia in high-fat diet-induced obese mice is associated with enhanced insulin translation in islets. Biochem Biophys Res Commun. 2015;458:681–686. doi:10.1016/j.bbrc.2015.02.024
29. Saravanan S, Pari L. Protective effect of thymol on high fat diet induced diabetic nephropathy in C57BL/6J mice. Chem Biol Interact. 2016;245:1–11. doi:10.1016/j.cbi.2015.11.033
30. Wu CC, Hung CN, Shin YC, Wang C-J, Huang H-P. Myrciaria cauliflora extracts attenuate diabetic nephropathy involving the Ras signaling pathway in streptozotocin/nicotinamide mice on a high fat diet. J Food Drug Anal. 2016;24:136–146. doi:10.1016/j.jfda.2015.10.001
31. Li X, Wang L, Gao X, et al. Mechanisms of protective effect of Ramulus Mori polysaccharides on renal injury in high-fatdiet/streptozotocin-induced diabetic rats. Cell Physiol Biochem. 2015;37:2125–2134. doi:10.1159/000438570
32. Chu C, Li D, Zhang S, et al. Role of silibinin in the management of diabetes mellitus and its complications. Arch Pharm Res. 2018;41:785–796. doi:10.1007/s12272-018-1047-x
33. Khazim K, Gorin Y, Cavaglieri RC, Abboud HE, Fanti P. The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol Renal Physiol. 2013;305:F691–F700. doi:10.1152/ajprenal.00028.2013
34. Kim JS, Han NK, Kim SH, Lee H-J. Silibinin attenuates radiation-induced intestinal fibrosis and reverses epithelial-to-mesenchymal transition. Oncotarget. 2017;8:69386–69397. doi:10.18632/oncotarget.20624
35. Son Y, Lee HJ, Rho JK, et al. The ameliorative effect of silibinin against radiation-induced lung injury: protection of normal tissue without decreasing therapeutic efficacy in lung cancer. BMC Pulm Med. 2015;15:68. doi:10.1186/s12890-015-0055-6
36. Ezhilarasan D, Karthikeyan S, Vivekanandan P. Ameliorative effect of silibinin against N-nitrosodimethylamine-induced hepatic fibrosis in rats. Environ Toxicol Pharmacol. 2012;34:1004–1013. doi:10.1016/j.etap.2012.07.004
37. Moynagh PN. The NF-κB pathway. J Cell Sci. 2005;118:4589–4592. doi:10.1242/jcs.02579
38. Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi:10.1172/JCI11914
39. Tian L, Li W, Wang T. Therapeutic effects of silibinin on LPS-induced acute lung injury by inhibiting NLRP3 and NF-κB signaling pathways. Microb Pathog. 2017;108:104–108. doi:10.1016/j.micpath.2017.05.011
40. Raina K, Agarwal C, Agarwal R. Effect of silibinin in human colorectal cancer cells: targeting the activation of NF-κB signaling. Mol Carcinog. 2013;52:195–206. doi:10.1002/mc.21843
41. Lv W, Booz GW, Wang Y, Fan F, Roman RJ. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018;820:65–76. doi:10.1016/j.ejphar.2017.12.016
42. Huang J, Chen Z, Li J, et al. Protein kinase CK2α catalytic subunit ameliorates diabetic renal inflammatory fibrosis via NF-κB signaling pathway. Biochem Pharmacol. 2017;132:102–117. doi:10.1016/j.bcp.2017.02.016
43. Huang J, Li J, Chen Z, et al. Sphingosine kinase 1 mediates diabetic renal fibrosis via NF-κB signaling pathway: involvement of CK2α. Oncotarget. 2017;8:88988–89004. doi:10.18632/oncotarget.21640
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.