Back to Journals » Clinical Ophthalmology » Volume 10
Silent information regulator T1 in aqueous humor of patients with cataract
Authors Kondo A, Goto M, Mimura T, Matsubara M
Received 10 November 2015
Accepted for publication 16 December 2015
Published 15 February 2016 Volume 2016:10 Pages 307—312
DOI https://doi.org/10.2147/OPTH.S100213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Aki Kondo,1 Mari Goto,2 Tatsuya Mimura,1 Masao Matsubara1
1Department of Ophthalmology, Tokyo Women’s Medical University Medical Center East, 2Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, Tokyo, Japan
Purpose: Silent information regulator T1 (SIRT1), a member of the sirtuin family, has a preventive role in various ocular diseases. We evaluated the relations between the aqueous humor level of SIRT1 and age, sex, systemic diseases, the severity of lens opacity, and other factors.
Setting: This study was conducted at a university teaching hospital in Tokyo, Japan.
Design: This study was designed based on the consecutive case series.
Methods: Aqueous humor samples were obtained from 29 eyes of the 21 consecutive patients undergoing surgery for age-related cataract (ARC). SIRT1 levels were determined by enzyme-linked immunosorbent assay.
Results: Aqueous humor levels of SIRT1 showed a positive correlation with visual acuity (logarithm of the minimum angle of resolution) and with the severity of nuclear cataract (r=0.32 and 0.30, respectively, P<0.05). However, only visual acuity was correlated with SIRT1 according to the stepwise multiple regression analysis (P<0.05).
Conclusion: These findings suggest that SIRT1 may have an effect on the formation of ARC, acting as a defensive factor against ARC.
Keywords: SIRT1, sirtuin, cataract surgery, oxidative stress, resveratrol, ocular aging
Introduction
The sirtuins are a highly conserved family of nicotinamide adenine dinucleotide-dependent histone deacetylases that are involved in regulating the life span of diverse organisms. The human genome encodes seven different sirtuins (silent information regulator T1 [SIRT1]–7), which have a common catalytic core domain but distinct N- and C-terminal extensions. It has been suggested that dysfunction of sirtuins is associated with various age-related diseases, including cancer, type II diabetes, obesity-associated metabolic diseases, neurodegeneration, and cardiac aging, as well as the response to environmental stress. SIRT1 is one of the targets of resveratrol, a polyphenol SIRT1 activator that increases the lifespan and protects various organs from aging.1 The role of sirtuins in ocular aging has been examined in a number of animal studies.1 However, few authors have investigated the relation between SIRT1 and age-related cataract (ARC), even though it is the most common age-related ophthalmic disease leading to blindness, and (to our knowledge) there have been no studies on the relation between ARC and SIRT1 in aqueous humor. In persons with ARC, progressive cloudiness or opacity of the lens or capsule occurs with aging and leads to the impairment of vision. It is the leading cause of blindness worldwide, being responsible for binocular blindness in an estimated 17 million individuals. There is currently no effective method for preventing the onset/progression of cataract.2,3
Against this background, we hypothesized that SIRT1 may have an effect on the formation of ARC. Accordingly, this study was performed to measure the aqueous humor level of SIRT1 in patients with ARC and to evaluate the correlations of SIRT1 with various demographic, clinical, and ocular factors.
Patients and methods
Aqueous humor samples were obtained from 29 eyes of the 21 patients with ARC who underwent cataract surgery between March and October 2013 at the Department of Ophthalmology, Tokyo Women’s Medical University Medical Center East. Written informed consent was obtained from all patients, and the protocol of this study was approved by the Ethics Committee of Tokyo Women’s Medical University. The mean age of the patients was 78.3±3.7 years (range 65–92 years). Samples were obtained from 12 eyes of the nine males and 17 eyes of the 12 females. Prior to surgery, the skin around the eye was disinfected with popiyodon solution 10% (Yoshida Seiyaku Inc., Tokyo, Japan), and the conjunctival sac was washed with fourfold diluted PA·IODO Ophthalmic and Eye washing Solution (Nihon Tengan Kenkyujyo, Aichi, Japan) under topical anesthesia with 4% lidocaine hydrochloride ophthalmic solution. The eyelids were draped with Tegaderm transparent film dressing (3M, Tokyo, Japan). After placing a lid retractor, paracentesis was performed at the limbus with a 27 G needle to obtain aqueous humor samples, which were stored in 1.5 mL microtubes in a freezer at −18°C. Information on the history of hypertension, diabetes, hypercholesterolemia, heart disease, cerebrovascular disease, and oral antiplatelet drug therapy was obtained from the patient, a family member, or the family doctor. Preoperative examination of visual acuity (logarithm of the minimum angle of resolution [logMAR]) was performed using a 5 m distance visual acuity chart (Inami Inc., Tokyo, Japan). The axial length, preoperative anterior chamber depth, and lens thickness were measured using an Ultrasonic A/B Scanner and Biometer (UD-6000; Tomey Inc., Nagoya, Japan), while the corneal endothelial cell density was determined with a Specular Microscope XIII (NSP-9900II; Konan Medical Inc., Hyougo, Japan). The type and severity of each cataract were graded and recorded according to a modified version of the Lens Opacities Classification System III (LOCS III).4 Six slit lamp images were used for grading the nuclear color, while five nuclear retroillumination images were used to grade cortical cataract and posterior subcapsular cataract. Each LOCS III scale is a decimal scale ranging from 0.1 (completely clear or colorless lens) to 5.9 (maximum value on the cataract scales indicating complete opacification of the cortex or posterior capsule) or 6.9 (maximum value on the nuclear color scale indicating advanced opacification and brunescence of the nucleus).4 Anterior subcapsular cataract was graded as 0 (clear) or 1 (present) on slit lamp images.
Enzyme-linked immunosorbent assay
SIRT1 levels in aqueous humor were determined by enzyme-linked immunosorbent assay (ELISA) with a Sirtuin 1 (human intracellular) ELISA Kit (BioVision Inc., Milpitas, CA, USA) according to the manufacturer’s instructions. The assay was performed as follows.
The human SIRT1 quality control sample was reconstituted with 1 mL of distilled water, and the human SIRT1 standard was also reconstituted with 1 mL of distilled water to obtain a stock solution (20 ng/mL). Then, the standard curve was prepared by making twofold serial dilutions with 1× diluent. First, 300 μL of the SIRT1 standard (4 ng/mL) was added to 300 μL of 1× diluent to obtain a 2 ng/mL SIRT1 solution, and this 300 μL of the 2 ng/mL SIRT1 solution was added to 300 μL of 1× diluent to obtain a 1 ng/mL SIRT1 solution. This process was repeated further to obtain SIRT1 solutions of 0.5 ng/mL, 0.25 ng/mL, 0.125 ng/mL, 0.0625 ng/mL, and 0.03125 ng/mL.
Assay protocol
The required number of eight-well assay strips were inserted into the frame, followed by the addition of 100 μL of standards, samples, and quality control samples to the appropriate wells in duplicate. The plate was covered and incubated for 1 hour at 37°C, followed by aspiration and washing three times with 300 μL of 1× washing buffer. Then, 100 μL of detection antibody was added to each well, and the incubation and washing were repeated as earlier. Next, 100 μL of 1× detector was added to each well, and the plate was covered, incubated for 1 hour at 37°C, aspirated, and washed five times with 300 μL of 1× washing buffer. Then the plate was inverted and tapped on a stack of paper towels, 100 μL of tetramethylbenzidine substrate solution was added to each well, and the color was developed at room temperature in the dark for 10 minutes. The reaction was stopped by adding 100 μL of stop solution to each well, and the optical density was measured at 450 nm using an ELISA plate reader within 30 minutes.
Statistical analysis
Univariate analysis was performed to investigate the correlation between the SIRT1 level in aqueous humor and demographic factors (age and sex), clinical factors (hypertension, diabetes, hypercholesterolemia, heart disease, cerebrovascular disease, and antiplatelet therapy), and ocular factors (preoperative visual acuity [logMAR], axial length, corneal endothelial cell density, anterior chamber depth, lens thickness, nuclear color, cortical cataract, posterior subcapsular cataract, and anterior subcapsular cataract). Stepwise multiple regression analysis was also performed.
Results
Aqueous humor levels of SIRT1 are shown in Table 1. The correlation between the aqueous humor level of SIRT1 and age, sex, hypertension, diabetes, hypercholesterolemia, heart disease, cerebrovascular disease, oral antiplatelet therapy, preoperative visual acuity (logMAR), axial length, preoperative corneal endothelial cell density, preoperative anterior chamber depth, lens thickness, nuclear color, cortical cataract, posterior subcapsular cataract, and anterior subcapsular cataract was −0.03, 0.03, −0.14, −0.13, −0.01, −0.06, 0.01, 0.19, 0.32, −0.02, −0.15, 0.06, −0.31, 0.30, −0.14, 0.02, and 0.22, respectively (one-tailed Pearson’s correlation coefficients) (Table 2). Preoperative visual acuity (logMAR) and nuclear color showed a significant positive correlation (Figures 1 and 2). Thus, the aqueous humor level of SIRT1 became higher as preoperative visual acuity got worse and the nuclear cataract became harder.
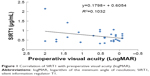  | Figure 1 Correlation of SIRT1 with preoperative visual acuity (logMAR). |
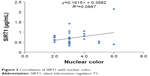  | Figure 2 Correlation of SIRT1 with nuclear color. |
However, only visual acuity (logMAR) was selected by stepwise multiple regression analysis, while the other factors did not show a significant correlation with the SIRT1 level in aqueous humor.
Discussion
In this study, we found that the aqueous humor level of SIRT1 increased as preoperative visual acuity declined and the nuclear cataract became more severe. To our knowledge, this is the first study that has shown SIRT1 in the aqueous humor may be associated with ARC and may regulate the senescence of lens cells in patients with cataract.
Jaliffa et al performed a detailed examination of the localization of SIRT1 in adult mouse eyes by immunohistochemistry and Western blotting, and they identified SIRT1 in the nucleus and/or cytoplasm of cells in all normal ocular structures, including the cornea, lens, iris, ciliary body, and retina.5 In the lens, SIRT1 was principally localized in the nuclei of epithelial cells and fiber cells, while it was not detected in the lens capsule.
In humans, expression of SIRT1 has also been detected in the lens epithelium of patients with ARC2,3 and in the retina.6 Lin et al reported that SIRT1 expression was lower in patients ≥51 years old with lens opacity than in those <51 years old and was negatively correlated with age,3 suggesting that decreased SIRT1 expression in the lens epithelium is associated with a higher grade of cataract and an increased age. Interestingly, Zheng and Lu reported that SIRT1 expression in the lens epithelium decreases with age but is higher in patients with ARC >50 years old than in persons >50 years old without cataract, and they suggested that SIRT1 may have a protective effect against ARC.2 Moreover, an increase in SIRT1 expression in response to oxidative stress and its protective effect against such stress have been observed in retinal pigment epithelial cells.7
In this study, we showed that the aqueous humor level of SIRT1 was positively correlated with the progression of ARC. Our findings support the notion that SIRT1 expression may increase to compensate the insufficient defenses against factors, such as ultraviolet light and oxidative stress, thus playing a protective role against factors that promote ARC. Accordingly, it is possible that SIRT1 may have an effect on the development and/or progression of ARC. Zheng et al2 and Lin et al3 reported the relationship of SIRT1 with lens epithelial cells, but we did not investigate lens epithelial cells in this study.
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is found in grapes and other plants,8 and it has been reported to activate SIRT19 by inducing the deacetylation of peroxisome proliferator-activated receptor gamma coactivator-1α.10 Doganay et al reported that resveratrol prevented cataract formation in a rat model,11 and Pearson et al reported that resveratrol-fed elderly mice showed a marked reduction in ARC.12 These findings suggest that the activation of SIRT1 by resveratrol may have a protective effect against cataract.
In human and mouse livers, Biel et al reported that the loss of SIRT1 led to defective autophagy, mitochondrial dysfunction, and death of hepatocytes after ischemia–reperfusion injury, which is a major cause of morbidity and mortality after liver surgery.13 In addition, Kaur et al reported that the neuroprotective effect of ischemic postconditioning, an important adaptive technique for limiting cerebral ischemia–reperfusion injury, probably involves the activation of SIRT1 in mice.14 Furthermore, Wu et al reported that SIRT1 is a potential new target for the treatment of hepatocellular carcinoma.15
A SIRT1 activator (SRT1720), which is much more potent than resveratrol, has been identified and has been suggested as a potential drug for the treatment of diabetes.16 It is possible that SIRT1 activators may also be useful for the prevention/treatment of ARC.
This study had the following limitations. The number of patients was small, and there were many explanatory variables, which might explain why nuclear color was not selected by stepwise multiple regression analysis despite showing a significant positive correlation. In addition, there were nine patients with diabetes, one patient with age-related macular degeneration, and one patient with pigment degeneration, and there is a possibility that these conditions may have had an effect on the SIRT1 level.
Conclusion
Although animal studies have shown that SIRT1 regulates ocular aging and protects ocular tissues from oxidative stress, suggesting that it is an attractive target for the prevention of ocular aging, it is uncertain how closely animal data apply to humans.1 The SIRT1 activator is currently being investigated for its use in treating cardiovascular disease, malignancy, diabetes, and Alzheimer’s disease.1 The role of SIRT1 in ocular aging should be further clarified by more clinical studies, since there is a possibility that the activation of SIRT1 could be effective for the treatment of cataract.
SIRT1 may be involved in the regulation of cellular senescence and may have a protective effect against oxidative stress. Expression of SIRT1 has been detected in the retina and in the lens epithelium of patients with ARC.
In patients with ARC, the aqueous humor level of SIRT1 was positively correlated with the progression of cataract. Ocular expression of SIRT1 may increase as a defense against factors that promote ARC.
Disclosure
The authors report no conflicts of interest in this work.
References
Mimura T, Kaji Y, Noma H, Funatsu H, Okamoto S. The role of SIRT1 in ocular aging. Exp Eye Res. 2013;116:17–26. | ||
Zheng T, Lu Y. Changes in SIRT1 expression and its downstream pathways in age-related cataract in humans. Curr Eye Res. 2011;36:449–455. | ||
Lin T, Peng C, Chiou S, et al. Severity of lens opacity, age, and correlation of the level of silent information regulator T1 expression in age-related cataract. J Cataract Refract Surg. 2011;37:1270–1274. | ||
Chylack LT Jr, Wolfe JK, Singer DM, et al. The lens opacities classification system III. Arch Ophthalmol. 1993;111:831–836. | ||
Jaliffa C, Ameqrane I, Dansault A, et al. Sirt1 involvement in rd10 mouse retinal degeneration. Invest Ophthalmol Vis Sci. 2003;50:3562–3572. | ||
Maloney SC, Antecka E, Odashiro AN, et al. Expression of SIRT1 and DBC1 in developing and adult retinas. Stem Cells Int. 2012;2012:8. | ||
Wu Z, Lauer TW, Sick A, Hackett SF, Campochiaro PA. Oxidative stress modulates complement factor H expression in retinal pigmented epithelial cells by acetylation of FOXO3. J Biol Chem. 2007;282:22414–22425. | ||
Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. | ||
Costa Cdos S, Rohde F, Hammes TO, et al. Resveratrol upregulated SIRT1, FOXO1, and adiponectin and downregulated PPARgamma1-3 mRNA expression in human visceral adipocytes. Obes Surg. 2011;21: 356–361. | ||
Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PCG-1alpha. Cell. 2006;127:1109–1122. | ||
Doganay S, Borazan M, Iraz M, Cigremis Y. The effect of resveratrol in experimental cataract model formed by sodium selenite. Curr Eye Res. 2006;31:147–153. | ||
Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. | ||
Biel TG, Lee S, Flores-Toro JA, et al. Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse livers in a mitofusin 2-dependent manner. Cell Death Differ. Epub 2015 Jul 17. | ||
Kaur H, Kumar A, Jaggi AS, Singh N. Pharmacologic investigations on the role of Sirt-1 in neuroprotective mechanism of postconditioning in mice. J Surg Res. 2015;197:191–200. | ||
Wu Y, Meng X, Huang C, Li J. Emerging role of silent information regulator 1 (SIRT1) in hepatocellular carcinoma: a potential therapeutic target. Tumour Biol. 2015;36:4063–4074. | ||
Milne JC, Lambert PD, Schenk S, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


